Abstract
PURPOSE
MammaPrint (MP) determines distant metastatic risk and may improve patient selection for extended endocrine therapy (EET). This study examined MP in predicting extended letrozole therapy (ELT) benefit in patients with early-stage breast cancer (BC) from the NSABP B-42 trial.
PATIENTS AND METHODS
MP was tested in 1,866 patients randomly assigned to receive ELT or placebo. The primary end point was distant recurrence (DR). Secondary end points were disease-free survival (DFS) and BC-free interval (BCFI). Tumors were classified as MP high risk (MP-HR) or low risk (MP-LR). MP-LR tumors were further classified as ultralow risk (MP-UL) or low non-ultralow risk (MP-LNUL).
RESULTS
There was no statistically significant difference in ELT benefit on DR between MP-HR and MP-LR (interaction P = .38). MP-LR tumors (n = 1,160) exhibited a statistically significant 10-year benefit of 3.7% for DR (hazard ratio [HR], 0.43 [95% CI, 0.25 to 0.74]; P = .002), whereas MP-HR tumors (n = 706) exhibited a nonsignificant 2.4% benefit (HR, 0.65 [95% CI, 0.34 to 1.24]; P = .19). The 10-year ELT benefit was significant for DFS (7.8%) and BCFI (7.0%) for MP-LR tumors, whereas MP-HR tumors did not significantly benefit (interaction DFS: P = .015, BCFI: P = .006). In exploratory analysis, the 10-year ELT benefit was significant and more pronounced in MP-LNUL (n = 908) tumors: 4.0% for DR, 9.5% for DFS, and 7.9% for BCFI; the benefit in MP-UL (n = 252) tumors was not significant: 3% for DR, 1.8% for DFS, and 4.1% for BCFI.
CONCLUSION
The primary hypothesis of predictive ability of MP on DR was not confirmed. However, the secondary outcomes demonstrated MP was predictive of ELT response and identified a subset of patients with early-stage hormone receptor–positive BC (MP-LR) with improved outcomes from ELT. These data could have important clinical implications in patient selection beyond clinical risk assessment for EET.
INTRODUCTION
Patients with hormone receptor–positive (HR+), early-stage breast cancer (BC) are at risk of recurrence up to 20 years after diagnosis.1,2 Extended endocrine therapy (EET) has been shown to improve disease-free survival (DFS).3,4 Clinical trials have evaluated the optimal duration of EET beyond 5 years after initial hormone therapy in this patient population. NSABP B-42 was a randomized, double-blind, placebo-controlled, phase III trial that evaluated the effectiveness of EET on improving DFS in postmenopausal women with early, HR+ BC. Patients who were disease-free after 5 years of treatment with an aromatase inhibitor (AI) or tamoxifen followed by an AI were randomly assigned to receive 5 years of letrozole or placebo. In the original results with 6.9 years of median follow-up, the extended letrozole therapy (ELT) showed a beneficial effect on DFS, although it did not reach statistical significance as predefined in the protocol (hazard ratio [HR], 0.85 [95% CI, 0.73 to 0.999]; P = .048).5 In the updated results with 10.3 years of median follow-up, the beneficial effect of letrozole on DFS persisted (HR, 0.85 [95% CI, 0.74 to 0.96]; P = .01).6 Additionally, ELT provided significant reduction in the rates of BC-free interval (BCFI) events and distant recurrence (DR) events. There was no benefit from ELT on overall survival.
CONTEXT
Key Objectives
Patients with early-stage HR+ breast cancer (BC) remain at risk for late recurrence.
Knowledge Generated
The original NSABP B-42 trial in postmenopausal women with HR+ early-stage BC showed that extended endocrine therapy (EET) modestly reduces the risk of recurrence. This report shows there was no difference in benefit of EET on distant recurrence between women whose tumors scored high risk versus low risk on the MammaPrint (MP) assay.
Relevance (G.F. Fleming)
At this time scores on the MP assay should not be used to select which patients with HR+ early-stage BC should receive shorter versus longer durations of ET. These data raise the possibility that patients whose tumors score in the low-but-not -ultralow category may have most benefit from prolonged treatment in terms of disease-free survival and BC-free interval; this requires further validation.*
*Relevance section written by JCO Associate Editor Gini F. Fleming, MD, FASCO.
With modest ELT effect, it remains unclear which patients benefit from ELT and which may be receiving unnecessary ELT with its associated toxicities. Genomic classifiers that predict risk of recurrence may identify patients who receive benefit from ELT and assist with EET recommendations. The 70-gene MammaPrint (MP) test is a prognostic genomic assay that classifies tumors as high risk (MP-HR) or low risk (MP-LR) of DR.7-9 In the phase III MINDACT trial,7 patients with BC who were assessed as clinically high risk but classified as MP-LR had excellent outcomes without chemotherapy at 8 years of follow-up. Furthermore, within MP-LR tumors, MP can differentiate between patients with an ultralow risk (MP-UL) and low non-ultralow risk (MP-LNUL) of DR.10 The STO-3 trial demonstrated that postmenopausal patients with node-negative BC and MP-UL result had a 20-year BC-specific survival (BCSS) rate of 97% with 2 or more years of ET compared with 94% in untreated patients, indicating excellent outcome with little to no ET.11 By contrast, patients with MP-LNUL tumors exhibited a 50% risk reduction in BCSS events from tamoxifen treatment.10 Here, we hypothesized that genomic analysis with MP will predict benefit from ELT in NSABP B-42 patients, allowing better patient selection of those who will benefit from ELT.
PATIENTS AND METHODS
Patient Population and Correlative Study Design
All eligible B-42 patients with clinical follow-up and available untreated primary tumor tissue were included in this prospective-retrospective correlative study. Tumor blocks were sent to Agendia (Irvine, CA) and then the MP test scores were generated while being blinded to clinical outcome. The results of the MP test were merged with the clinical data for analysis.
The primary end point for this correlative study was DR, defined as time to DR. Secondary prespecified end points included DFS, defined as time to BC recurrence, second primary malignancy, or death, and BCFI, defined as time to local, regional, or DR, or contralateral BC as a first event. All time-to-event end points were measured from date of random assignment to date of diagnosis of the specified event. Event-free patients were censored at the date of last follow-up. Second primary cancers (nonbreast) and death without evidence of recurrence were treated as censored events for BCFI. Clinical assessment was required for determining patients' status for all end points.
The primary objective of the study was to determine the utility of MP to identify NSABP B-42 patients who would be more likely to benefit from ELT with reduced DR rate. It was hypothesized that BC samples with an MP-HR index would identify patients likely to benefit from ELT as measured by DR, whereas those with MP-LR would not show a significant ELT benefit. Some of the secondary objectives included the predictive ability of MP in terms of ELT benefit on DFS and BCFI and the identification of any clinicopathologic covariables, which may increase the clinical utility of MP. In an exploratory analysis, we evaluated whether a further range within the MP-LR index provides additional predictive ability.
Statistical Considerations
For the primary analysis, patients were classified as either MP-HR (MP score ≤0.000) or MP-LR (MP score >0.000). Exploratory analyses were performed for MP-LR subcategories: MP-UL (MP score >0.355) and MP-LNUL (MP score >0.000, ≤0.355). Patients included in the translational MP cohort were compared with other excluded B-42 patients in terms of patient and tumor characteristics, as well as treatment effect. The distribution of characteristics was also compared between treatment groups within the translational MP cohort. Kaplan-Meier (KM) technique was used to estimate 10-year event rates for all end points. Differences in the event rate between treatment groups at 10 years post–random assignment were computed as the 10-year rate in the placebo minus the 10-year rate in the ELT group. HR and corresponding 95% CIs were calculated on the basis of the stratified Cox proportional hazards model. Stratification factors included original stratification factors from the parent B-42 trial, that is, pathological node status at diagnosis (negative v positive), prior tamoxifen use (no v yes), and lowest bone mineral density T-score in the lumbosacral spine, total hip, or femoral neck (≤–2.0 or >–2.0 SD). The stratified log-rank test evaluated the differences between the two treatment groups. The likelihood ratio test evaluated treatment-by-MP risk group interaction. Similar analyses were repeated for subgroups of patients defined by node status. The assumption of proportionality of hazards for each time-to-event end point was tested. If not satisfied, a change point for the relative risk technique was used to identify the optimal time point to divide the time interval into the regions of proportionality.5 All reported P-values are two-sided, using P values <0.05 to indicate statistical significance with no adjustment for multiplicity as was predefined in the statistical analyses plan. All primary, secondary, and exploratory aims and analyses were prespecified in the statistical analyses plan. The statistical analyses were performed using SAS (v9.4). The data cutoff for reported analyses was April 30, 2020.
Laboratory Methods
MP was performed according to established protocols as previously described.12,13 RNA was isolated from formalin-fixed paraffin-embedded tissue at Agendia while being blinded for clinicopathologic data. Microarray gene expression data was generated using custom-designed arrays (Agilent Technologies, Santa Clara, CA).
RESULTS
Patient Characteristics
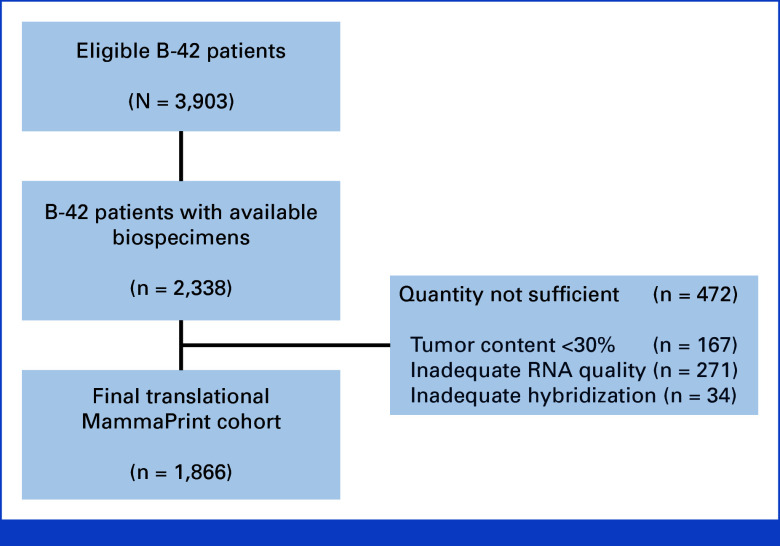
There were 3,966 patients randomly assigned in the B-42 trial to placebo or letrozole. Among them, 63 were excluded because of having either no clinical assessment or not being at risk for the primary DFS end point for the B-42 parent trial. Blocks were available for 2,338 patients who consented, with approval by a local Human Investigations Committee and in accordance with assurances filed with and approved by the Department of Health and Human Services, for the future research with the final translational MP cohort consisting of 1,866 patients (Fig 1). There were no differences in the distributions of patient and tumor characteristics between the translational MP cohort and the excluded B-42 cohort, except for the small differences in the human epidermal growth factor receptor 2 status and no differences between treatment groups within the translational MP cohort (Table 1). Compared with the excluded B-42 population, the MP cohort had slightly better prognosis in terms of DR (P = .036) with a more pronounced ELT effect on the DR rate (MP cohort: HR, 0.50 [95% CI, 0.33 to 0.75]; excluded cohort: HR, 0.92 [95% CI, 0.66 to 1.29]; treatment-by-inclusion status interaction P = .03). The median follow-up time for patients included in the MP cohort was 10.4 years, comparable with the excluded cohort (10.2 years). Among 1,866 patients, 706 (37.8%) were MP-HR and 1,160 (62.2%) were MP-LR (MP-UL: 252 [13.5%], MP-LNUL: 908 [48.7%]).
FIG 1.
REMARK diagram shows 2,338 eligible B-42 patients with available biospecimens. Insufficient quantity and quality of RNA excluded 472 tumors leading to a final translational MammaPrint cohort of 1,866 patients.
TABLE 1.
Distribution of Demographic, Clinical, and Treatment Characteristics of Patients in NSABP B-42 According to Inclusion in the Translational MP Cohort
| Characteristic | Translational MP Cohort | P a | Translational MP Cohort (n = 1,866) | Excluded B-42 Cohort (n = 2,037) | P b | Overall B-42 Population (N = 3,903) | |
|---|---|---|---|---|---|---|---|
| Placebo (n = 950) | Letrozole (n = 916) | ||||||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||
| Age at random assignment, yrs | |||||||
| <60 | 306 (32.2) | 317 (34.6) | .27 | 623 (33.4) | 721 (35.4) | .19 | 1,344 (34.4) |
| ≥60 | 644 (67.8) | 599 (65.4) | 1,243 (66.6) | 1,316 (64.6) | 2,559 (65.6) | ||
| Pathologic nodal status | |||||||
| Negative | 547 (57.6) | 506 (55.2) | .31 | 1,053 (56.4) | 1,187 (58.3) | .25 | 2,240 (57.4) |
| Positive | 403 (42.4) | 410 (44.8) | 813 (43.6) | 850 (41.7) | 1,663 (42.6) | ||
| Lowest BMD T-score | |||||||
| ≤–2.0 | 237 (24.9) | 232 (25.3) | .85 | 469 (25.1) | 485 (23.8) | .34 | 954 (24.4) |
| >–2.0 | 713 (75.1) | 684 (74.7) | 1,397 (74.9) | 1,552 (76.2) | 2,949 (75.6) | ||
| Received prior tamoxifen | |||||||
| No | 581 (61.2) | 559 (61.0) | .95 | 1,140 (61.1) | 1,237 (60.7) | .81 | 2,377 (60.9) |
| Yes | 369 (38.8) | 357 (39.0) | 726 (38.9) | 800 (39.3) | 1,526 (39.1) | ||
| HER2 status | |||||||
| Negative | 742 (78.1) | 737 (80.5) | .15 | 1,479 (79.3) | 1,562 (76.7) | .04 | 3,041 (77.9) |
| Positive | 134 (14.1) | 128 (14.0) | 262 (14.0) | 297 (14.6) | 559 (14.3) | ||
| Not done/unknown | 74 (7.8) | 51 (5.6) | 125 (6.7) | 178 (8.7) | 303 (7.8) | ||
| Surgery type | |||||||
| Lumpectomy | 583 (61.4) | 538 (58.7) | .25 | 1,121 (60.1) | 1,253 (61.5) | .36 | 2,374 (60.8) |
| Mastectomy | 367 (38.6) | 378 (41.3) | 745 (39.9) | 784 (38.5) | 1,529 (39.2) | ||
| Treatment | |||||||
| Placebo | 950 (50.9) | 1,003 (49.2) | .30 | 1,953 (50.0) | |||
| Letrozole | 916 (49.1) | 1,034 (50.8) | 1,950 (50.0) | ||||
Abbreviations: BMD, bone mineral density; HER2, human epidermal growth factor receptor 2; MP, MammaPrint.
Comparison between placebo and letrozole.
Comparison between the translational MP cohort and excluded B-42 population.
MP and ELT Benefit
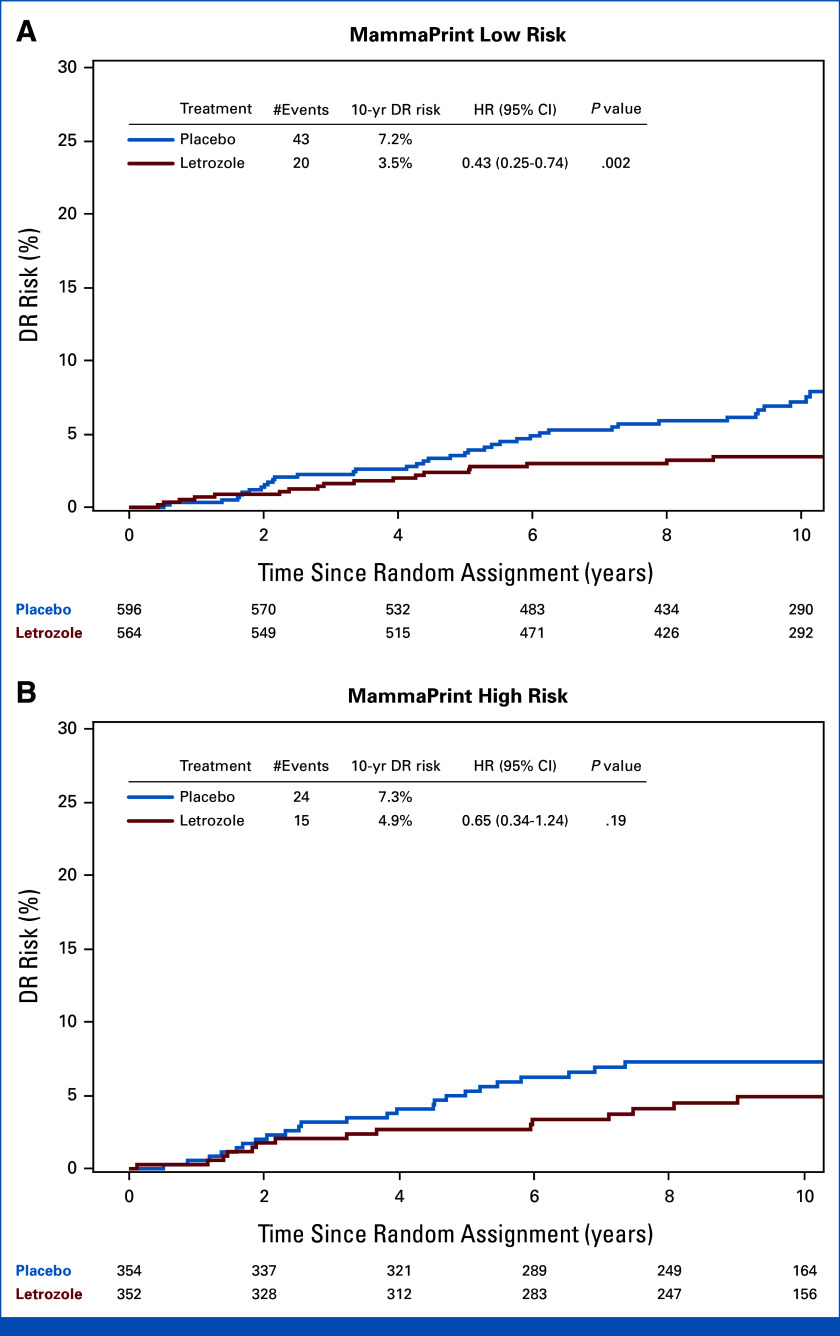
There were 102 DR events in the translational MP cohort. There was no statistically significant difference in terms of effect of letrozole on the DR rate between MP-HR and MP-LR groups (treatment-by-MP risk group interaction P = .38). However, the ELT effect was more pronounced in MP-LR (HR, 0.43 [95% CI, 0.25 to 0.74]; P = .002) than in MP-HR (HR, 0.65 [95% CI, 0.34 to 1.24]; P = .19; Table 2, Fig 2). Among patients with MP-LR tumors, the DR risk was 3.5% for patients treated with ELT and 7.2% for patients in the placebo group, demonstrating an ELT benefit of 3.7% at 10 years post–random assignment (Table 2, Fig 2A). In patients with MP-HR tumors, a 10-year DR risk of 4.9% and 7.3% was observed in the ELT and placebo group, respectively, translating to an ELT benefit of 2.4% (Table 2, Fig 2B). Similar results were observed in the adjusted analyses (not presented).
TABLE 2.
The Extended Letrozole Therapy Effect in the Subgroups of Patients Defined by MP Risk Categories
| End Point | MP Risk Group | Letrozole | Placebo | Difference (%) | HR (95% CI) | P | P Interaction | ||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | 10-year Event Rate (%) | No. of Events | 10-year Event Rate (%) | ||||||
| DR | Low | 20 | 3.5 | 43 | 7.2 | 3.7 | 0.43 (0.25 to 0.74) | .002 | .38 |
| High | 15 | 4.9 | 24 | 7.3 | 2.4 | 0.65 (0.34 to 1.24) | .19 | ||
| DFSa | Low | 113 | 20.3 | 165 | 28.1 | 7.8 | 0.67 (0.52 to 0.85) | <.001 | .015 |
| High | 91 | 28.8 | 88 | 27.2 | -1.6 | 1.10 (0.82 to 1.47) | .55 | ||
| BCFIb | Low | 44 | 8.4 | 83 | 15.4 | 7.0 | 0.51 (0.35 to 0.74) | <.001 | .006 |
| High | 42 | 14.6 | 38 | 11.6 | -3.0 | 1.15 (0.74 to 1.79) | .53 | ||
NOTE. No. of patients in high risk (placebo: 354, letrozole: 352), low risk (placebo: 596, letrozole: 564). Difference is computed as the 10-year event rate in the placebo group minus the 10-year event rate in the letrozole group.
Abbreviations: BCFI, BC-free interval; DFS, disease-free survival; DR, distant recurrence; HR, hazard ratio; MP, MammaPrint.
DFS event is local, regional, DR, second primary cancer, or death.
BCFI event is local, regional, DR, or contralateral BC, as a first event.
FIG 2.
Prediction of ELT benefit by MP based on DR. K-M analysis of risk of DR comparing extended letrozole versus placebo in patients with (A) MP low-risk (n = 1,160) tumors and patients with (B) MP high-risk (n = 706) tumors. DR, distant recurrence; ELT, extended letrozole therapy; HR, hazard ratio; K-M, Kaplan-Meier; MP, MammaPrint.
Differences in the ELT effect for secondary end points, DFS, and BCFI, were dependent on MP classification. There were 457 DFS events observed (Appendix Table A1, online only). For DFS, there was a statistically significant ELT benefit in MP-LR (HR, 0.67 [95% CI, 0.52 to 0.85]; P < .001), but not MP-HR (HR, 1.10 [95% CI, 0.82 to 1.47]; P = .55; treatment-by-MP risk group interaction P = .015; Table 2, Figs 3A and 3B). Among MP-LR tumors, the 10-year rate of DFS events was 20.3% in patients treated with ELT compared with 28.1% in the placebo group, resulting in a 7.8% benefit from ELT, whereas among MP-HR tumors, the 10-year rate of DFS events was 28.8% in patients treated with ELT and 27.2% in patients treated with placebo (–1.6% difference; Table 2, Figs 3A and 3B). Similar findings were observed for BCFI (Table 2, Figs 3C and 3D). The analysis of primary and secondary end points by nodal status is presented in Appendix Table A2.
FIG 3.
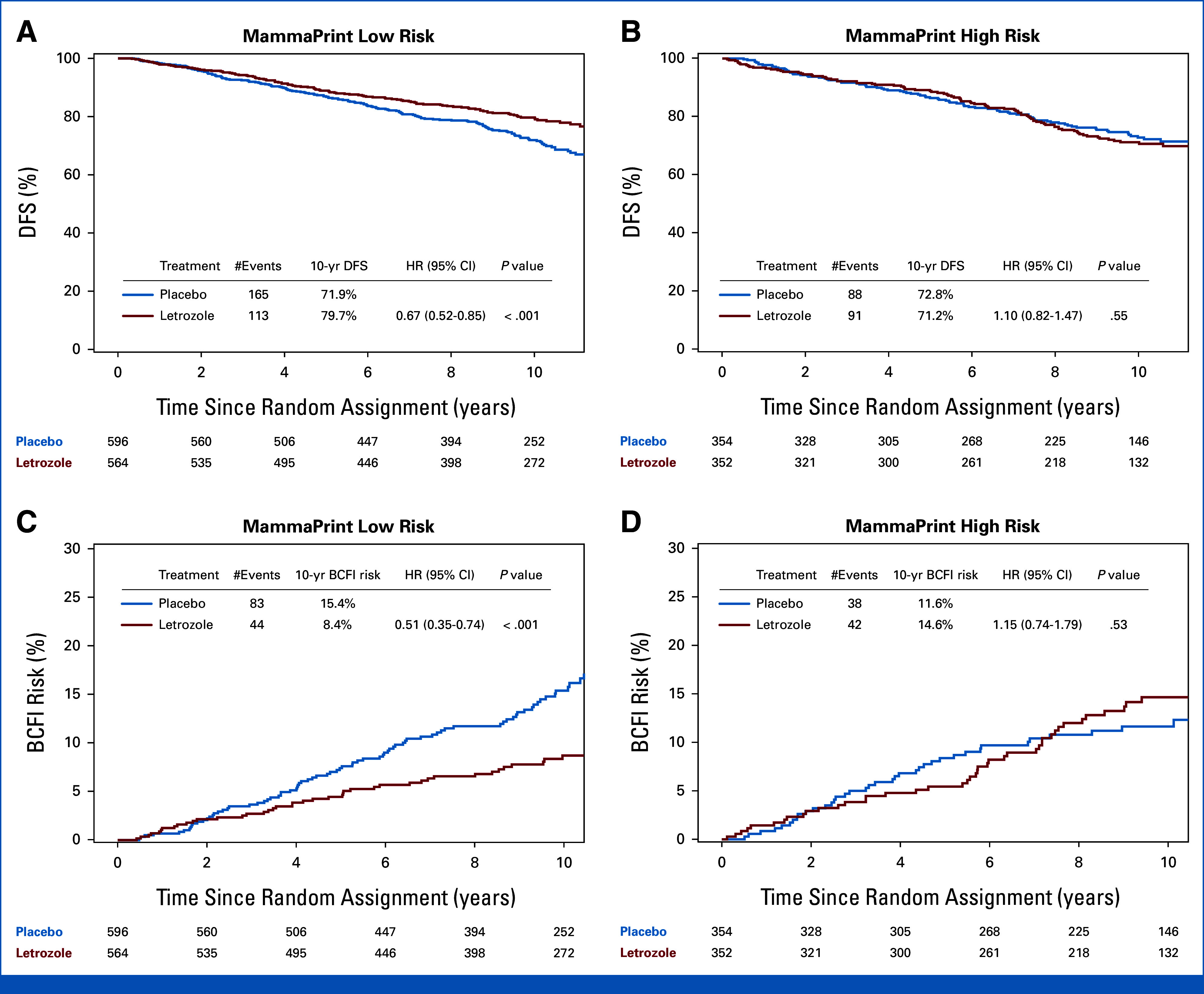
Prediction of ELT benefit by MP on the basis of DFS and BCFI event rate. K-M analysis of (A, B) DFS and (C, D) BCFI comparing ELT versus placebo in patients with MP low-risk (n = 1,160) and patients with MP high-risk (n = 706) tumors. BCFI, breast cancer-free interval; DFS, disease-free survival; ELT, extended letrozole therapy; HR, hazard ratio; K-M, Kaplan-Meier; MP, MammaPrint.
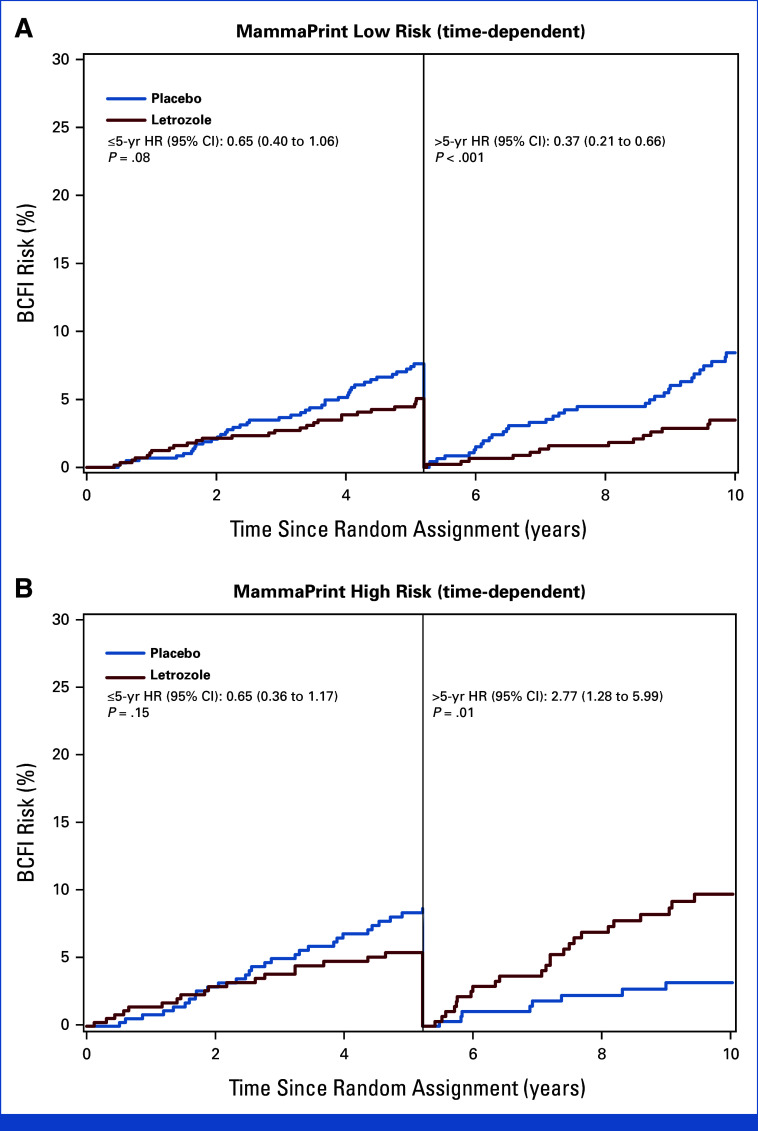
For BCFI, the assumption of hazards proportionality between treatment groups was not satisfied for the MP-HR subgroup. On the basis of the MP-HR subgroup, we identified a change point of 5.2 years. The ELT benefit before 5.2 years was similar between MP-HR and MP-LR subgroups. However, after 5.2 years, the ELT effect was statistically significantly different between MP-HR and MP-LR patients: MP-HR (HR, 2.77 [95% CI, 1.28 to 5.99]; P = .01) versus MP-LR (HR, 0.37 [95% CI, 0.21 to 0.66]; P < .001; treatment-by-MP risk group interaction P < .001; Appendix Fig A1).
MP and ELT Benefit in Low-Risk Subgroups
In the exploratory analysis of MP-LR subgroups, the ELT effect was more pronounced for patients with MP-LNUL tumors than for MP-UL tumors for DR and for the secondary end points of DFS and BCFI (Appendix Table A3). Statistically significant benefit from ELT was shown in MP-LNUL tumors for DR risk (HR, 0.42 [95% CI, 0.24 to 0.77]; P = .003), but not in MP-UL tumors (HR, 0.53 [95% CI, 0.13 to 2.15]; P = .37). However, this difference in the ELT effect was not statistically significant (treatment-by-MP-LR subgroup interaction P = .89). The DR risk was 3.6% in MP-LNUL patients treated with ELT versus 7.6% in the placebo group (4.0% difference) and 2.9% in MP-UL patients treated with ELT versus 5.8% in the placebo group (3.0% difference) at 10 years post–random assignment.
Similarly, for DFS, the ELT benefit was more pronounced in MP-LNUL tumors (HR, 0.64 [95% CI, 0.49 to 0.83]; P < .001) than MP-UL tumors (HR, 0.82 [95% CI, 0.45 to 1.48]; P = .50). However, the difference was not statistically significant (treatment-by-MP-LR subgroup interaction P = .52). The 10-year rate of DFS events was 21.1% in MP-LNUL tumors treated with ELT compared with 30.6% in the placebo group (9.5% difference) and was 17.5% in MP-UL patients treated with ELT versus 19.3% in the placebo group (1.8% difference). Similar results were observed for BCFI: MP-LNUL tumors (HR, 0.48 [95% CI, 0.32 to 0.73]; P < .001), MP-UL tumors (HR, 0.67 [95% CI, 0.28 to 1.65]; P = .38; treatment-to-MP-LR subgroup interaction P = .59). The BCFI risk at 10 years was 8.7% and 16.6% for patients with MP-LNUL tumors treated with ELT or placebo, respectively (7.9% difference) and was 7.3% and 11.4% in MP-UL patients treated with ELT or placebo, respectively (4.1% difference; Appendix Table A3).
DISCUSSION
Hormone receptor–positive BC is associated with a persistent long-term risk of recurrence. The modest benefit along with the adverse effects of EET beyond 5 years underscore the need to improve the identification of individual patients at an increased risk of late DR and those who derive benefit from EET. Results from this prospective-retrospective translational study suggest that significant ELT benefit in the NSABP B-42 trial could be dependent on genomic classification by MP.
Patients with MP-LR tumors had statistically significantly improved rates of DR, DFS, and BCFI when treated with ELT, whereas MP-HR patients did not derive statistically significant benefit from ELT as we had originally hypothesized. The treatment-by-genomic risk group interaction was not statistically significant for the primary end point of DR, but it was for the secondary end points of DFS and BCFI. The difference in ELT benefit for MP-HR tumors between these end points may be attributed to somewhat higher rates of locoregional recurrence and second primary breast events in ELT MP-HR patients. For women with MP-HR tumors, most recurrences occur in the first 5 years after diagnosis, during which they significantly benefit from ET.14 This is supported by previous analyses in the STO-3 trial,11 which demonstrated that patients with MP-HR tumors exhibited an 18% benefit in BC-specific survival at 20 years postdiagnosis after receiving 2-5 years of adjuvant ET. These patients, however, may have intrinsic decreased sensitivity to hormone therapy and did not benefit from EET, as evidenced by the results of this study. Although information on adjuvant chemotherapy use was not collected in the B-42 trial, it is likely that more patients with MP-HR tumors received adjuvant chemotherapy compared with patients with MP-LR tumors, which may explain the similar recurrence rates between the two groups in the placebo arm.
Further analysis within MP-LR subgroups revealed that MP-UL breast cancers do not derive statistically significant benefit from ELT. Although the small number of DR events in the MP-UL group (3 with ELT v 6 with placebo) limits the power of detecting a significant difference in outcomes from ELT, previous reports have shown no significant difference in 20-year BC-specific survival between MP-UL patients who received 2-5 years of tamoxifen versus those who did not.10,11 Furthermore, several survival analyses of patients enrolled in FOCUS, IKA, and MINDACT, and the STO-3 trials report that an MP-UL classification in postmenopausal women with node-negative HR+ BC identifies indolent tumors with very low risk of recurrence in the first 5-10 years postdiagnosis despite limited to no endocrine treatment.10,11,15-17 The recurrence rates 15 years postdiagnosis were higher in the NSABP B-42 translational MP cohort compared with previous studies for MP-UL patients, even though patients had to be disease-free 5 years from diagnosis to enter the B-42 trial. However, this may likely be due to the inclusion of higher clinical risk patients in the NSABP B-42 cohort (approximately 42% were node-positive).
Patients with MP-LNUL tumors, which represent nearly half (48.7%) of the NSABP B-42 translational MP cohort, appear to derive the majority of ELT benefit. A significant treatment-by-MP risk group interaction was found for DFS and BCFI, and there was a relative risk reduction in DR by 58%, in DFS events by 36%, and in BCFI events by 52% for MP-LNUL tumors with ELT. In the overall NSABP B-42 translational MP cohort (n = 1,866 patients), there was a benefit of 4.3% in DFS, 3.3% in BCFI, and 3.3% in DR with ELT. Patients with MP-LNUL tumors (n = 908) exhibited a benefit of 9.5% in DFS, 7.9% in BCFI, and 4.0% in DR with ELT. Therefore, MP biomarker effects were associated with more than a 2-2.5-fold improvement in DFS and BCFI outcomes on the basis of benefit in MP-LNUL patients relative to that observed in the overall translational MP cohort. These results further confirm that women with MP-LNUL tumor biology continue to have risk of recurrence beyond 5 years and therefore benefit from additional adjuvant ET.
The results of the primary and secondary end points analyses by node status were similar to the overall cohort with more pronounced differences among patients with node-positive BC. Of note, the results should be interpreted with caution because of the small number of events in the subgroups and wide confidence intervals.
Similar findings on the effect of MP on EET benefit to those observed in our study were recently reported at the 2022 San Antonio Breast Cancer Symposium from the IDEAL trial,18 which also evaluated ELT in postmenopausal patients with HR+ early-stage BC.19 Patients who had completed 5 years of adjuvant ET were randomly assigned to 2.5 or 5 years of letrozole. Rates of DFS events were similar between groups (HR, 0.92 [95% CI, 0.74 to 1.16]; P = .49). MP was performed in tumors from 515 patients, who had no events after 2.5 years of ELT. Patients with MP-LR tumors had a statistically significant benefit from ELT for the primary end point of DR plus one of the secondary end points of recurrence-free interval (RFI), although patients with MP-HR tumors did not. The ELT benefit-by-MP risk group interaction was not statistically significant for DR or BCFI, but it was for RFI.18 In the IDEAL trial, another biomarker, the Breast Cancer Index (BCI) HOXB13/IL17BR ratio (H/I), or BCI (H/I), was shown to be predictive for EET benefit.20 The association between BCI (H/I) and ELT benefit was also recently evaluated in the B-42 trial.21 Although the results did not confirm a significant interaction between BCI (H/I) and ELT for the primary end point of RFI, a time-dependent analysis for DR demonstrated that BCI (H/I)-high patients had a statistically significant benefit from ELT after 4 years, whereas BCI (H/I)-low patients did not.
Notable limitations of our correlative study include its prospective-retrospective nature and a lack of multiplicity adjustment for all secondary and exploratory analyses. Although clinical guidelines for evaluation of genomic classifiers recognize the importance of using archival specimens in a prospectively defined randomized study, implementation of MP in clinical practice to inform EET recommendation warrants validation in other independent clinical trials.22 One such trial was recently presented, as discussed above.18 Another limitation includes the exploratory nature of the analyses within the MP-LR subgroup (MP-UL and MP-LNUL). In addition, patients included in this correlative study constitute a subset of the original B-42 study and therefore might not be a representative sample. Although characteristics were comparable between the translational MP cohort and excluded B-42 cohort, the translational MP cohort had somewhat better prognosis in terms of DR with a more pronounced ELT effect on the DR compared with the excluded B-42 patient cohort. The relatively low DR event rate may have resulted in the loss of statistical power. Finally, the NSABP B-42 study reported more second primary breast cancers than observed in other trials, which may have contributed to the observed treatment effect.
Overall, these findings may expand the clinical utility of MP beyond prognostic indication because they provide the first evidence suggesting that MP is predictive of EET benefit. Future analyses incorporating clinicopathologic characteristics could also further optimize patient selection. The confirmation of the MP genomic classification utility will allow many postmenopausal women with HR+ BC to avoid unnecessary treatment and focus on patients who require additional adjuvant endocrine treatment.
ACKNOWLEDGMENT
We thank the patients and their families and the staff members of NSABP/NRG Oncology/SDMC. The authors would also like to acknowledge the contributions of Mark L. Graham, MD. We also thank Wendy L. Rea, BA, Editorial Associate, for editorial assistance with preparation and submission of the manuscript. She is an employee of NSABP Foundation and was not compensated beyond her normal salary for this work.
APPENDIX
FIG A1.
Time-dependent analyses of BCFI in patients with (A) MP low-risk (n = 1,160) and (B) MP high-risk (n = 706) tumors. BCFI, breast cancer-free interval; HR, hazard ratio; MP, MammaPrint.
TABLE A1.
Distribution of Event Type by MP Group and Treatment Arm
| Event | MP-LR | MP-HR | ||
|---|---|---|---|---|
| Placebo (n = 596) | Letrozole (n = 564) | Placebo (n = 354) | Letrozole (n = 352) | |
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Distant recurrence | 43 (7.2) | 20 (3.5) | 24 (6.8) | 15 (4.3) |
| BCFI eventa | 83 (13.9) | 44 (7.8) | 38 (10.7) | 42 (11.9) |
| Distant recurrence | 37 (6.2) | 15 (2.7) | 20 (5.6) | 15 (4.3) |
| Locoregional recurrence | 12 (2.0) | 10 (1.8) | 9 (2.5) | 15 (4.3) |
| Breast second primary | 34 (5.7) | 19 (3.4) | 9 (2.5) | 12 (3.4) |
| DFS eventb | 165 (27.7) | 113 (20.0) | 88 (24.9) | 91 (25.9) |
| Distant recurrence | 37 (6.2) | 15 (2.7) | 20 (5.6) | 15 (4.3) |
| Locoregional recurrence | 12 (2.0) | 10 (1.8) | 9 (2.5) | 15 (4.3) |
| Second primary | 85 (14.3) | 52 (9.2) | 40 (11.3) | 42 (11.9) |
| Breast | 34 (5.7) | 19 (3.4) | 9 (2.5) | 12 (3.4) |
| Other | 51 (8.6) | 33 (5.9) | 31 (8.8) | 30 (8.5) |
| Death | 31 (5.2) | 36 (6.4) | 19 (5.4) | 19 (5.4) |
Abbreviations: MP-HR, MammaPrint high risk; MP-LR, MammaPrint low risk.
BCFI event is local, regional, DR, or contralateral BC, as a first event.
DFS event is local, regional, DR, second primary cancer, or death.
TABLE A2.
The Extended Letrozole Therapy Effect in the Subgroups of Patients Defined by Node Status and MP Risk Categories
| End Point | MP Risk Group | Letrozole | Placebo | Difference (%) | HR (95% CI) | P | P Interaction | ||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | 10-Year Event Rate (%) | No. of Events | 10-Year Event Rate (%) | ||||||
| Node negative | |||||||||
| DR | Low | 8 | 2.4 | 13 | 3.3 | 0.8 | 0.62 (0.25 to 1.56) | .31 | .98 |
| High | 3 | 1.9 | 5 | 2.9 | 1.0 | 0.67 (0.16 to 2.84) | .58 | ||
| DFS | Low | 64 | 20.1 | 85 | 23.9 | 3.8 | 0.81 (0.59 to 1.13) | .21 | .17 |
| High | 48 | 28.9 | 44 | 26.2 | -2.8 | 1.20 (0.79 to 1.81) | .40 | ||
| BCFI | Low | 22 | 7.7 | 39 | 11.6 | 4.0 | 0.59 (0.35 to 1.01) | .051 | .21 |
| High | 15 | 10.2 | 16 | 9.2 | –1.1 | 1.06 (0.52 to 2.16) | .87 | ||
| Node positive | |||||||||
| DR | Low | 12 | 4.9 | 30 | 13.5 | 8.6 | 0.36 (0.18 to 0.71) | .002 | .26 |
| High | 12 | 7.9 | 19 | 12.0 | 4.1 | 0.64 (0.31 to 1.33) | .23 | ||
| DFS | Low | 49 | 20.5 | 80 | 34.6 | 14.1 | 0.53 (0.37 to 0.76) | <.001 | .027 |
| High | 43 | 28.9 | 44 | 27.9 | –1.0 | 1.00 (0.65 to 1.53) | .99 | ||
| BCFI | Low | 22 | 9.3 | 44 | 21.4 | 12.1 | 0.44 (0.27 to 0.74) | .002 | .01 |
| High | 27 | 18.9 | 22 | 14.2 | –4.7 | 1.22 (0.69 to 2.14) | .50 | ||
NOTE. No. of node–negative patients: High risk (placebo: 182, letrozole: 183), low risk (placebo: 365, letrozole: 323), No. of node–positive patients: High risk (placebo: 172, letrozole: 169), low risk (placebo: 231, letrozole: 241). Difference is computed as the 10-year event rate in the placebo group minus the 10-year event rate in the letrozole group.
Abbreviations: BCFI, breast cancer-free interval; DFS, disease-free survival; DR, distant recurrence; HR, hazard ratio; MP, MammaPrint.
TABLE A3.
The Extended Letrozole Therapy Effect in the Subgroups of Patients Defined by MP-Risk Categories
| End Point | MP Risk Group | Letrozole | Placebo | Difference (%) | HR (95% CI) | P | P Treatment-by-Three Group Interaction | ||
|---|---|---|---|---|---|---|---|---|---|
| No. of Events | 10-Year Event Rate (%) | No. of Events | 10-Year Event Rate (%) | ||||||
| DR | UL | 3 | 2.9 | 6 | 5.8 | 3.0 | 0.53 (0.13 to 2.15) | .37 | .69 |
| LNUL | 17 | 3.6 | 37 | 7.6 | 4.0 | 0.42 (0.24 to 0.77) | .003 | ||
| High | 15 | 4.9 | 24 | 7.3 | 2.4 | 0.65 (0.34 to 1.24) | .19 | ||
| DFS | UL | 20 | 17.5 | 26 | 19.3 | 1.8 | 0.82 (0.45 to 1.48) | .50 | .042 |
| LNUL | 93 | 21.1 | 139 | 30.6 | 9.5 | 0.64 (0.49 to 0.83) | <.001 | ||
| High | 91 | 28.8 | 88 | 27.2 | -1.6 | 1.10 (0.82 to 1.47) | .55 | ||
| BCFI | UL | 8 | 7.3 | 13 | 11.4 | 4.1 | 0.67 (0.28 to 1.65) | .38 | .021 |
| LNUL | 36 | 8.7 | 70 | 16.6 | 7.9 | 0.48 (0.32 to 0.73) | <.001 | ||
| High | 42 | 14.6 | 38 | 11.6 | –3.0 | 1.15 (0.74 to 1.79) | .53 | ||
NOTE. No. of patients in MammaPrint high risk (placebo: 354, letrozole: 352), ultralow risk (UL; placebo: 133, letrozole: 119), low non-ultralow risk (LNUL; placebo: 463, letrozole: 445). Difference is computed as the 10-year event rate in the placebo group minus the 10-year event rate in the letrozole group.
Abbreviations: BCFI, breast cancer-free interval; DFS, disease-free survival; DR, distant recurrence; HR, hazard ratio; MP, MammaPrint.
Priya Rastogi
Travel, Accommodations, Expenses: Genentech/Roche, Lilly, AstraZeneca
Hanna Bandos
Consulting or Advisory Role: IntraOp Medical, Hologic
Research Funding: Hologic (Inst), Koios Medical (Inst), AstraZeneca (Inst)
Peter C. Lucas
Stock and Other Ownership Interests: Amgen
Patents, Royalties, Other Intellectual Property: Patent No PCT/US2022/021720, “Small molecules and their use as MALT1 inhibitors”
Laura J. van ‘t Veer
Employment: Agendia
Stock and Other Ownership Interests: Agendia, Exai Bio
Charles E. Geyer Jr
Consulting or Advisory Role: Exact Sciences
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: AbbVie, Genentech/Roche, Daiichi-Sankyo, AstraZeneca
Stephen K.L. Chia
Honoraria: Novartis, Pfizer, Lilly, AstraZeneca, Merck, Gilead Sciences
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Novartis (Inst)
Adam M. Brufsky
Consulting or Advisory Role: Pfizer, Genentech/Roche, Agendia, Novartis, Lilly, Puma Biotechnology, Merck, Myriad Pharmaceuticals, Eisai, Seagen, Daiichi Sankyo/Lilly, OncLive, Michael J. Hennessy Associates, Gilead Sciences, General Electric
Research Funding: Roche/Genentech (Inst), AstraZeneca/Daiichi Sankyo (Inst), Merck (Inst), Novartis (Inst), Gilead Sciences (Inst), Lilly (Inst), Puma Biotechnology (Inst)
Expert Testimony: Pfizer
Janice M. Walshe
Honoraria: Novartis
Consulting or Advisory Role: Gilead Sciences
Travel, Accommodations, Expenses: Novartis
Soonmyung Paik
Employment: Theragen Bio
Leadership: Theragen Bio, ImmuneOncia
Stock and Other Ownership Interests: ImmuneOncia
Sandra M. Swain
Leadership: Seagen
Stock and Other Ownership Interests: Seagen
Honoraria: Chugai/Roche
Consulting or Advisory Role: Genentech/Roche, Daiichi Sankyo, Molecular Templates, AstraZeneca, Aventis Pharma, Jaguar Health
Travel, Accommodations, Expenses: Daichi Sankyo, Aventis Pharma, Genentech/Roche, Chugai/Roche
Other Relationship: Roche, AstraZeneca
Uncompensated Relationships: Genentech/Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/801195
Andrea R. Menicucci
Employment: Agendia
M. William Audeh
Employment: Agendia
Leadership: Agendia
Stock and Other Ownership Interests: Agendia
Consulting or Advisory Role: Celanese, Private Health, Quantum Health
Research Funding: Agendia
Travel, Accommodations, Expenses: Agendia
Norman Wolmark
Travel, Accommodations, Expenses: Komen Award, SABCS, 2022; American Surgical Association Mediallion for the Advancement of Surgical Care, 2024
Eleftherios P. Mamounas
Stock and Other Ownership Interests: Moderna Therapeutics
Honoraria: Genentech/Roche, PrecisCa, Exact Sciences, Merck
Consulting or Advisory Role: bioTheranostics, Roche/Genentech, Merck, PrecisCa, Exact Sciences, Tersera, Sanofi/Aventis, Novartis, Delphi Diagnostics
Speakers' Bureau: Genentech/Roche, Exact Sciences, Merck
Travel, Accommodations, Expenses: SBI Pharmaceuticals
Jennifer Wei
Employment: Agendia
Stock and Other Ownership Interests: Agendia
No other potential conflicts of interest were reported.
SUPPORT
Supported by the National Institutes of Health grant numbers NCI U10CA180868, -180822, and UG1CA189867; The Korea Health Technology R&D Project; Novartis, which provided letrozole and placebo to all study sites during the course of the study; and Agendia, Inc.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.01995.
AUTHOR CONTRIBUTIONS
Conception and design: Priya Rastogi, Hanna Bandos, Laura J. van ‘t Veer, Louis Fehrenbacher, Stephen K.L. Chia, Soonmyung Paik, Sandra M. Swain, M. William Audeh, Norman Wolmark, Eleftherios P. Mamounas
Financial support: Norman Wolmark
Administrative support: M. William Audeh, Norman Wolmark
Provision of study materials or patients: Peter C. Lucas, Charles E. Geyer, Jr, Louis Fehrenbacher, Stephen K.L. Chia, Adam M. Brufsky, Janice M. Walshe, Shaker R. Dakhil, Soonmyung Paik, Eleftherios P. Mamounas
Collection and assembly of data: Hanna Bandos, Laura J. van ‘t Veer, Jia-Perng J. Wei, Charles E. Geyer, Jr, Louis Fehrenbacher, Stephen K.L. Chia, Adam M. Brufsky, Janice M. Walshe, Gamini S. Soori, Shaker R. Dakhil, Soonmyung Paik, Eleftherios P. Mamounas
Data analysis and interpretation: Hanna Bandos, Peter C. Lucas, Jia-Perng J. Wei, Charles E. Geyer, Jr, Louis Fehrenbacher, Janice M. Walshe, Soonmyung Paik, Andrea R. Menicucci, M. William Audeh, Eleftherios P. Mamounas
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Utility of the 70-Gene MammaPrint Assay for Prediction of Benefit From Extended Letrozole Therapy in the NRG Oncology/NSABP B-42 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Priya Rastogi
Travel, Accommodations, Expenses: Genentech/Roche, Lilly, AstraZeneca
Hanna Bandos
Consulting or Advisory Role: IntraOp Medical, Hologic
Research Funding: Hologic (Inst), Koios Medical (Inst), AstraZeneca (Inst)
Peter C. Lucas
Stock and Other Ownership Interests: Amgen
Patents, Royalties, Other Intellectual Property: Patent No PCT/US2022/021720, “Small molecules and their use as MALT1 inhibitors”
Laura J. van ‘t Veer
Employment: Agendia
Stock and Other Ownership Interests: Agendia, Exai Bio
Charles E. Geyer Jr
Consulting or Advisory Role: Exact Sciences
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Daiichi Sankyo/Astra Zeneca (Inst), AbbVie (Inst)
Travel, Accommodations, Expenses: AbbVie, Genentech/Roche, Daiichi-Sankyo, AstraZeneca
Stephen K.L. Chia
Honoraria: Novartis, Pfizer, Lilly, AstraZeneca, Merck, Gilead Sciences
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Novartis (Inst)
Adam M. Brufsky
Consulting or Advisory Role: Pfizer, Genentech/Roche, Agendia, Novartis, Lilly, Puma Biotechnology, Merck, Myriad Pharmaceuticals, Eisai, Seagen, Daiichi Sankyo/Lilly, OncLive, Michael J. Hennessy Associates, Gilead Sciences, General Electric
Research Funding: Roche/Genentech (Inst), AstraZeneca/Daiichi Sankyo (Inst), Merck (Inst), Novartis (Inst), Gilead Sciences (Inst), Lilly (Inst), Puma Biotechnology (Inst)
Expert Testimony: Pfizer
Janice M. Walshe
Honoraria: Novartis
Consulting or Advisory Role: Gilead Sciences
Travel, Accommodations, Expenses: Novartis
Soonmyung Paik
Employment: Theragen Bio
Leadership: Theragen Bio, ImmuneOncia
Stock and Other Ownership Interests: ImmuneOncia
Sandra M. Swain
Leadership: Seagen
Stock and Other Ownership Interests: Seagen
Honoraria: Chugai/Roche
Consulting or Advisory Role: Genentech/Roche, Daiichi Sankyo, Molecular Templates, AstraZeneca, Aventis Pharma, Jaguar Health
Travel, Accommodations, Expenses: Daichi Sankyo, Aventis Pharma, Genentech/Roche, Chugai/Roche
Other Relationship: Roche, AstraZeneca
Uncompensated Relationships: Genentech/Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/801195
Andrea R. Menicucci
Employment: Agendia
M. William Audeh
Employment: Agendia
Leadership: Agendia
Stock and Other Ownership Interests: Agendia
Consulting or Advisory Role: Celanese, Private Health, Quantum Health
Research Funding: Agendia
Travel, Accommodations, Expenses: Agendia
Norman Wolmark
Travel, Accommodations, Expenses: Komen Award, SABCS, 2022; American Surgical Association Mediallion for the Advancement of Surgical Care, 2024
Eleftherios P. Mamounas
Stock and Other Ownership Interests: Moderna Therapeutics
Honoraria: Genentech/Roche, PrecisCa, Exact Sciences, Merck
Consulting or Advisory Role: bioTheranostics, Roche/Genentech, Merck, PrecisCa, Exact Sciences, Tersera, Sanofi/Aventis, Novartis, Delphi Diagnostics
Speakers' Bureau: Genentech/Roche, Exact Sciences, Merck
Travel, Accommodations, Expenses: SBI Pharmaceuticals
Jennifer Wei
Employment: Agendia
Stock and Other Ownership Interests: Agendia
No other potential conflicts of interest were reported.
REFERENCES
- 1. Colleoni M, Sun Z, Price KN, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J Clin Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. Erratum in: Lancet 2013; 381:804. Erratum in: Lancet 2017; 389:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 5. Mamounas EP, Bandos H, Lembersky BC, et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:88–99. doi: 10.1016/S1470-2045(18)30621-1. Erratum in: Lancet Oncol 2019; 20(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mamounas EP, Bandos H, Rastogi P, et al. Ten-year update: NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-42 randomized trial: Extended letrozole therapy in early-stage breast cancer. J Natl Cancer Inst. 2023;115:1302–1309. doi: 10.1093/jnci/djad078. Erratum in: J Natl Cancer Inst. 2023 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piccart M, van 't Veer LJ, Poncet C, et al. 70-gene signature as an aid for treatment decisions in early breast cancer: Updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–488. doi: 10.1016/S1470-2045(21)00007-3. [DOI] [PubMed] [Google Scholar]
- 8. van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 9. Cardoso F, van't Veer LJ, Bogaerts J, et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 10. Esserman LJ, Yau C, Thompson CK, et al. Use of molecular tools to identify patients with indolent breast cancers with ultralow risk over 2 decades. JAMA Oncol. 2017;3:1503–1510. doi: 10.1001/jamaoncol.2017.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van 't Veer LJ, Yau C, Yu NY, et al. Tamoxifen therapy benefit for patients with 70-gene signature high and low risk. Breast Cancer Res Treat. 2017;166:593–601. doi: 10.1007/s10549-017-4428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krijgsman O, Roepman P, Zwart W, et al. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat. 2012;133:37–47. doi: 10.1007/s10549-011-1683-z. [DOI] [PubMed] [Google Scholar]
- 13. Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278. doi: 10.1186/1471-2164-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansson A, Dar H, van 't Veer LJ, et al. Twenty-year benefit from adjuvant goserelin and tamoxifen in premenopausal patients with breast cancer in a controlled randomized clinical trial. J Clin Oncol. 2022;40:4071–4082. doi: 10.1200/JCO.21.02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lopes Cardozo JMN, Drukker CA, Rutgers JT, et al. Outcome of patients with an ultralow-risk 70-gene signature in the MINDACT Trial. J Clin Oncol. 2022;40:1335–1345. doi: 10.1200/JCO.21.02019. [DOI] [PubMed] [Google Scholar]
- 16. Noordhoek I, Bastiaannet E, de Glas NA, et al. Validation of the 70-gene signature test (MammaPrint) to identify patients with breast cancer aged ≥70 years with ultralow risk of distant recurrence: A population-based cohort study. J Geriatr Oncol. 2022;13:1172–1177. doi: 10.1016/j.jgo.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 17. Opdam M, van der Noort V, Kleijn M, et al. Limiting systemic endocrine overtreatment in postmenopausal breast cancer patients with an ultralow classification of the 70-gene signature. Breast Cancer Res Treat. 2022;194:265–278. doi: 10.1007/s10549-022-06618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liefers G-J, Meershoek-Klein Kranenbarg E, Duijm-de Carpentier M, et al. Utility of the 70-gene MammaPrint test for prediction of extended endocrine therapy benefit in patients with early-stage breast cancer in the IDEAL Trial. San Antonio Breast Cancer Symposium 2022; Abstr #GS5-10.
- 19. Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; Results of the IDEAL Trial (BOOG 2006-05) JNCI: Journal Natl Cancer Inst. 2018;110:40–48. doi: 10.1093/jnci/djx134. [DOI] [PubMed] [Google Scholar]
- 20. Noordhoek I, Treuner K, Putter H, et al. Breast cancer index predicts extended endocrine benefit to individualize selection of patients with HR+ early-stage breast cancer for 10 Years of endocrine therapy. Clin Cancer Res. 2021;27:311–319. doi: 10.1158/1078-0432.CCR-20-2737. [DOI] [PubMed] [Google Scholar]
- 21. Mamounas EP, Bandos H, Rastogi P, et al. Breast cancer index and prediction of extended aromatase inhibitor therapy benefit in hormone receptor-positive breast cancer from the NRG Oncology/NSABP B-42 trial. Clin Cancer Res. 2024;30:1984–1991. doi: 10.1158/1078-0432.CCR-23-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.23.01995.