Abstract
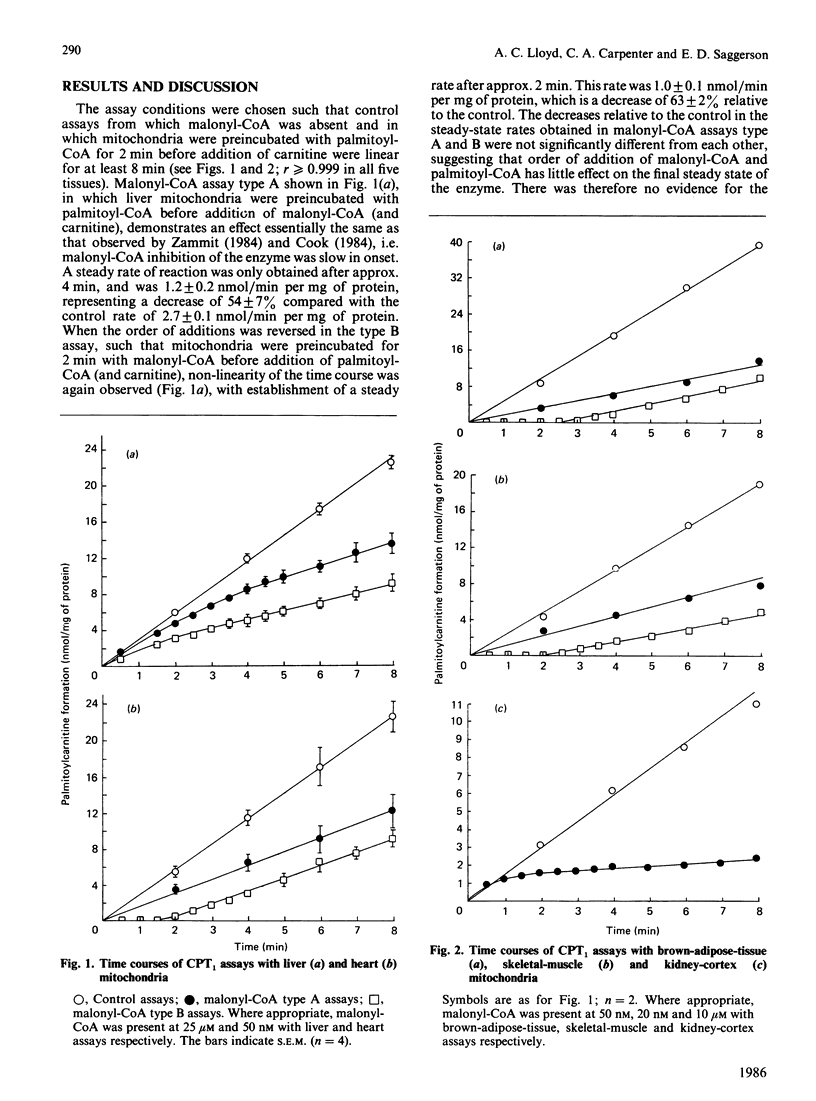
In the presence of malonyl-CoA, the overt form of carnitine palmitoyltransferase (CPT1) in mitochondria from rat liver, kidney cortex, heart, skeletal muscle and brown adipose tissue shows non-linear time courses, suggesting hysteretic behaviour. The pattern of this hysteresis is similar in heart, skeletal muscle and brown adipose tissue, but the hysteretic behaviour of the enzyme in these three tissues differs markedly from that seen in liver and kidney.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergseth S., Lund H., Bremer J. Is carnitine palmitoyltransferase inhibited by a malonyl-CoA-binding unit in the mitochondria? Biochem Soc Trans. 1986 Aug;14(4):671–672. doi: 10.1042/bst0140671. [DOI] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G. A. Involvement of hysteretic effects in the inhibition of carnitine palmitoyltransferase by malonyl-CoA. Biochem J. 1984 Dec 15;224(3):1015–1018. doi: 10.1042/bj2241015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R., Bird M. I., Saggerson E. D. Effects of DL-2-bromopalmitoyl-CoA and bromoacetyl-CoA in rat liver and heart mitochondria. Inhibition of carnitine palmitoyltransferase and displacement of [14C]malonyl-CoA from mitochondrial binding sites. Biochem J. 1985 Aug 15;230(1):169–179. doi: 10.1042/bj2300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden C. Slow transitions and hysteretic behavior in enzymes. Annu Rev Biochem. 1979;48:471–489. doi: 10.1146/annurev.bi.48.070179.002351. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Binding of [14C]malonyl-CoA to rat liver mitochondria after blocking of the active site of carnitine palmitoyltransferase I. Displacement of low-affinity binding by palmitoyl-CoA. Biochem J. 1986 Jan 15;233(2):589–593. doi: 10.1042/bj2330589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase and carnitine octanoyltransferase activities in liver, kidney cortex, adipocyte, lactating mammary gland, skeletal muscle and heart. FEBS Lett. 1981 Jul 6;129(2):229–232. doi: 10.1016/0014-5793(81)80171-8. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Carnitine palmitoyltransferase in liver and five extrahepatic tissues in the rat. Inhibition by DL-2-bromopalmitoyl-CoA and effect of hypothyroidism. Biochem J. 1986 May 15;236(1):137–141. doi: 10.1042/bj2360137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Sensitivity of brown-adipose-tissue carnitine palmitoyltransferase to inhibition by malonyl-CoA. Biochem J. 1982 Apr 15;204(1):373–375. doi: 10.1042/bj2040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. W., Cook G. A., Harris R. A. Effect of pH on malonyl-CoA inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 May 15;212(2):521–524. doi: 10.1042/bj2120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Increased sensitivity of carnitine palmitoyltransferase I activity to malonyl-CoA inhibition after preincubation of intact rat liver mitochondria with micromolar concentrations of malonyl-CoA in vitro. Biochem J. 1983 Mar 15;210(3):953–956. doi: 10.1042/bj2100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Time-dependence of inhibition of carnitine palmitoyltransferase I by malonyl-CoA in mitochondria isolated from livers of fed or starved rats. Evidence for transition of the enzyme between states of low and high affinity for malonyl-CoA. Biochem J. 1984 Mar 1;218(2):379–386. doi: 10.1042/bj2180379. [DOI] [PMC free article] [PubMed] [Google Scholar]


