Abstract
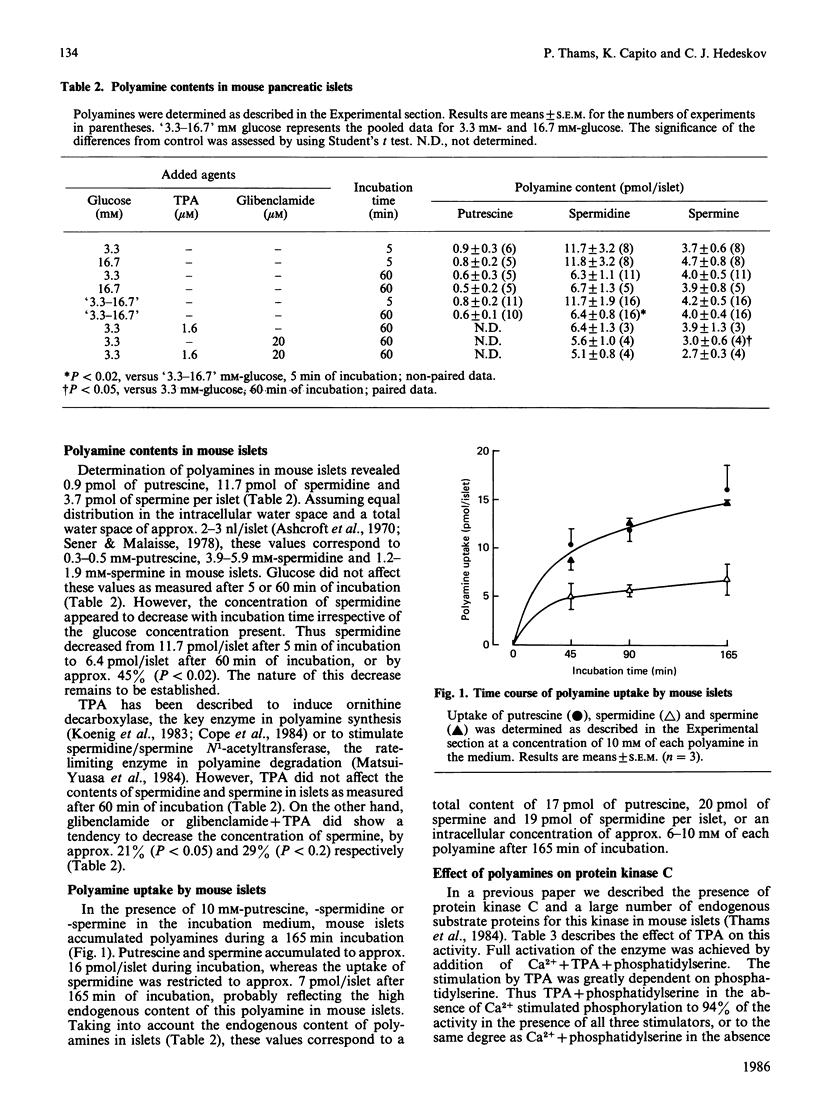
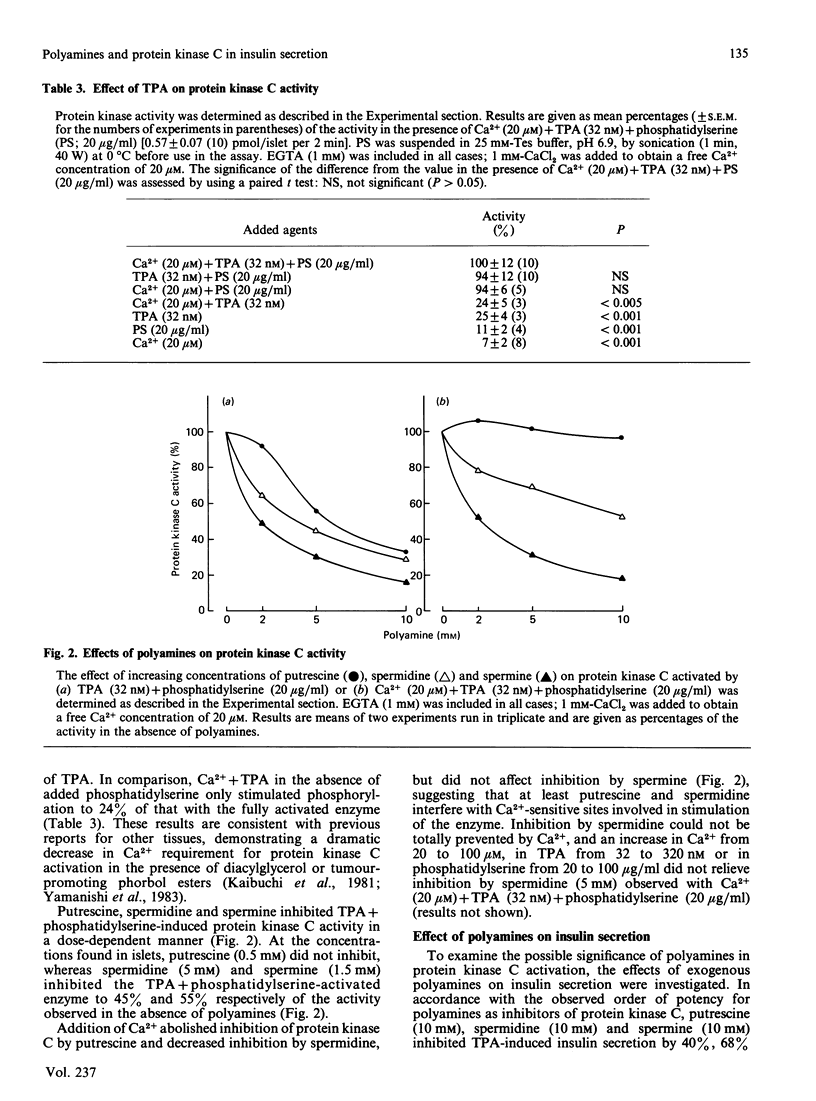
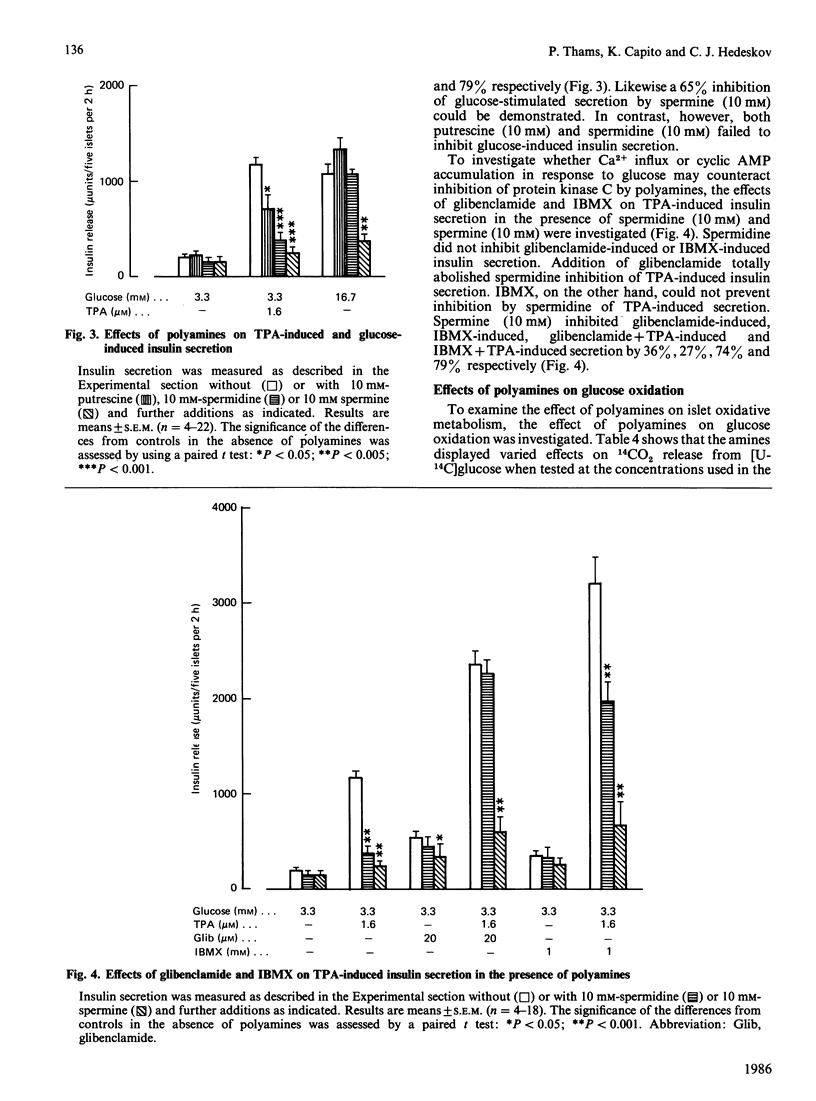
The occurrence and function of polyamines in protein kinase C activation and insulin secretion in mouse pancreatic islets were studied. Determination of polyamines in mouse islets revealed 0.9 +/- 0.3 (mean +/- S.E.M., n = 6) pmol of putrescine, 11.7 +/- 3.2 (8) pmol of spermidine and 3.7 +/- 0.6 (8) pmol of spermine per islet, corresponding to intracellular concentrations of 0.3-0.5 mM-putrescine, 3.9-5.9 mM-spermidine and 1.2-1.9 mM-spermine in mouse islets. Stimulation of insulin secretion by glucose, the phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) or the sulphonylurea glibenclamide did not affect these polyamine contents. In accordance with a role for protein kinase C in insulin secretion, TPA stimulated both protein kinase C activity and insulin secretion. Stimulation of insulin secretion by TPA was dependent on a non-stimulatory concentration of glucose and was further potentiated by stimulatory concentrations of glucose, glibenclamide or 3-isobutyl-1-methylxanthine, suggesting that protein kinase C activation, Ca2+ mobilization and cyclic AMP accumulation are all needed for full secretory response of mouse islets. Spermidine (5 mM) and spermine (1.5 mM) at concentrations found in islets inhibited protein kinase C stimulated by TPA + phosphatidylserine by 55% and 45% respectively. Putrescine (0.5 mM) was without effect, but inhibited the enzyme at higher concentrations (2-10 mM). Inhibition of protein kinase C by polyamines showed competition with Ca2+, and Ca2+ influx in response to glucose or glibenclamide prevented inhibition of insulin secretion by exogenous polyamines at concentrations where they did not affect glucose oxidation. It is suggested that inhibition of protein kinase C by polyamines may be of significance for regulation of insulin secretion in vivo and that Ca2+ influx may function by displacing inhibitory polyamines bound to phosphatidylserine in membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Hedeskov C. J., Randle P. J. Glucose metabolism in mouse pancreatic islets. Biochem J. 1970 Jun;118(1):143–154. doi: 10.1042/bj1180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungay P. J., Potter J. M., Griffin M. A role for polyamines in stimulus-secretion coupling in the pancreatic beta-cell. Biosci Rep. 1984 Oct;4(10):869–877. doi: 10.1007/BF01138169. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Christie M. R., Ashcroft S. J. Cyclic AMP-dependent protein phosphorylation and insulin secretion in intact islets of Langerhans. Biochem J. 1984 Feb 15;218(1):87–99. doi: 10.1042/bj2180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll-Garcia E., Gill J. R. Insulin release by isolated pancreatic islets of the mouse incubated in vitro. Diabetologia. 1969 Apr;5(2):61–66. doi: 10.1007/BF01211999. [DOI] [PubMed] [Google Scholar]
- Cope F. O., Conrad E. A., Staller J. M., Boutwell R. K. Induction of mouse brain ornithine decarboxylase by 12-O-tetradecanoylphorbol-13-acetate is independent of TPA receptor concentration. Cancer Lett. 1984 Jul;23(3):331–342. doi: 10.1016/0304-3835(84)90101-0. [DOI] [PubMed] [Google Scholar]
- Gagliardino J. J., Harrison D. E., Christie M. R., Gagliardino E. E., Ashcroft S. J. Evidence for the participation of calmodulin in stimulus-secretion coupling in the pancreatic beta-cell. Biochem J. 1980 Dec 15;192(3):919–927. doi: 10.1042/bj1920919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe E., Hellman B., Sehlin J., Täljedal B. Interaction of sulfonylurea with the pancreatic B-cell. Experientia. 1984 Oct 15;40(10):1126–1134. doi: 10.1007/BF01971460. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Ashcroft S. J. Effects of Ca2+, calmodulin and cyclic AMP on the phosphorylation of endogenous proteins by homogenates of rt islets of langerhans. Biochim Biophys Acta. 1982 Feb 2;714(2):313–319. doi: 10.1016/0304-4165(82)90339-7. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Effects of trifluoperazine and pimozide on stimulus-secretion coupling in pancreatic B-cells. Suggestion for a role of calmodulin? Biochem J. 1981 Jun 15;196(3):771–780. doi: 10.1042/bj1960771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. The interplay between cyclic AMP and ions in the stimulus-secretion coupling in pancreatic B-cells. Arch Int Physiol Biochim. 1985 May;93(1):37–48. doi: 10.3109/13813458509104514. [DOI] [PubMed] [Google Scholar]
- Janjic D., Wollheim C. B., Siegel E. G., Krausz Y., Sharp G. W. Sites of action of trifluoperazine in the inhibition of glucose-stimulated insulin release. Diabetes. 1981 Nov;30(11):960–966. doi: 10.2337/diab.30.11.960. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Koenig H., Goldstone A. D., Lu C. Y. Beta-adrenergic stimulation of Ca2+ fluxes, endocytosis, hexose transport, and amino acid transport in mouse kidney cortex is mediated by polyamine synthesis. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7210–7214. doi: 10.1073/pnas.80.23.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Ashcroft S. J. Identification and characterization of Ca2+-phospholipid-dependent protein kinase in rat islets and hamster beta-cells. Biochem J. 1984 Apr 15;219(2):547–551. doi: 10.1042/bj2190547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Lebrun P., Herchuelz A., Sener A., Malaisse-Lagae F. Synergistic effect of a tumor-promoting phorbol ester and a hypoglycemic sulfonylurea upon insulin release. Endocrinology. 1983 Nov;113(5):1870–1877. doi: 10.1210/endo-113-5-1870. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Herchuelz A., Carpinelli A. R., Poloczek P., Winand J., Castagna M. Insulinotropic effect of the tumor promoter 12-O-tetradecanoylphorbol-13-acetate in rat pancreatic islets. Cancer Res. 1980 Oct;40(10):3827–3831. [PubMed] [Google Scholar]
- Matsui-Yuasa I., Otani S., Shu Z. W., Morisawa S. Phorbol esters stimulate spermidine/spermine N1-acetyltransferase activity in mitogen-stimulated bovine lymphocytes. FEBS Lett. 1984 Dec 10;178(2):297–300. doi: 10.1016/0014-5793(84)80620-1. [DOI] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H. Effects of polyamines and polyanions on a cyclic nucleotide-independent and a cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1977 Jul 21;498(1):294–305. doi: 10.1016/0304-4165(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Paulus T. J., Davis R. H. A double-isotope derivative assay for polyamines. Methods Enzymol. 1983;94:36–42. doi: 10.1016/s0076-6879(83)94007-7. [DOI] [PubMed] [Google Scholar]
- Qi D. F., Schatzman R. C., Mazzei G. J., Turner R. S., Raynor R. L., Liao S., Kuo J. F. Polyamines inhibit phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J. 1983 Aug 1;213(2):281–288. doi: 10.1042/bj2130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Schuber F., Hong K., Düzgünes N., Papahadjopoulos D. Polyamines as modulators of membrane fusion: aggregation and fusion of liposomes. Biochemistry. 1983 Dec 20;22(26):6134–6140. doi: 10.1021/bi00295a015. [DOI] [PubMed] [Google Scholar]
- Sener A., Malaisse W. J. The metabolism of glucose in pancreatic islets. Diabete Metab. 1978 Jun;4(2):127–133. [PubMed] [Google Scholar]
- Sugden M. C., Christie M. R., Ashcroft S. J. Presence and possible role of calcium-dependent regulator (calmodulin) in rat islets of Langerhans. FEBS Lett. 1979 Sep 1;105(1):95–100. doi: 10.1016/0014-5793(79)80894-7. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tanigawa K., Kuzuya H., Imura H., Taniguchi H., Baba S., Takai Y., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase in rat pancreas islets of langerhans. Its possible role in glucose-induced insulin release. FEBS Lett. 1982 Feb 22;138(2):183–186. doi: 10.1016/0014-5793(82)80436-5. [DOI] [PubMed] [Google Scholar]
- Thams P., Capito K., Hedeskov C. J. Endogenous substrate proteins for Ca2+-calmodulin-dependent, Ca2+-phospholipid-dependent and cyclic AMP-dependent protein kinases in mouse pancreatic islets. Biochem J. 1984 Jul 1;221(1):247–253. doi: 10.1042/bj2210247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M. A., Steffes M. W., Estensen R. D. Phorbol myristate acetate: effect of a tumor promoter on insulin release from isolated rat islets of Langerhans. Endocrinology. 1978 Mar;102(3):706–711. doi: 10.1210/endo-102-3-706. [DOI] [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]
- Yamamoto M., Criss W. E., Takai Y., Yamamura H., Nishizuka Y. A hepatic soluble cyclic nucleotide-independent protein kinase. Stimulation by basic polypeptides. J Biol Chem. 1979 Jun 25;254(12):5049–5052. [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]


