Abstract
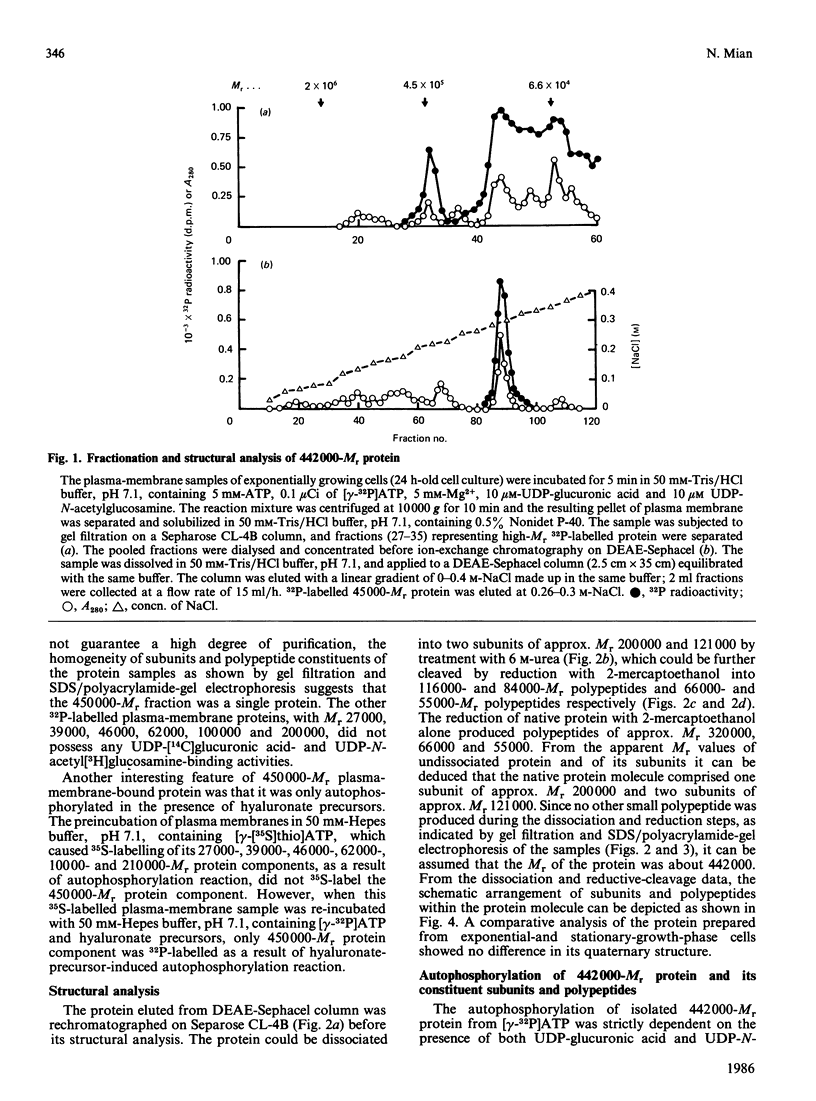
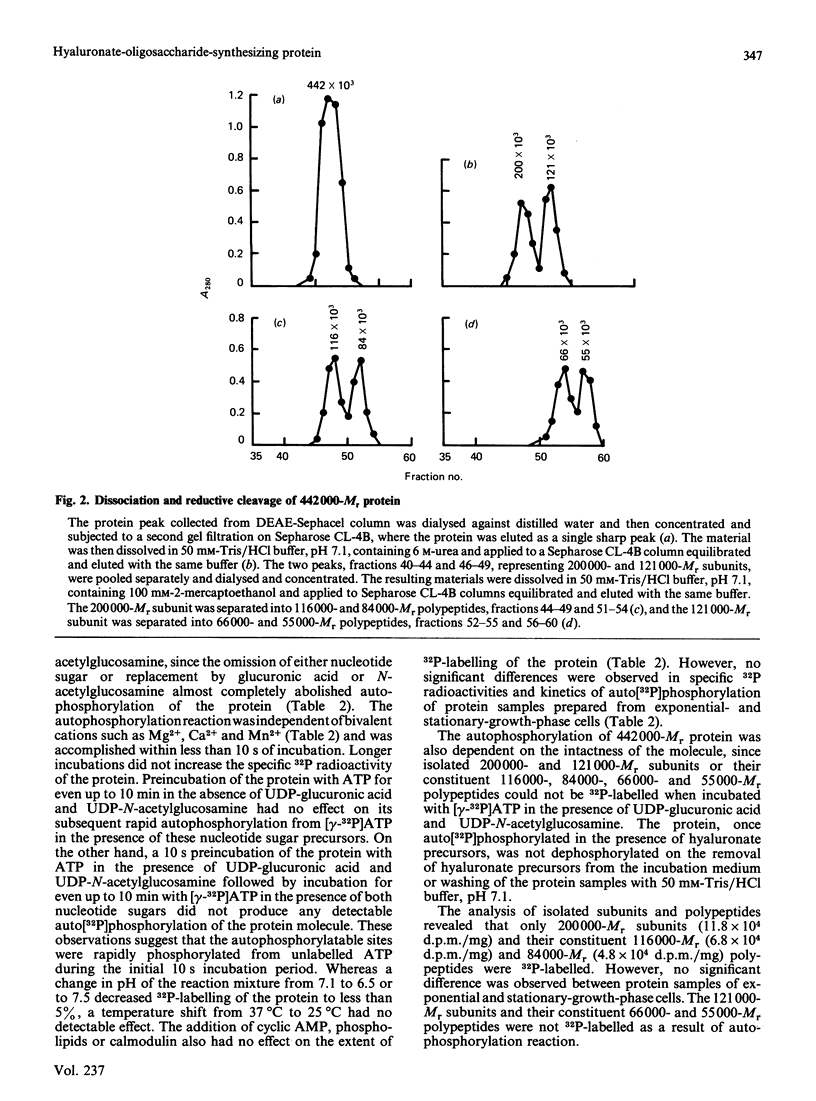
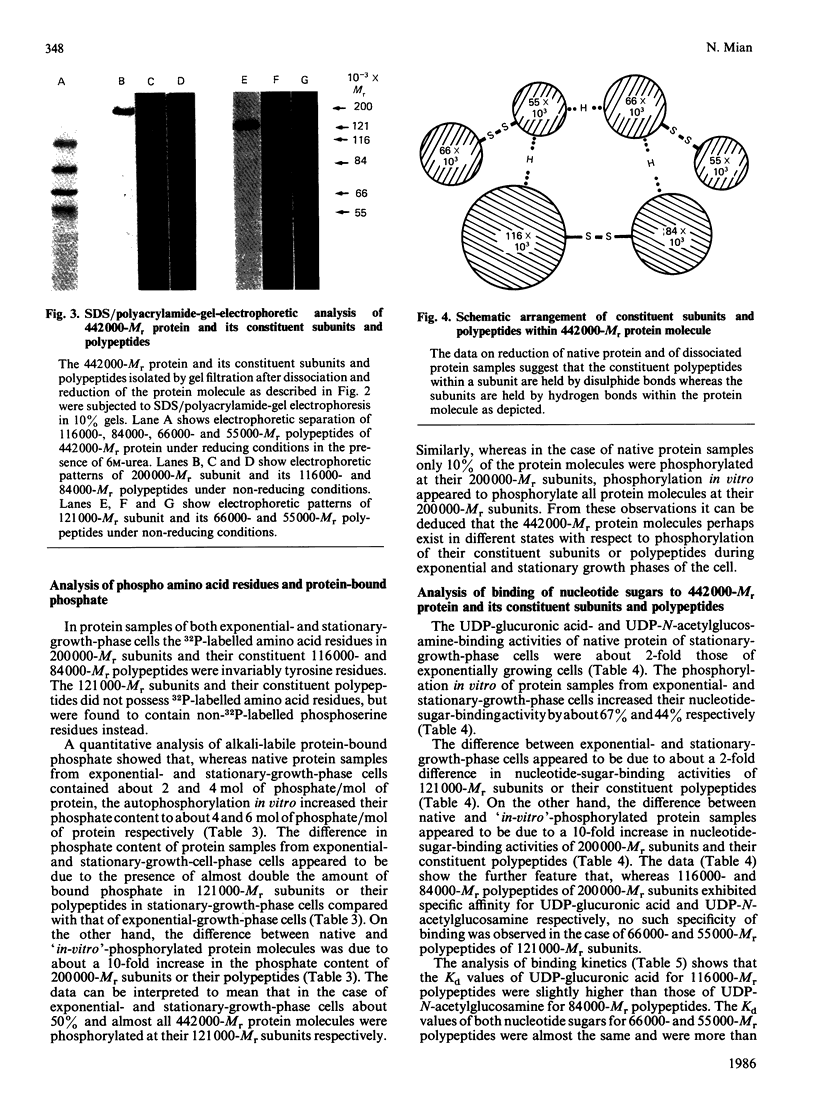
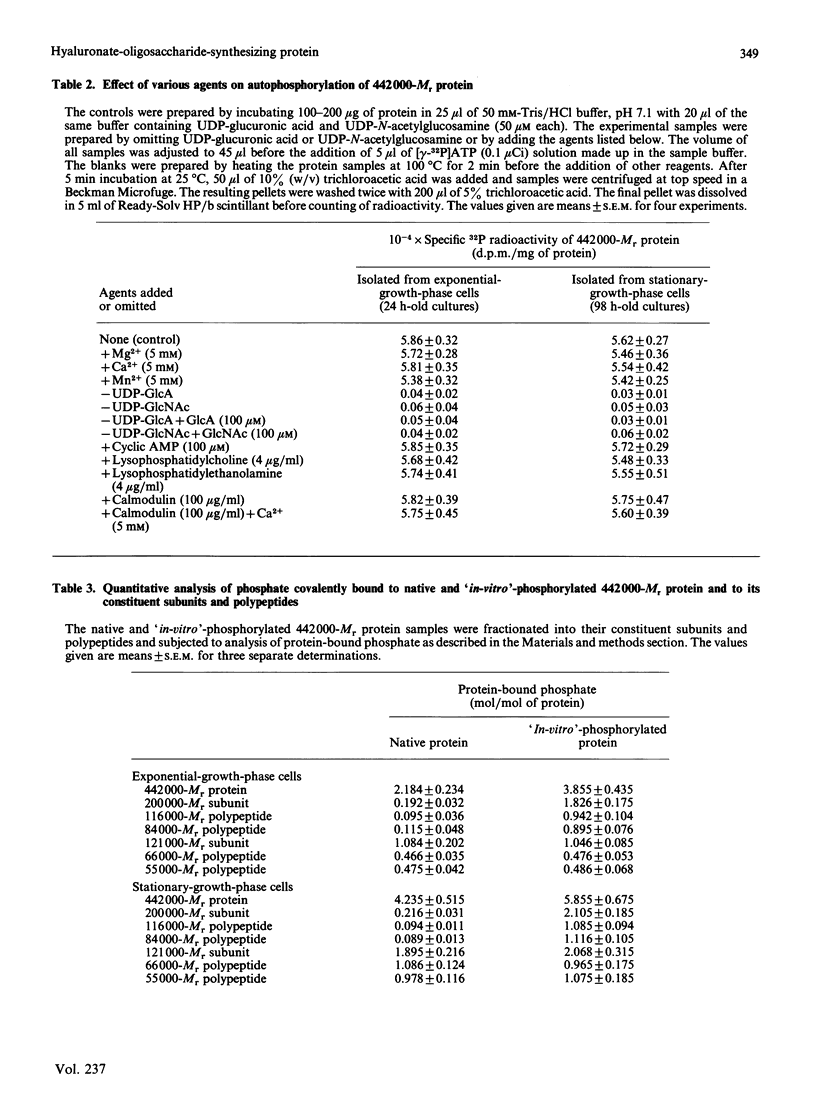
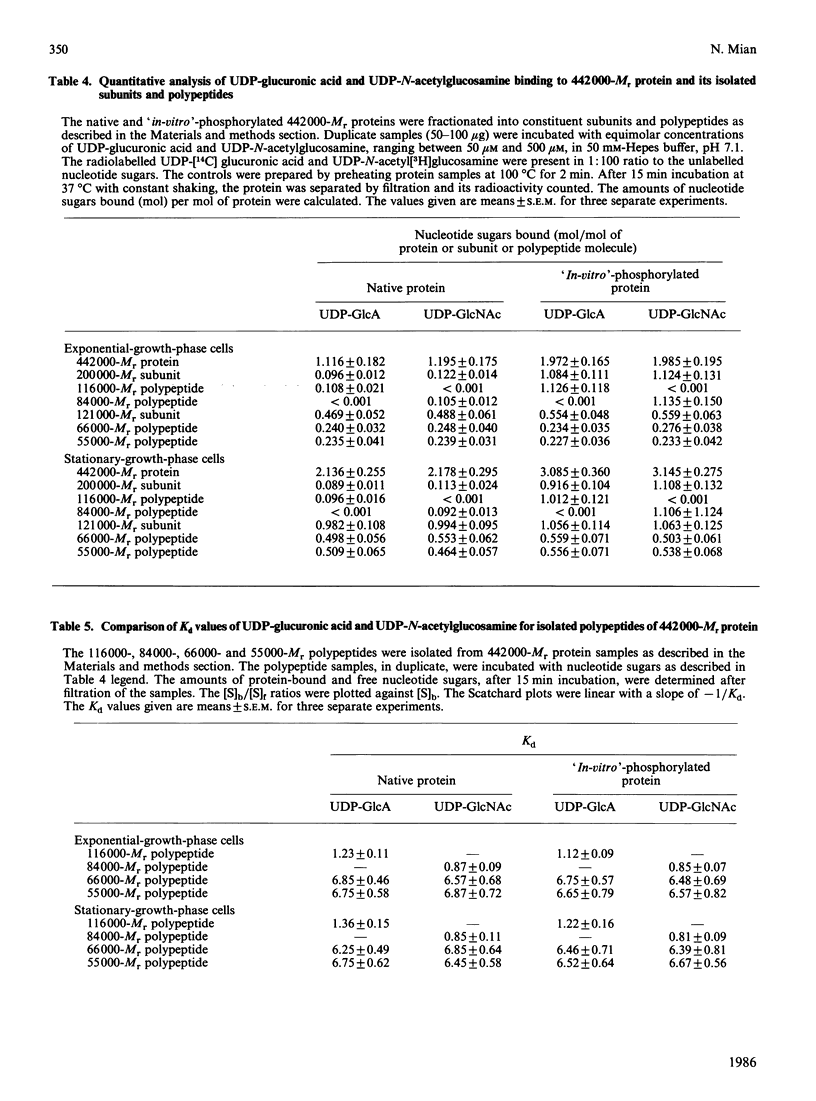
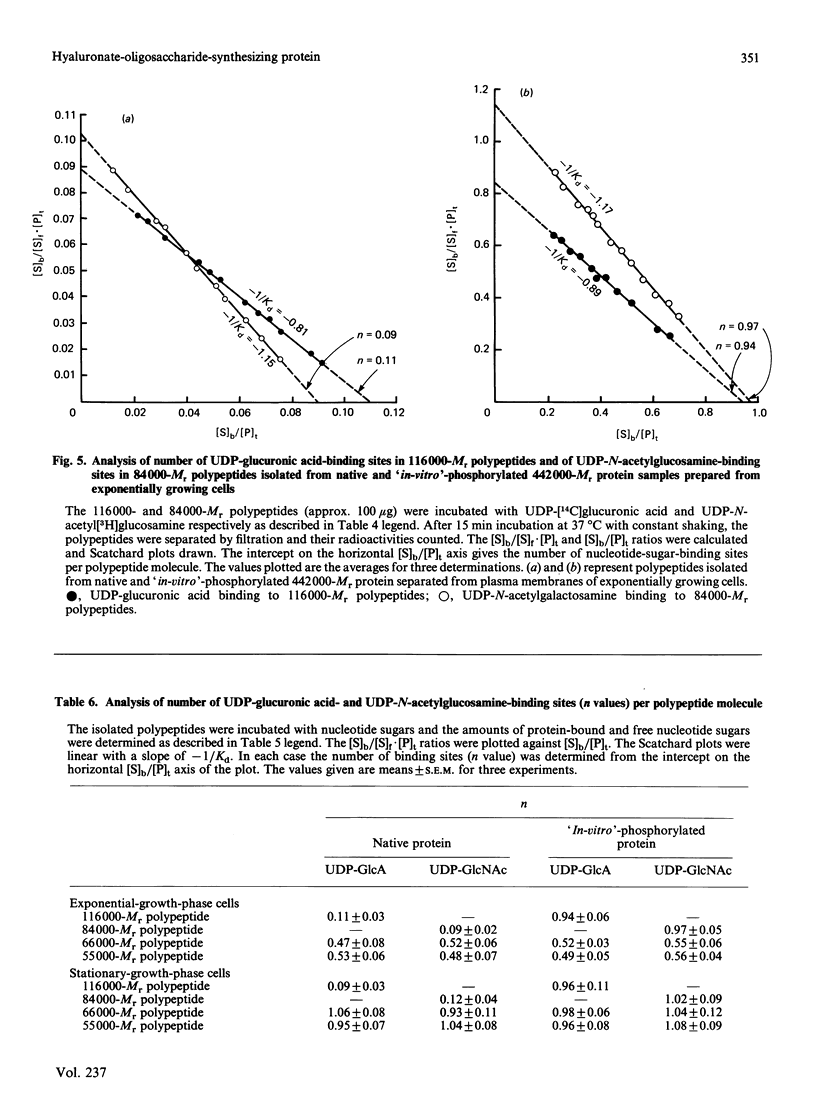
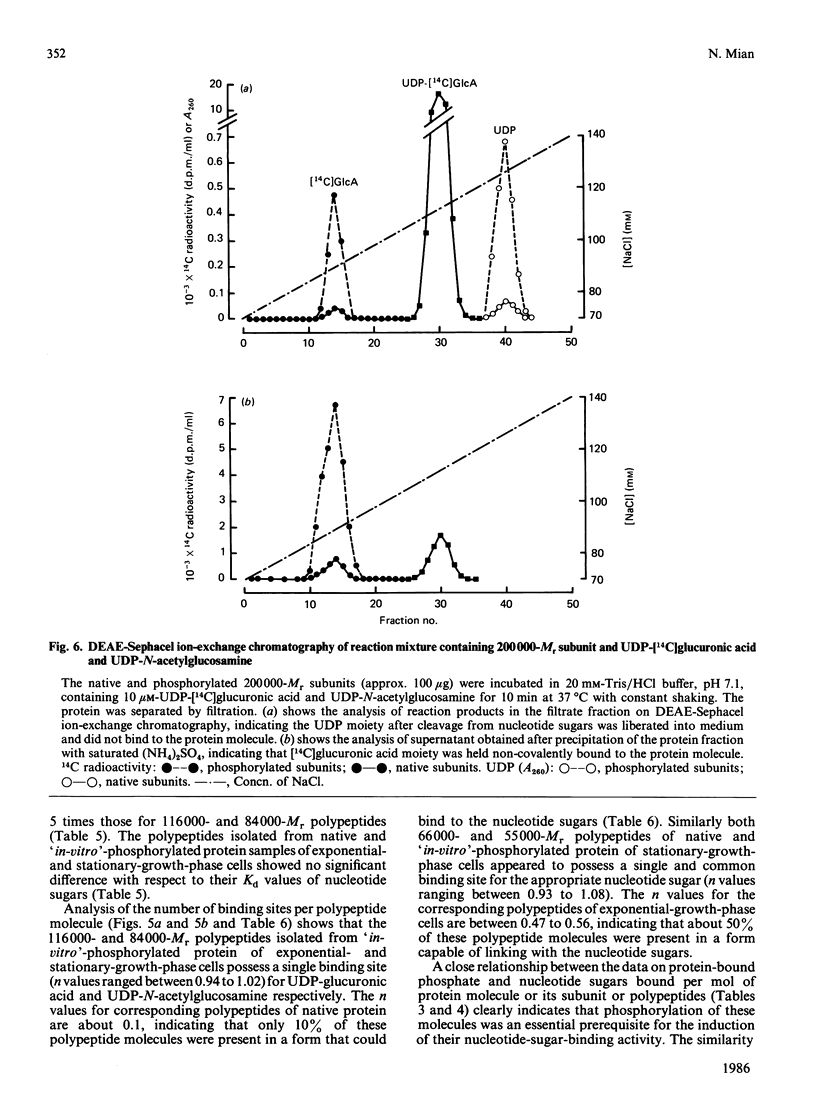
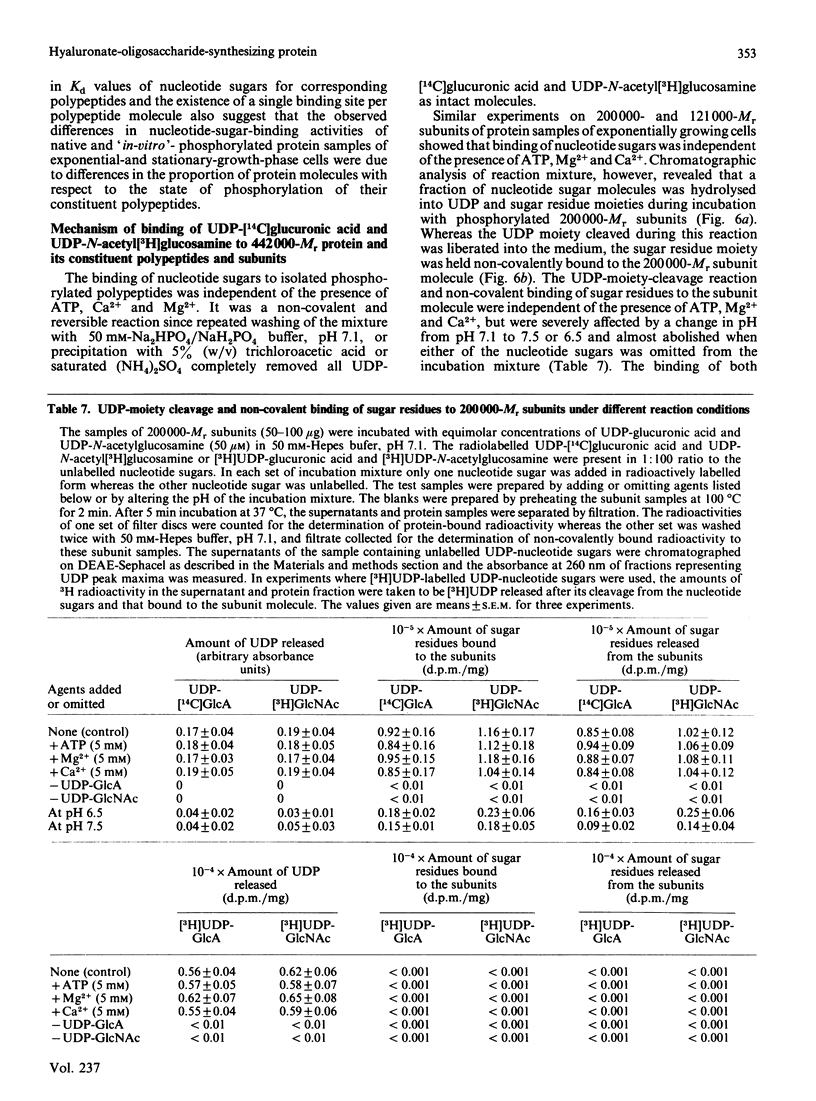
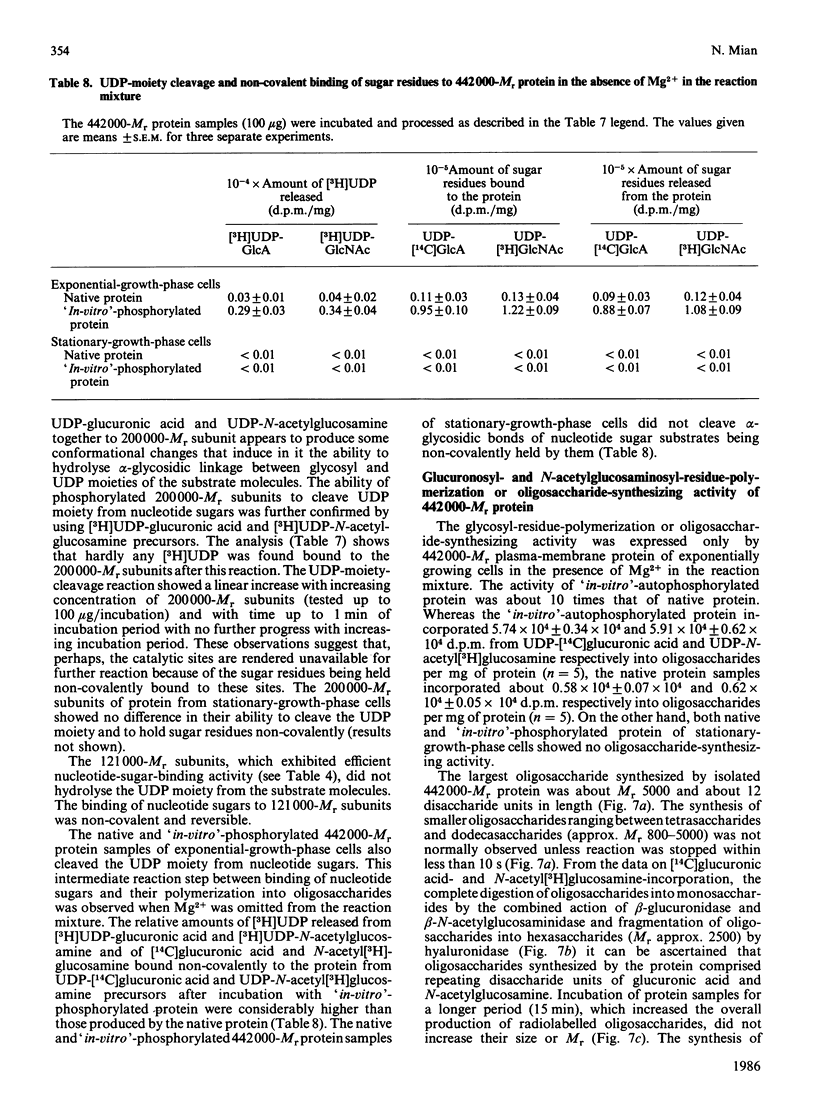
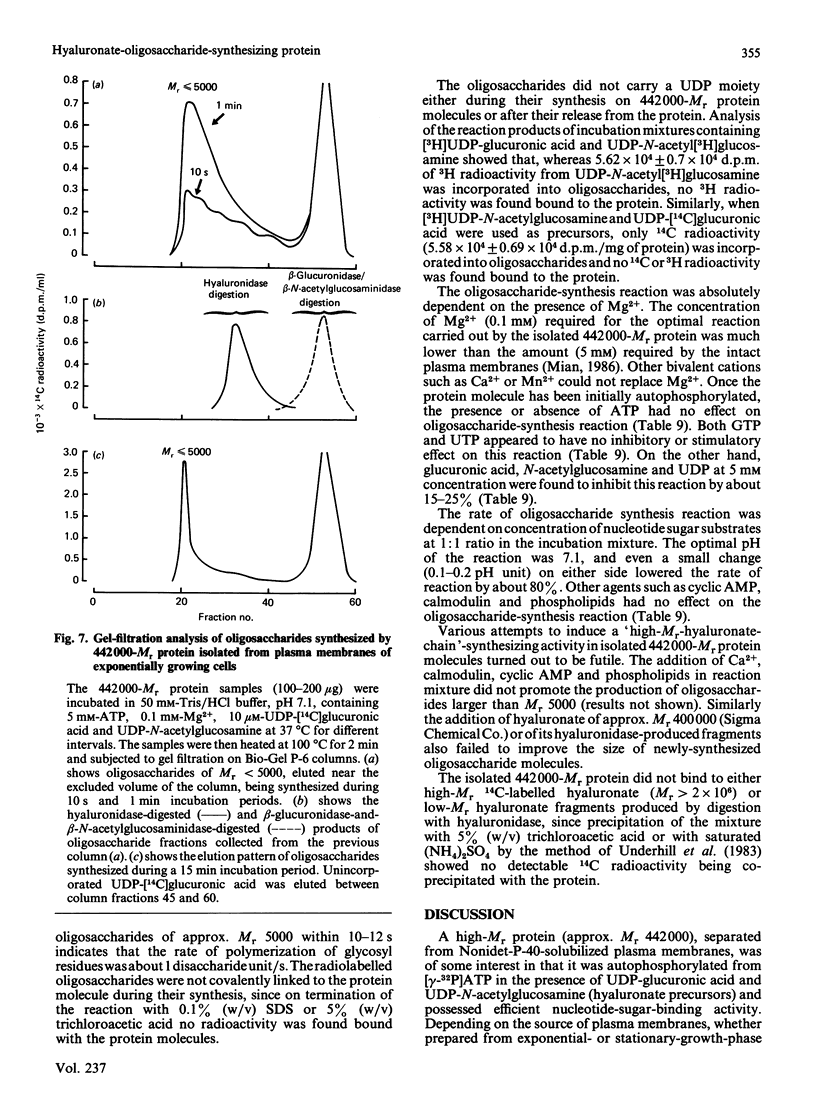
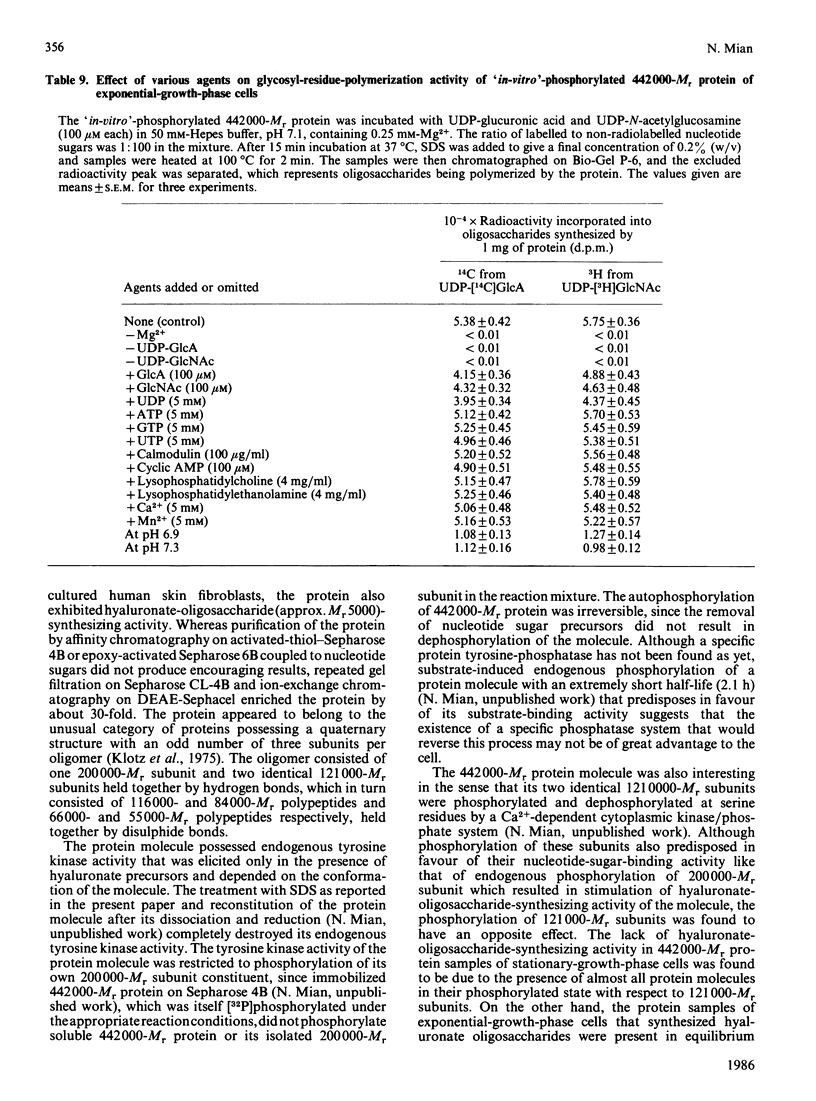
A high-Mr phosphoprotein (Mr 442,000) was purified from Nonidet-P-40-solubilized plasma membranes of cultured human skin fibroblasts. The protein comprised one 200,000-Mr subunit consisting of 116,000- and 84,000-Mr polypeptides and two identical 121,000-Mr subunits each consisting of 66,000- and 55,000-Mr polypeptides. The 200,000-Mr subunit and its polypeptides contained phosphotyrosine residues and were also [32P]phosphorylated at these residues from [gamma-32P]ATP in vitro by an intrinsic tyrosine kinase activity of the protein molecule in response to the presence of hyaluronate precursors, UDP-glucuronic acid and UDP-N-acetylglucosamine. The 121,000-Mr subunits and their polypeptides contained phosphoserine residues that could not be [32P]phosphorylated during autophosphorylation of the protein in vitro. The protein molecules separated from exponential- and stationary-growth-phase cells were identical in their quaternary structure, but appeared to exist in different proportions with respect to the state of phosphorylation of their 121,000-Mr subunits during different growth phases of the cell. Phosphorylation of polypeptides appeared to predispose in favour of their UDP-glucuronic acid- and UDP-N-acetylglucosamine-binding activities. The phosphorylated 116,000- and 84,000-Mr polypeptides of 200,000-Mr subunits possessed a single binding site for UDP-glucuronic acid and UDP-N-acetylglucosamine respectively. The phosphorylated 200,000-Mr subunit could also cleave the UDP moiety from UDP-glucuronic acid and UDP-N-acetylglucosamine precursors. The phosphorylated 121,000-Mr subunit possessed two binding sites with equal affinity towards UDP-glucuronic acid and UDP-N-acetylglucosamine but did not possess UDP-moiety-cleavage activity. The phosphorylation of 200,000-Mr subunit by an intrinsic kinase activity of the protein molecule appeared to elicit its oligosaccharide-synthesizing activity, whereas phosphorylation of 121,000-Mr subunits, presumably carried out in vivo, abolished this activity of the protein molecule. The oligosaccharides synthesized by the protein were about Mr 5000 and about 12 disaccharide units in length. Neither nucleotide sugars nor glycosyl residues nor newly synthesized oligosaccharides were bound covalently to the protein molecule. The UDP moiety of nucleotide sugar precursors did not constitute a link between protein molecule and oligosaccharide during its synthesis. Although isolated 442,000-Mr protein did not synthesize high-Mr hyaluronate in vitro, this protein molecule can be considered as a constituent of membrane-bound hyaluronate synthase complex because of its observed properties.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., KALCKAR H. M., MAXWELL E. S., STROMINGER J. L. Enzymatic formation of uridine diphosphoglucuronic acid. J Biol Chem. 1957 Jan;224(1):79–90. [PubMed] [Google Scholar]
- Appel A., Horwitz A. L., Dorfman A. Cell-free synthesis of hyaluronic acid in Marfan syndrome. J Biol Chem. 1979 Dec 10;254(23):12199–12203. [PubMed] [Google Scholar]
- Hart G. W., Lennarz W. J. Effects of tunicamycin on the biosynthesis of glycosaminoglycans by embryonic chick cornea. J Biol Chem. 1978 Aug 25;253(16):5795–5801. [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977 Jul 25;252(14):4777–4785. [PubMed] [Google Scholar]
- Kleine T. O. Biosynthesis of proteoglycans: an approach to locate it in different membrane systems. Int Rev Connect Tissue Res. 1981;9:27–98. doi: 10.1016/b978-0-12-363709-3.50008-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li H. C., Felmly D. A. A rapid paper chromatographic assay for protein kinase. Anal Biochem. 1973 Mar;52(1):300–304. doi: 10.1016/0003-2697(73)90352-7. [DOI] [PubMed] [Google Scholar]
- Longas M. O., Meyer K. Sequential hydrolysis of hyaluronate by beta-glucuronidase and beta-N-acetylhexosaminidase. Biochem J. 1981 Aug 1;197(2):275–282. doi: 10.1042/bj1970275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A., CIFONELLI J. A., DORFMAN A. The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. J Biol Chem. 1959 Sep;234:2343–2350. [PubMed] [Google Scholar]
- Mapleson J. L., Buchwald M. Effect of cycloheximide and dexamethasone phosphate on hyaluronic acid synthesis and secretion in cultured human skin fibroblasts. J Cell Physiol. 1981 Nov;109(2):215–222. doi: 10.1002/jcp.1041090204. [DOI] [PubMed] [Google Scholar]
- Mian N. Analysis of cell-growth-phase-related variations in hyaluronate synthase activity of isolated plasma-membrane fractions of cultured human skin fibroblasts. Biochem J. 1986 Jul 15;237(2):333–342. doi: 10.1042/bj2370333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Hyaluronate is synthesized at plasma membranes. Biochem J. 1984 Jun 1;220(2):597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Characterization of the synthase. Biochem J. 1983 Apr 1;211(1):181–189. doi: 10.1042/bj2110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Mechanism of chain growth. Biochem J. 1983 Apr 1;211(1):191–198. doi: 10.1042/bj2110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. K., Mendicino J. Synthesis of UDP-N-[1-14C]acetyl D-glucosamine and UDP-N-[1-14C]acetyl-D-galactosamine from [1-14C]acetate. Anal Biochem. 1978 Dec;91(2):490–495. doi: 10.1016/0003-2697(78)90535-3. [DOI] [PubMed] [Google Scholar]
- Shatton J. B., Ward C., Williams A., Weinhouse S. A microcolorimetric assay of inorganic pyrophosphatase. Anal Biochem. 1983 Apr 1;130(1):114–119. doi: 10.1016/0003-2697(83)90657-7. [DOI] [PubMed] [Google Scholar]
- Stoolmiller A. C., Dorfman A. The biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem. 1969 Jan 25;244(2):236–246. [PubMed] [Google Scholar]
- Sugahara K., Schwartz N. B., Dorfman A. Biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem. 1979 Jul 25;254(14):6252–6261. [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Induction of hyaluronic acid synthetase activity in rat fibroblasts by medium change of confluent cultures. J Cell Physiol. 1975 Aug;86(1):121–130. doi: 10.1002/jcp.1040860114. [DOI] [PubMed] [Google Scholar]
- Underhill C. B., Chi-Rosso G., Toole B. P. Effects of detergent solubilization on the hyaluronate-binding protein from membranes of simian virus 40-transformed 3T3 cells. J Biol Chem. 1983 Jul 10;258(13):8086–8091. [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. Detection of phosphate esters on paper chromatograms. Nature. 1953 Mar 21;171(4351):529–530. doi: 10.1038/171529a0. [DOI] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Altered metabolism of heparan sulfate in simian virus 40 transformed cloned mouse cells. J Biol Chem. 1978 Jul 25;253(14):5109–5120. [PubMed] [Google Scholar]



