Abstract
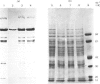
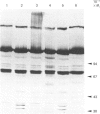
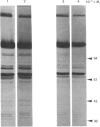
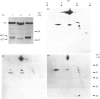
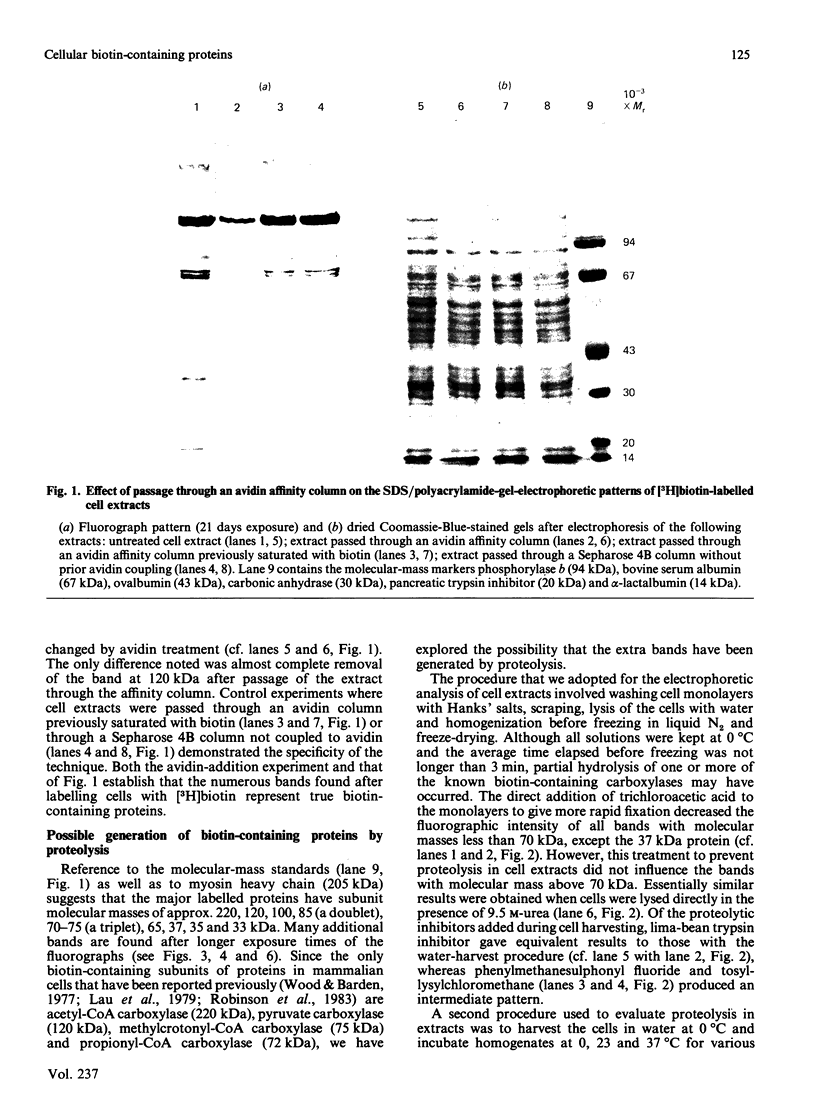
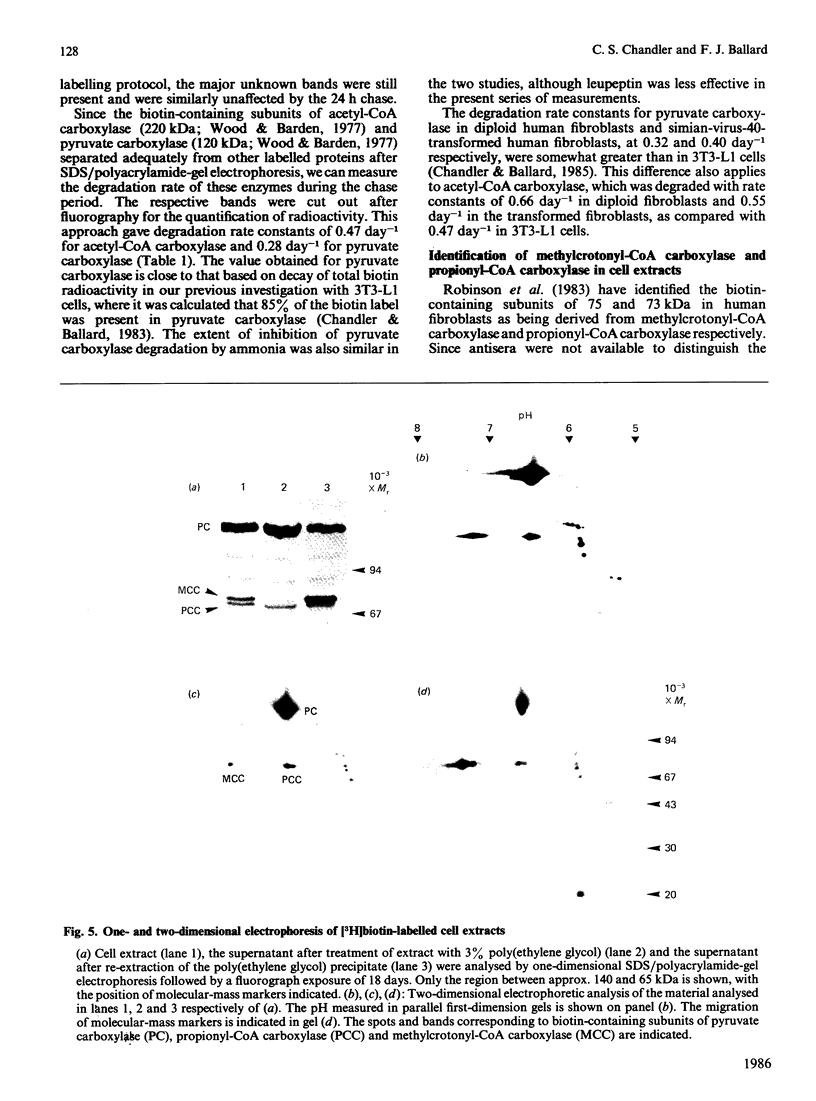
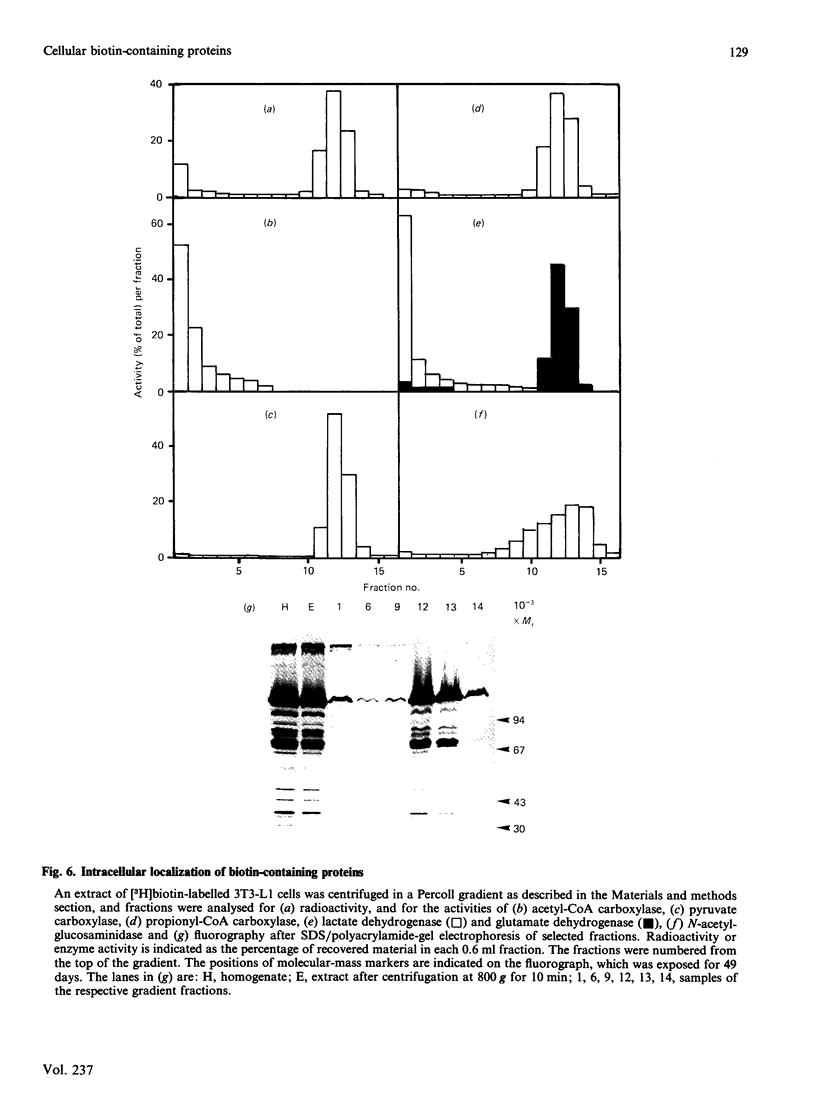
Extracts of 3T3-L1 cells prepared after labelling the monolayer cultures with [3H]biotin contained numerous protein bands that were detected by fluorography of dried SDS/polyacrylamide electrophoresis gels. All labelled proteins in the extracts could be removed by avidin affinity chromatography. The biotin-containing subunits of acetyl-CoA carboxylase, pyruvate carboxylase, methylcrotonyl-CoA carboxylase and propionyl-CoA carboxylase, with molecular masses of approx. 220, 120, 75 and 72 kDa respectively, were detected together with minor bands at 100, 85 and 37 kDa that did not appear to be partial degradation products. Additional labelled bands increased in amount during incubation of cell extracts or did not occur in extracts prepared with trichloroacetic acid, 9.5 M-urea or proteolytic inhibitors, and were tentatively classified as partial degradation products. The unknown bands were not removed by incubation of cell monolayers for 24 h, a treatment that gave degradation rate constants of 0.47 day-1 for acetyl-CoA carboxylase and 0.28 day-1 for pyruvate carboxylase. Upon two-dimensional electrophoresis, pyruvate carboxylase, methylcrotonyl-CoA carboxylase and propionyl-CoA carboxylase had isoelectric points of 6.4, 7.2 and 6.4 respectively. Several additional discrete spots with isoelectric points below 6.2 were also present. All the unknown biotin-containing proteins banded with intact mitochondria during density-gradient centrifugation. We conclude that several unknown biotin-containing proteins are present in the mitochondria of 3T3-L1 cells, whereas others are partial breakdown products of mitochondrial proteolysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard F. J. The development of gluconeogenesis in rat liver. Controlling factors in the newborn. Biochem J. 1971 Sep;124(2):265–274. doi: 10.1042/bj1240265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Distribution and degradation of biotin-containing carboxylases in human cell lines. Biochem J. 1985 Dec 1;232(2):385–393. doi: 10.1042/bj2320385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Inhibition of pyruvate carboxylase degradation and total protein breakdown by lysosomotropic agents in 3T3-L1 cells. Biochem J. 1983 Mar 15;210(3):845–853. doi: 10.1042/bj2100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freytag S. O., Utter M. F. Induction of pyruvate carboxylase apoenzyme and holoenzyme in 3T3-L1 cells during differentiation. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1321–1325. doi: 10.1073/pnas.77.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzner H. G., Ahmad P. M., Zegadlo J., Ahmad F. Immunofluorescent localization of acetyl CoA carboxylase, fatty acid synthetase and pyruvate carboxylase during the adipocyte conversion of 3T3 fibroblasts. Cell Biol Int Rep. 1980 May;4(5):497–508. doi: 10.1016/0309-1651(80)90037-5. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Grimaldi P., Négrel R., Ailhaud G. Induction of the triglyceride pathway enzymes and of lipolytic enzymes during differentiation in a 'preadipocyte' cell line. Eur J Biochem. 1978 Mar 15;84(2):369–376. doi: 10.1111/j.1432-1033.1978.tb12177.x. [DOI] [PubMed] [Google Scholar]
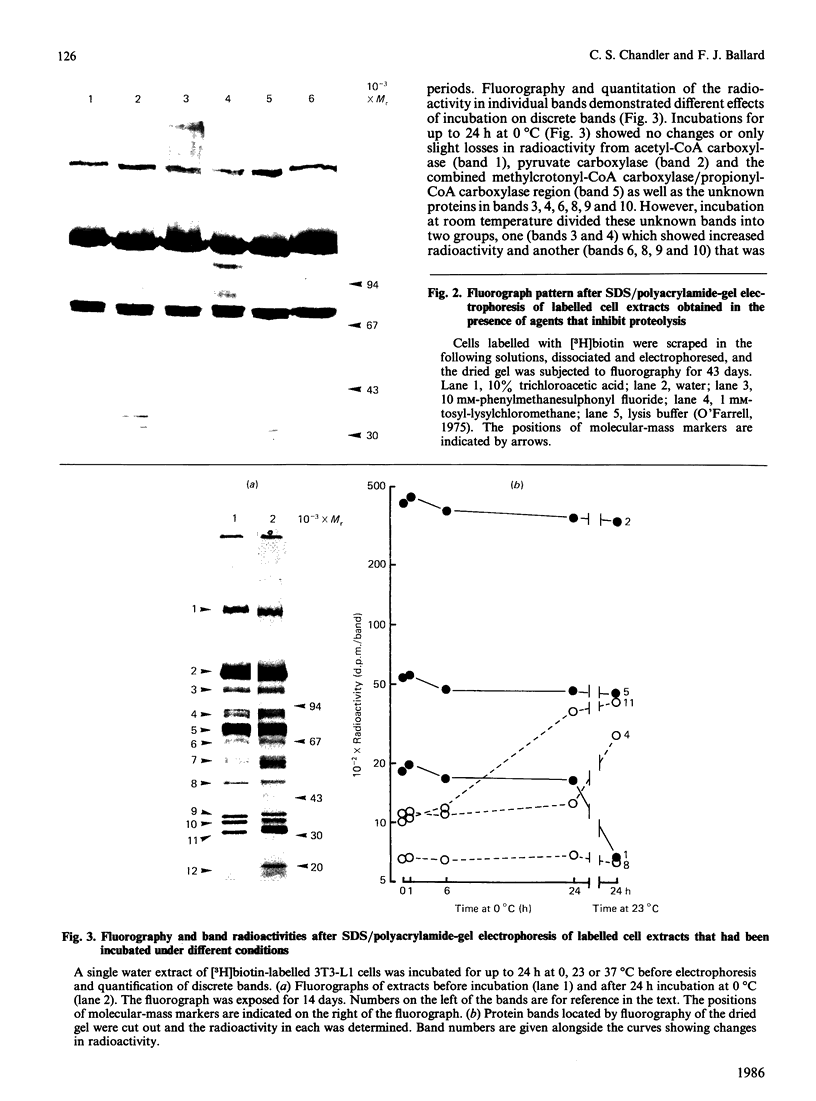
- Hopgood M. F., Ballard F. J. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. Biochem J. 1973 Jun;134(2):445–453. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. W., Utter M. F., Patel M. S. Induction of pyruvate dehydrogenase in 3T3-L1 cells during differentiation. J Biol Chem. 1983 Feb 25;258(4):2315–2320. [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Increasing activity of enzymes on pathway of triacylglycerol synthesis during adipose conversion of 3T3 cells. J Biol Chem. 1977 Mar 25;252(6):2158–2160. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lau E. P., Cochran B. C., Fall R. R. Isolation of 3-methylcrotonyl-coenzyme A carboxylase from bovine kidney. Arch Biochem Biophys. 1980 Dec;205(2):352–359. doi: 10.1016/0003-9861(80)90117-4. [DOI] [PubMed] [Google Scholar]
- Lau E. P., Cochran B. C., Munson L., Fall R. R. Bovine kidney 3-methylcrotonyl-CoA and propionyl-CoA carboxylases: each enzyme contains nonidentical subunits. Proc Natl Acad Sci U S A. 1979 Jan;76(1):214–218. doi: 10.1073/pnas.76.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Role of pyruvate carboxylase in fatty acid synthesis: alterations during preadipocyte differentiation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):720–725. doi: 10.1016/0006-291x(77)91171-8. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
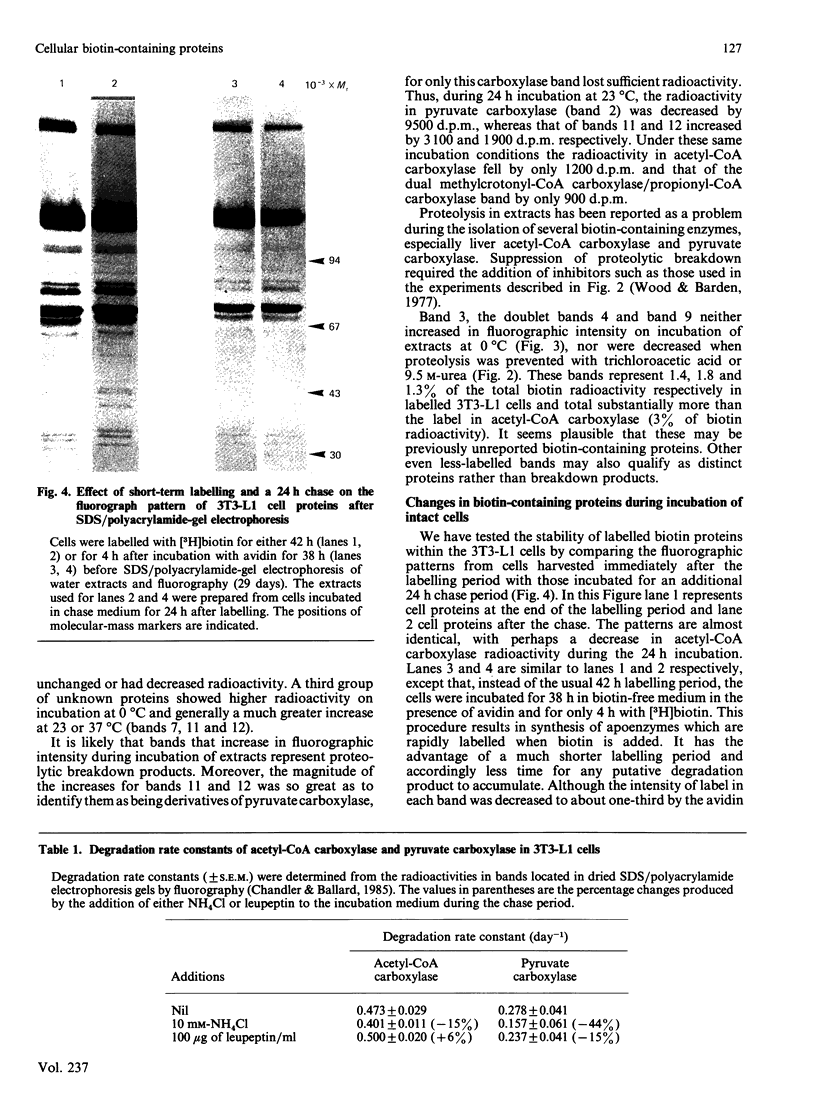
- Moss J., Lane M. D. The biotin-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Plattner H., Henning R. Isolation of hepatocyte-derived rat liver lysosomal fractions. Exp Cell Res. 1975 Mar 15;91(2):333–343. doi: 10.1016/0014-4827(75)90112-3. [DOI] [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Oei J., Saunders M., Gravel R. [3H]biotin-labeled proteins in cultured human skin fibroblasts from patients with pyruvate carboxylase deficiency. J Biol Chem. 1983 May 25;258(10):6660–6664. [PubMed] [Google Scholar]
- Swack J. A., Zander G. L., Utter M. F. Use of avidin-sepharose to isolate and idenify biotin polypeptides from crude extracts. Anal Biochem. 1978 Jun 15;87(1):114–126. doi: 10.1016/0003-2697(78)90575-4. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]