Full text
PDF





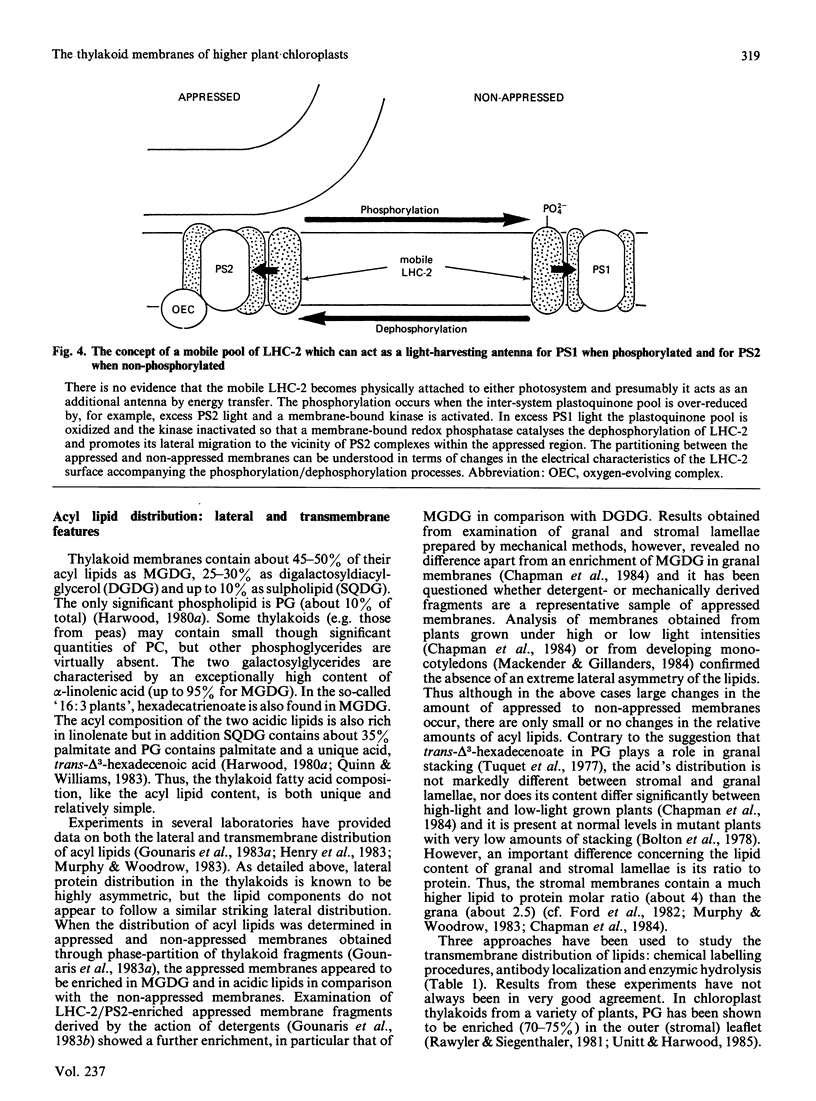







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund H. E., Andersson B., Albertsson P. A. Isolation of photosystem II enriched membrane vesicles from spinach chloroplasts by phase partition. Biochim Biophys Acta. 1976 Dec 6;449(3):525–535. doi: 10.1016/0005-2728(76)90161-4. [DOI] [PubMed] [Google Scholar]
- Andersson B., Anderson J. M. Lateral heterogeneity in the distribution of chlorophyll-protein complexes of the thylakoid membranes of spinach chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):427–440. doi: 10.1016/0005-2728(80)90078-x. [DOI] [PubMed] [Google Scholar]
- Barber J., Chow W. S., Scoufflaire C., Lannoye R. The relationship between thylakoid stacking and salt induced chlorophyll fluorescence changes. Biochim Biophys Acta. 1980 Jun 10;591(1):92–103. doi: 10.1016/0005-2728(80)90223-6. [DOI] [PubMed] [Google Scholar]
- Barber J. Membrane surface charges and potentials in relation to photosynthesis. Biochim Biophys Acta. 1980 Dec;594(4):253–308. doi: 10.1016/0304-4173(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Bennett J. Regulation of photosynthesis by reversible phosphorylation of the light-harvesting chlorophyll a/b protein. Biochem J. 1983 Apr 15;212(1):1–13. doi: 10.1042/bj2120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg S., Dodge S., Krogmann D. W., Dilley R. A. Chloroplast grana membrane carboxyl groups: their involvement in membrane association. Plant Physiol. 1974 Apr;53(4):619–627. doi: 10.1104/pp.53.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D. F., Hruza D. S., Baker P. Y. Effects of dietary protein level on hepatic microsomal mixed-function oxidase systems during aging in two generations of Syrian hamsters. Toxicol Appl Pharmacol. 1983 Mar 30;68(1):77–86. doi: 10.1016/0041-008x(83)90356-3. [DOI] [PubMed] [Google Scholar]
- Bishop D. G., Kenrick J. R., Bayston J. H., Macpherson A. S., Johns S. R. Monolayer properties of chloroplast lipids. Biochim Biophys Acta. 1980 Nov 4;602(2):248–259. doi: 10.1016/0005-2736(80)90308-9. [DOI] [PubMed] [Google Scholar]
- Bitria Ibars A., Alsius Camps M., Guillot Miravet J. Trasplante hepático y la enfermería de U.C.I. Rev Enferm. 1985 Sep;8(86):59–64. [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem. 1983 Nov 10;258(21):13281–13286. [PubMed] [Google Scholar]
- Bohnenberger E., Sandermann H., Jr Lipid dependence of diacylglycerol kinase from Escherichia coli. Eur J Biochem. 1983 May 16;132(3):645–650. doi: 10.1111/j.1432-1033.1983.tb07412.x. [DOI] [PubMed] [Google Scholar]
- Bolton P., Wharfe J., Harwood J. L. The lipid composition of a barley mutant lacking chlorophyll b. Biochem J. 1978 Jul 15;174(1):67–72. doi: 10.1042/bj1740067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Brasseur R., De Meutter J., Goormaghtigh E., Ruysschaert J. M. Mode of organization of galactolipids: a conformational analysis. Biochem Biophys Res Commun. 1983 Sep 15;115(2):666–672. doi: 10.1016/s0006-291x(83)80196-x. [DOI] [PubMed] [Google Scholar]
- Burke J. J., Wilson R. F., Swafford J. R. Characterization of Chloroplasts Isolated from Triazine-Susceptible and Triazine-Resistant Biotypes of Brassica campestris L. Plant Physiol. 1982 Jul;70(1):24–29. doi: 10.1104/pp.70.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani O., Barber J., Malkin S. Evidence that phosphorylation and dephosphorylation regulate the distribution of excitation energy between the two photosystems of photosynthesis in vivo: Photoacoustic and fluorimetric study of an intact leaf. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1614–1618. doi: 10.1073/pnas.81.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R. Structure and expression of a pea nuclear gene encoding a chlorophyll a/b-binding polypeptide. Proc Natl Acad Sci U S A. 1984 May;81(10):2960–2964. doi: 10.1073/pnas.81.10.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey I., Kekwick R. G. The characteristics of some components of the fatty acid synthetase system in the plastids from the mesocarp of avocado (Persea americana) fruit. Eur J Biochem. 1982 Apr;123(3):553–561. doi: 10.1111/j.1432-1033.1982.tb06568.x. [DOI] [PubMed] [Google Scholar]
- Chapman D. J., De-Felice J., Barber J. Growth temperature effects on thylakoid membrane lipid and protein content of pea chloroplasts. Plant Physiol. 1983 May;72(1):225–228. doi: 10.1104/pp.72.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. D., Hind G. Isolation of a five-polypeptide cytochrome b-f complex from spinach chloroplasts. J Biol Chem. 1983 Sep 10;258(17):10348–10354. [PubMed] [Google Scholar]
- Cocucci M., Ballarin-Denti A. Effect of polar lipids on ATPase activity of membrane preparations from germinating radish seeds. Plant Physiol. 1981 Aug;68(2):377–381. doi: 10.1104/pp.68.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington J. M., Johns S. R., Leslie D. R., Willing R. I., Bishop D. G. 13C nuclear magnetic resonance studies of the composition and fluidity of several chloroplast monogalactosyldiacylglycerols. Biochim Biophys Acta. 1981 Mar 23;663(3):653–660. doi: 10.1016/0005-2760(81)90076-x. [DOI] [PubMed] [Google Scholar]
- De Grip W. J., Drenthe E. H., Van Echteld C. J., De Kruijff B., Verkleij A. J. A possible role of rhodopsin in maintaining bilayer structure in the photoreceptor membrane. Biochim Biophys Acta. 1979 Dec 12;558(3):330–337. doi: 10.1016/0005-2736(79)90269-4. [DOI] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Hurt E., Hauska G. A cytochrome f/b6 complex of five polypeptides with plastoquinol-plastocyanin-oxidoreductase activity from spinach chloroplasts. Eur J Biochem. 1981 Jul;117(3):591–595. doi: 10.1111/j.1432-1033.1981.tb06379.x. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Mitchell D. J., Ninham B. W. Theory of self-assembly of lipid bilayers and vesicles. Biochim Biophys Acta. 1977 Oct 17;470(2):185–201. doi: 10.1016/0005-2736(77)90099-2. [DOI] [PubMed] [Google Scholar]
- Jolliot A., Justin A. M., Bimont E., Mazliak P. Regulation by Lipids of Plant Microsomal Enzymes: III. Phospholipid Dependence of the Cytidine-Diphospho-Choline Phosphotransferase of Potato Microsomes. Plant Physiol. 1982 Jul;70(1):206–210. doi: 10.1104/pp.70.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. V., Harwood J. L. Desaturation of linoleic acid from exogenous lipids by isolated chloroplasts. Biochem J. 1980 Sep 15;190(3):851–854. doi: 10.1042/bj1900851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppinger-Sparace K. F., Mudd J. B., Bishop D. G. Biosynthesis of sulfoquinovosyldiacylglycerol in higher plants: the incorporation of 35SO4 by intact chloroplasts. Arch Biochem Biophys. 1985 Aug 1;240(2):859–865. doi: 10.1016/0003-9861(85)90096-7. [DOI] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Murata N., Yamaya J. Temperature-dependent phase behavior of phosphatidylglycerols from chilling-sensitive and chilling-resistant plants. Plant Physiol. 1984 Apr;74(4):1016–1024. doi: 10.1104/pp.74.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J., Forrester J. A. Surface charges on chloroplast membranes as studied by particle electrophoresis. Biochim Biophys Acta. 1978 Oct 11;504(1):215–225. doi: 10.1016/0005-2728(78)90019-1. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y. Photosynthetic oxygen evolution does not require the participation of polypeptides of 16 and 24 kilodaltons. Biochem Biophys Res Commun. 1984 Apr 16;120(1):299–304. doi: 10.1016/0006-291x(84)91448-7. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai P., John J. B. Lipid composition of chloroplast membranes from weed biotypes differentially sensitive to triazine herbicides. Plant Physiol. 1981 Sep;68(3):585–587. doi: 10.1104/pp.68.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska L. J., Gross E. L. The effect of 1-ethyl-3(3-dimethylaminopropyl)carbodiimide on calcium binding and associated changes in chloroplast structure and chlorophyll a fluorescence in spinach chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):126–135. doi: 10.1016/0005-2728(75)90211-x. [DOI] [PubMed] [Google Scholar]
- Punnett T. Environmental control of photosynthetic enhancement. Science. 1971 Jan 22;171(3968):284–286. doi: 10.1126/science.171.3968.284. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Williams W. P. Plant lipids and their role in membrane function. Prog Biophys Mol Biol. 1978;34(2):109–173. doi: 10.1016/0079-6107(79)90016-6. [DOI] [PubMed] [Google Scholar]
- Rawyler A., Siegenthaler P. A. Transmembrane distribution of phospholipids and their involvement in electron transport, as revealed by phospholipids A2 treatment of spinach thylakoids. Biochim Biophys Acta. 1981 Apr 13;635(2):348–358. doi: 10.1016/0005-2728(81)90033-5. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Holland R., Slack C. R. On the control of long-chain-fatty acid synthesis in isolated intact spinach (Spinacia oleracea) chloroplasts. Biochem J. 1979 Nov 15;184(2):193–202. doi: 10.1042/bj1840193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughan P. G. Phosphatidylglycerol and chilling sensitivity in plants. Plant Physiol. 1985 Mar;77(3):740–746. doi: 10.1104/pp.77.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Sen A., Williams W. P., Brain A. P., Dickens M. J., Quinn P. J. Formation of inverted micelles in dispersions of mixed galactolipids. Nature. 1981 Oct 8;293(5832):488–490. doi: 10.1038/293488a0. [DOI] [PubMed] [Google Scholar]
- Sen A., Williams W. P., Quinn P. J. The structure and thermotropic properties of pure 1,2-diacylgalactosylglycerols in aqueous systems. Biochim Biophys Acta. 1981 Feb 23;663(2):380–389. doi: 10.1016/0005-2760(81)90167-3. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Isolation and function of spinach leaf beta-ketoacyl-[acyl-carrier-protein] synthases. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5808–5812. doi: 10.1073/pnas.79.19.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Purification and characterizations of beta-Ketoacyl-[acyl-carrier-protein] reductase, beta-hydroxyacyl-[acyl-carrier-protein] dehydrase, and enoyl-[acyl-carrier-protein] reductase from Spinacia oleracea leaves. Arch Biochem Biophys. 1982 Oct 1;218(1):77–91. doi: 10.1016/0003-9861(82)90323-x. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]
- Unitt M. D., Harwood J. L. Sidedness studies of thylakoid phosphatidylglycerol in higher plants. Biochem J. 1985 Jun 15;228(3):707–711. doi: 10.1042/bj2280707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos R. H., Ceccarelli E., Chan R. Evidence for the existence of a thylakoid intrinsic protein that binds ferredoxin-NADP+ oxidoreductase. J Biol Chem. 1984 Jul 10;259(13):8048–8051. [PubMed] [Google Scholar]
- Verkleij A. J., Momvers C., Leunissen-Bijvelt J., Ververgaert P. H. Lipidic intramembranous particles. Nature. 1979 May 10;279(5709):162–163. doi: 10.1038/279162a0. [DOI] [PubMed] [Google Scholar]
- Walker K. A., Harwood J. L. Localization of chloroplastic fatty acid synthesis de novo in the stroma. Biochem J. 1985 Mar 1;226(2):551–556. doi: 10.1042/bj2260551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire P. J., Kekwick R. G. The synthesis of fatty acids in avocado mesocarp and cauliflower bud tissue. Biochem J. 1975 Feb;146(2):425–437. doi: 10.1042/bj1460425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widger W. R., Cramer W. A., Herrmann R. G., Trebst A. Sequence homology and structural similarity between cytochrome b of mitochondrial complex III and the chloroplast b6-f complex: position of the cytochrome b hemes in the membrane. Proc Natl Acad Sci U S A. 1984 Feb;81(3):674–678. doi: 10.1073/pnas.81.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T. Coupling of quanta, electrons, fields, ions and phosphrylation in the functional membrane of photosynthesis. Results by pulse spectroscopic methods. Q Rev Biophys. 1971 Nov;4(4):365–477. doi: 10.1017/s0033583500000834. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kosuda T., Yanagawa H., Tachibana T., Shima K., Hosoda Y., Mikami R., Homma H. Long-term follow-up in sarcoidosis in Japan. Z Erkr Atmungsorgane. 1977 Aug;149(2):191–196. [PubMed] [Google Scholar]
- de Kruijff B., Verkley A. J., van Echteld C. J., Gerritsen W. J., Mombers C., Noordam P. C., de Gier J. The occurrence of lipidic particles in lipid bilayers as seen by 31P NMR and freeze-fracture electron-microscopy. Biochim Biophys Acta. 1979 Aug 7;555(2):200–209. doi: 10.1016/0005-2736(79)90160-3. [DOI] [PubMed] [Google Scholar]


