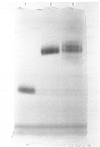
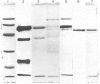
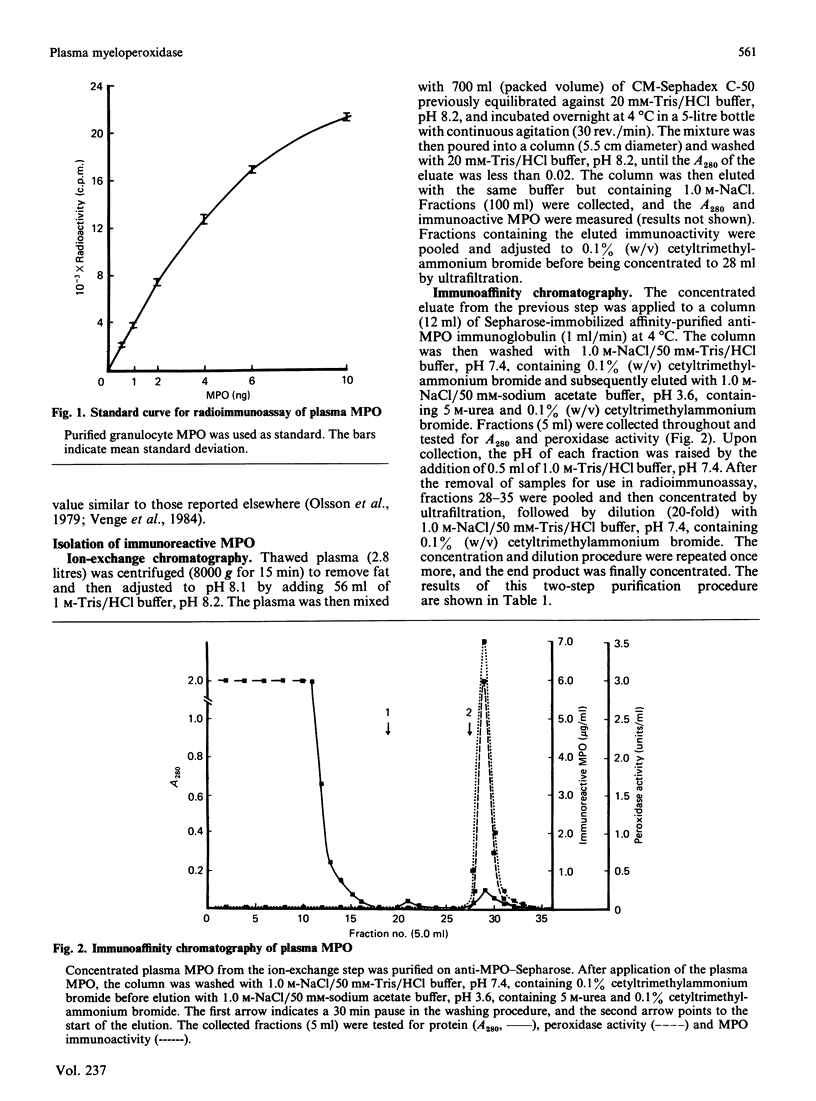
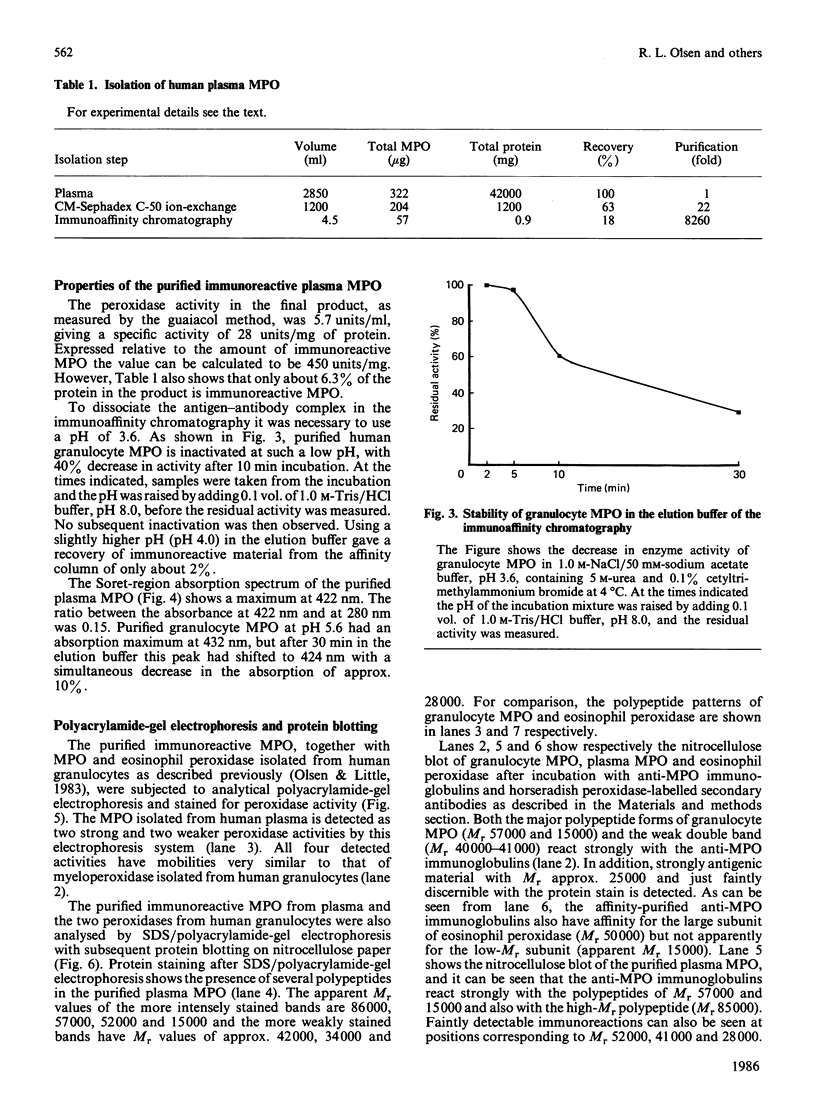
Abstract
A radioimmunoassay for myeloperoxidase was established with the use of affinity-purified anti-(human myeloperoxidase) immunoglobulins. By the use of ion-exchange followed by immunoaffinity chromatography a preparation of immunoreactive, catalytically active myeloperoxidase was obtained from fresh human plasma. In non-denaturing gel electrophoresis, the plasma preparation showed about four catalytically active components of mobility very similar to that of the granulocyte enzyme. SDS/polyacrylamide-gel electrophoresis combined with protein blotting showed that the two polypeptides of strongest antigenicity in the plasma preparation corresponded in Mr to the large and the small subunits of the granulocyte enzyme. In addition, the plasma preparation contained a higher-Mr immunoreactive polypeptide, possibly a precursor form of the enzyme, together with another of Mr similar to that of the large subunit of eosinophil peroxidase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. R., Atkin C. L., Eyre H. J. Intact form of myeloperoxidase from normal human neutrophils. Arch Biochem Biophys. 1982 Mar;214(1):273–283. doi: 10.1016/0003-9861(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentwood B. J., Henson P. M. The sequential release of granule constitutents from human neutrophils. J Immunol. 1980 Feb;124(2):855–862. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Christensen R. D., Rothstein G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood. 1982 Sep;60(3):618–622. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hansen N. E., Malmquist J., Thorell J. Plasma myeloperoxidase and lactoferrin measured by radioimmunoassay: relations to neutrophil kinetics. Acta Med Scand. 1975 Dec;198(6):437–443. doi: 10.1111/j.0954-6820.1975.tb19572.x. [DOI] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hasilik A., Pohlmann R., Olsen R. L., von Figura K. Myeloperoxidase is synthesized as larger phosphorylated precursor. EMBO J. 1984 Nov;3(11):2671–2676. doi: 10.1002/j.1460-2075.1984.tb02192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Voss B., Von Figura K. Transport and processing of lysosomal enzymes by smooth muscle cells and endothelial cells. Exp Cell Res. 1981 May;133(1):23–30. doi: 10.1016/0014-4827(81)90352-9. [DOI] [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971 Dec;107(6):1547–1557. [PubMed] [Google Scholar]
- Imort M., Zühlsdorf M., Feige U., Hasilik A., von Figura K. Biosynthesis and transport of lysosomal enzymes in human monocytes and macrophages. Effects of ammonium chloride, zymosan and tunicamycin. Biochem J. 1983 Sep 15;214(3):671–678. doi: 10.1042/bj2140671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Ranyard J., Pertcheck M. Myeloperoxidase: its structure and expression during myeloid differentiation. Blood. 1985 Feb;65(2):484–491. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malmquist J. Serum myeloperoxidase in leukaemia and polycythaemia vera. Scand J Haematol. 1972;9(4):311–317. doi: 10.1111/j.1600-0609.1972.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Oberg G., Lindmark G., Moberg L., Venge P. The peroxidase activity and cellular content of granule proteins in PMN during pregnancy. Br J Haematol. 1983 Dec;55(4):701–708. doi: 10.1111/j.1365-2141.1983.tb02853.x. [DOI] [PubMed] [Google Scholar]
- Odajima T., Yamazaki I. Myeloperoxidase of the leukocyte of normal blood. V. The spectral conversion of myeloperoxidase to a cytochrome oxidase like derivative. Biochim Biophys Acta. 1972 Oct 12;284(2):368–374. doi: 10.1016/0005-2744(72)90132-5. [DOI] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Comparative studies on oestrogen-induced rat uterus peroxidase and rat eosinophil peroxidase. Biochem J. 1982 Dec 1;207(3):613–616. doi: 10.1042/bj2070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1983 Mar 1;209(3):781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Purification of rat uterine peroxidase. Acta Chem Scand B. 1981;35(1):1–4. doi: 10.3891/acta.chem.scand.35b-0001. [DOI] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Studies on the subunits of human myeloperoxidase. Biochem J. 1984 Sep 15;222(3):701–709. doi: 10.1042/bj2220701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. The peroxidase activity of rat uterus. Eur J Biochem. 1979 Nov;101(2):333–339. doi: 10.1111/j.1432-1033.1979.tb19725.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9(5):483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Ohlsson K., Gustavsson A. Serum and plasma myeloperoxidase, elastase and lactoferrin content in acute myeloid leukaemia. Scand J Haematol. 1979 May;22(5):397–406. doi: 10.1111/j.1600-0609.1979.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Olsson I., Persson A. M., Strömberg K. Biosynthesis, transport and processing of myeloperoxidase in the human leukaemic promyelocytic cell line HL-60 and normal marrow cells. Biochem J. 1984 Nov 1;223(3):911–920. doi: 10.1042/bj2230911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C., Davies P., Allison A. C. The macrophage as a secretory cell. Int Rev Cytol. 1978;52:119–157. doi: 10.1016/s0074-7696(08)60755-x. [DOI] [PubMed] [Google Scholar]
- Skudlarek M. D., Swank R. T. Turnover of two lysosomal enzymes in macrophages. J Biol Chem. 1981 Oct 10;256(19):10137–10144. [PubMed] [Google Scholar]
- Sly W. S., Fischer H. D. The phosphomannosyl recognition system for intracellular and intercellular transport of lysosomal enzymes. J Cell Biochem. 1982;18(1):67–85. doi: 10.1002/jcb.1982.240180107. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Dalldorf F. G., Leffell M. S., Folds J. D., Welsh I. R., Cooney M. H., Martin L. E. Character of azurophil and specific granules purified from human polymorphonuclear leukocytes. Lab Invest. 1974 Jun;30(6):774–785. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venge P., Foucard T., Henriksen J., Håkansson L., Kreuger A. Serum-levels of lactoferrin, lysozyme and myeloperoxidase in normal, infection-prone and leukemic children. Clin Chim Acta. 1984 Jan 31;136(2-3):121–130. doi: 10.1016/0009-8981(84)90283-3. [DOI] [PubMed] [Google Scholar]
- Yamada M. Myeloperoxidase precursors in human myeloid leukemia HL-60 cells. J Biol Chem. 1982 Jun 10;257(11):5980–5982. [PubMed] [Google Scholar]
- Zühlsdorf M., Imort M., Hasilik A., von Figura K. Molecular forms of beta-hexosaminidase and cathepsin D in serum and urine of healthy subjects and patients with elevated activity of lysosomal enzymes. Biochem J. 1983 Sep 1;213(3):733–740. doi: 10.1042/bj2130733. [DOI] [PMC free article] [PubMed] [Google Scholar]