Abstract
Background
Scoliosis is defined as a lateral spine curvature of at least 10° with vertebral rotation, as seen on a posterior-anterior radiograph, often accompanied by reduced thoracic kyphosis. Scoliosis affects all age groups: idiopathic scoliosis is the most common spinal disorder in children and adolescents, while adult degenerative scoliosis typically affects individuals over fifty. In the United States, approximately 3 million new cases of scoliosis are diagnosed annually, with a predicted increase in part due to global aging. Despite its prevalence, the etiopathogenesis of scoliosis remains unclear.
Methods
This comprehensive review analyzes the literature on the etiopathogenetic evidence for both idiopathic and adult degenerative scoliosis. PubMed and Google Scholar databases were searched for studies on the genetic factors and etiopathogenetic mechanisms of scoliosis development and progression, with the search limited to articles in English.
Results
For idiopathic scoliosis, genetic factors are categorized into three groups: genes associated with susceptibility, disease progression, and both. We identify gene groups related to different biological processes and explore multifaceted pathogenesis of idiopathic scoliosis, including evolutionary adaptations to bipedalism and developmental and homeostatic spinal aberrations. For adult degenerative scoliosis, we segregate genetic and pathogenic evidence into categories of angiogenesis and inflammation, extracellular matrix degradation, neural associations, and hormonal influences. Finally, we compare findings in idiopathic scoliosis and adult degenerative scoliosis, discuss current limitations in scoliosis research, propose a new model for scoliosis etiopathogenesis, and highlight promising areas for future studies.
Conclusions
Scoliosis is a complex, multifaceted disease with largely enigmatic origins and mechanisms of progression, keeping it under continuous scientific scrutiny.
Keywords: Adolescent idiopathic scoliosis, Adult degenerative scoliosis, Aging, Degenerative spine disorder, Low back pain, Spinal deformity
Background
The spine is a functionally complex structure. Disorders compromising its integrity can lead to complications in multiple organ systems. Scoliosis is one such condition, where severe curvature may be associated with serious cardiovascular and pulmonary complications, chronic pain, and psychological stress [[1], [2], [3]]. The profound impact of scoliosis on patients’ quality of life is becoming increasingly recognized [4,5]. Patients with scoliosis often suffer from body image misperceptions and demonstrate a higher incidence of mood disorders [6]. This broad impact on patients' lives underscores the need for ongoing extensive scientific scrutiny into the etiopathogenesis of scoliosis.
According to the Scoliosis Research Society, the diagnosis of scoliosis involves measuring the Cobb angle, with a threshold of 10° or higher indicating the disease, though significant axial rotation can occur even below this angle [7,8]. The prevalence of scoliosis in the general population is around 2%–3%, with approximately 20% of cases being secondary to another disease [8]. The remaining 80% are cases of idiopathic scoliosis and adult degenerative scoliosis [8]. Idiopathic scoliosis is further subcategorized based on the age of the disease manifestation: infantile (first 3 years of life), juvenile (4–10 years old), and adolescent (10–18 years old). The latter is the predominant form of scoliosis in the pediatric population [9] and is the most common pediatric musculoskeletal disorder, affecting approximately 3% of school-aged children, which amounts to over 29 million children worldwide [10]. Progression is more common in girls during puberty and can lead to severe deformities and impaired quality of life if untreated [11]. Such risk is highest before peak growth velocity but decreases sharply after skeletal maturity [12].
In contrast to idiopathic scoliosis, adult degenerative scoliosis develops de novo in patients usually above fifty years of age with no previously detected spine deformity [13]. It is also more prevalent in the female population and is growing in incidence in parallel with the global aging [14]. Intense back and leg pain, leading to spinal dysfunction and disability in patients with adult degenerative scoliosis, can result from uneven muscle strain, facet joint arthritis, or nerve root compression [15].
Management of idiopathic scoliosis generally depends on severity of the curve progression and the symptomatology [9]. It ranges from conservative management with analgesics, orthoses, nerve blocks and physical therapy to possible surgery [[16], [17], [18]].
Fortunately, modern therapeutic interventions are highly successful, significantly improving patients' quality of life [19]. Surgical corrections of scoliotic deformities can be highly effective and improve patient's body image [20]. However, they carry a risk of complications in 5%-25% of cases [21]. Additionally, such surgical interventions result in a large financial burden [22]. In general, treatment of scoliosis has been associated with substantial consumption of healthcare resources [23,24]. Such socioeconomic consequences of scoliosis further highlight the need for improved disease understanding and consequent development of disease prevention strategies.
The current scope of knowledge indicates that idiopathic scoliosis likely results from multiple factors [7,25,26]. The pathogenesis of adult degenerative scoliosis involves a self-reinforcing cycle of asymmetric degeneration of intervertebral discs and facet joints, resulting in unbalanced spinal load distribution and abnormal spinal curvature [27]. However, as with idiopathic scoliosis, the etiopathogenetic mechanisms of adult degenerative scoliosis also remain poorly understood.
Previously, our group conducted a comprehensive literature review on the biological principles driving the development of adult degenerative scoliosis [14]. Building on that study, in this review we explore the evidence on the genetic and pathogenetic factors of both idiopathic and adult degenerative scoliosis. This approach aims to clarify the mechanisms involved in scoliosis, identifying those that are either common to both types or specific to either one. For clarity, the term “idiopathic scoliosis” will refer specifically to adolescent idiopathic scoliosis.
Methods
Literature search and study selection
A comprehensive literature search was conducted to identify studies examining the biological factors, including genetic and developmental aspects, as well as environmental influences on the development and progression of scoliosis. The search focused on studies related to idiopathic scoliosis and adult degenerative scoliosis, with studies on congenital scoliosis excluded, as it is regarded as a distinct category [28] outside the scope of this review.
Databases and search strategy
The databases PubMed and Google Scholar were used for the literature search. Keywords included terms related to “genetic factors in scoliosis,” “genetics of scoliosis,” “pathogenesis of scoliosis,” “biology of idiopathic scoliosis,” “biology of adult degenerative scoliosis,” “etiology of scoliosis”. No time restrictions were applied to the search, allowing for the inclusion of studies published across all years. Only articles published in English were considered.
Genetics of idiopathic scoliosis
The term “idiopathy” is translated from Greek to “a disease of its own kind,” referring to a condition that has an unknown cause [29]. This term is particularly relevant in the context of idiopathic scoliosis, where despite numerous pathogenetic theories proposed and extensive research conducted, no single hypothesis has gained universal acceptance, and the debate is continued [30].
The notion that idiopathic scoliosis might be a genetically predetermined disease was proposed over a century ago, and research into its hereditary aspects has been ongoing ever since [31]. Twin studies have been particularly insightful. They have shown that idiopathic scoliosis has a higher genetic correlation in monozygotic twins [73%] than in dizygotic twins [36%], with a noticeable association between disease severity and genetics in identical twins only [32]. Results from the Danish Twin Registry have underscored the strong genetic influence, showing a significantly higher concordance in identical twins [33]. These findings, along with evidence from later-arising genome-wide association studies (GWAS), highlighted the role of genetics in the development of idiopathic scoliosis.
However, not all the identified genetic correlations were found to have an impact on specific forms of curvature [32]. Differences in the progression of idiopathic scoliosis among family members further suggested that the genes modifying its progression are likely distinct from those increasing the disease susceptibility [34].
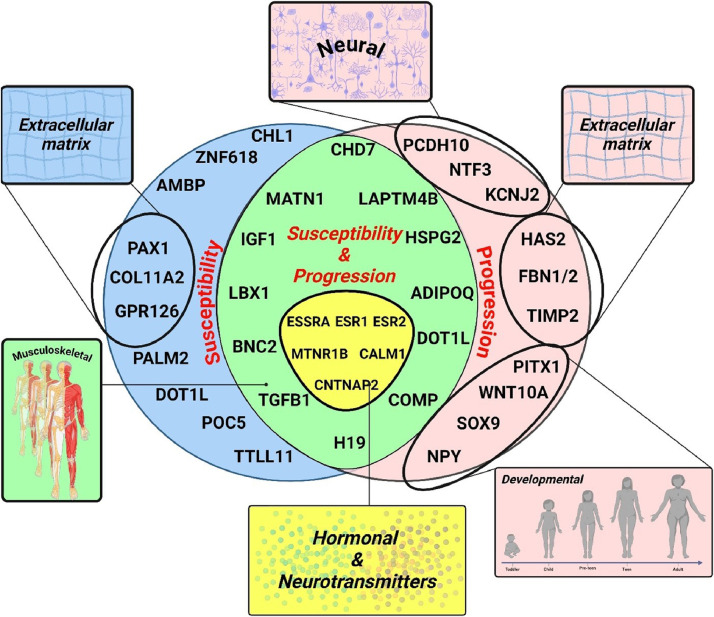
To facilitate perception, we categorize the identified genes as either idiopathic scoliosis susceptibility genes or genes that are associated with disease severity and progression (Fig. 1). The latter two are combined, considering their interdependence. Namely, severity of scoliosis at the time of diagnosis is a significant predictor of how much the curve might progress. This in part is 1 of the reasons why monitoring and managing scoliosis, especially during periods of rapid growth, is crucial to prevent significant worsening of the spinal curvature. Additionally, we group together a set of genes that have been shown to have a role in both susceptibility and scoliosis progression.
Fig. 1.
Genetics of idiopathic scoliosis.
Susceptibility genes
The first GWAS of adolescent idiopathic scoliosis identified a region on chromosome 3p26.3 near the cell adhesion molecule L1 like (CHL1) gene associated with adolescent idiopathic scoliosis risk, although no causal variation was found within CHL1 [35]. The latter encodes a neural cell adhesion molecule that participates in nervous system development [[36], [37], [38]]. Gene LOC642891 with an unidentified function in a proximity to CHL1 was also linked to adolescent idiopathic scoliosis [35]. In the 9q31.2–34.2 interval, SNPs rs4979321 (zinc finger protein 618 [ZNF618] gene), rs891725 (alpha-1-microglobulin/ bikunin precursor [AMBP] gene), rs1969944 (paralemmin 2 [PALM2] gene), and rs4836643 (intergenic) showed significant associations with idiopathic scoliosis. These findings suggest that adolescent idiopathic scoliosis risk arises from genetic heterogeneity rather than a single locus [35].
Genome-wide pathway burden analysis of exome sequence data reveals that extracellular matrix (ECM) genes significantly contribute to the polygenic inheritance of idiopathic scoliosis [39]. More broadly, independent studies have identified a group of genes that are functionally linked to the vertebral column structural integrity (COL11A2, GPR126 and PAX1).
Specifically, new coding variants in musculoskeletal collagen genes, particularly collagen type XI alpha 2 chain (COL11A2), increase idiopathic scoliosis risk by more than 2-fold [39]. The G-protein coupled receptor 126 (GPR126 [aka, ADGRG6]) gene within the chromosome 6q24.1 locus was also identified as a candidate for idiopathic scoliosis susceptibility through extensive GWAS analysis, replicated in Japanese, Chinese, and European-ancestry populations [40]. Activated by type IV collagen, GPR126 is crucial for the normal differentiation of promyelinating Schwann cells and proper myelination of axons [41]. Interestingly, variants of GPR126 have also been shown to regulate bone mass through cAMP-CREB signaling pathway [42].
In addition, using a functional fine-mapping strategy, researchers have identified a susceptibility locus for idiopathic scoliosis on chromosome 20p11.22, located downstream of the paired box 1 (PAX1) gene [43]. The PAX1 region has been previously linked to spinal development through research on naturally occurring undulated mouse strains [44]. Collectively, COL11A2, GPR126 and PAX1 may indicate a genetic signature predisposing to spine structural deformities.
Genetic linkage analyses combined with exome sequencing identified a rare missense variant (p. A446T) in the centriolar protein gene POC5 centriolar protein (POC5) that co-occurred with the disease in several families with multiple members affected with idiopathic scoliosis [45]. Using exome sequencing, researchers also identified an insertion, c.1569_1570insTT in the tubulin tyrosine ligase like 11 (TTLL11) gene as 1 of the potentially causative genes for idiopathic scoliosis [46]. Moreover, TTLL11 and POC5 are involved in similar biological processes of cytoskeleton organization and centrioles assembly respectively [47,48]. Both genes may play a role in the pathophysiology of idiopathic scoliosis.
Disease/curve progression genes
In this review of genes associated with the progression of idiopathic scoliosis, we identified 3 rather distinct patterns: genes with neural associations (NTF3, KCNJ2, PCDH10, NPY), genes involved in connective tissue and extracellular matrix homeostasis (FBN1/2, TIMP2, HAS2), and genes associated with spinal embryogenic development (SOX9, PITX1, WNT10A). This patterning may help future research aimed at uncovering the pathogenetic mechanisms underlying the development of idiopathic scoliosis.
Genes with neural associations
Variations in the neurotrophin 3 (NTF3) gene do not correlate with the incidence of idiopathic scoliosis, yet the promoter variation (rs11063714) is linked to the severity of the spinal curvature and influences the effectiveness of brace treatment [49]. This suggests that NTF3 may play a mitigating role in the progression of idiopathic scoliosis. Normally, it promotes nerve growth and survival of neurons [50].
In another study, variant rs12946942 located on chromosome 17q24.3 near potassium inwardly rectifying channel subfamily J member 2 (KCNJ2) gene, has been associated with scoliosis phenotypes [51]. The product of KCNJ2 had been suggested to have a role in establishing action potential waveform and excitability of neuronal and muscle tissues [52,53].
Average methylation of the protocadherin 10 (PCDH10) promoter was higher and gene expression was lower in idiopathic scoliosis patients compared to controls [54]. Additionally, high PCDH10 promoter methylation correlated with the Cobb angle of major curves in idiopathic scoliosis patients [54]. The product of PCDH10 has been shown to be critically involved in formation and maintenance of neural circuits and synapses, and regulations of actin assembly [55].
Genes involved in connective tissue and extracellular matrix homeostasis
Analysis of genetic linkage in eleven families with 52 scoliosis-affected members did not implicate fibrillin 1 (FBN1), elastin, or collagen type I alpha 2 chain (COL1A2) as relevant genes in these cases [56]. Conversely, a genome-wide study examining rare variant burden through exome sequencing identified FBN1 as significantly associated with idiopathic scoliosis [57]. The severity of scoliosis in idiopathic cases was linked to rare variants in FBN1 and FBN2 genes (p=.0012), a finding that was confirmed in a separate Han Chinese cohort (p=.0376) [57]. This indicates that rare genetic variants could serve as predictors for the progression of the spinal curve.
Earlier research indicated a potential link between a promoter polymorphism of the tissue inhibitor of metalloproteinases 2 (TIMP2) gene (rs8179090; −418 bp G/C) and scoliosis progression in a Chinese cohort [58], but this finding was not confirmed in a Japanese study [59]. In more recent studies, four different TIMP2 polymorphisms (rs11077401, rs2376999, rs2277700, and rs4789934) have been associated with an increased risk of developing the progressive form of idiopathic scoliosis [60]. Protein expressed by TIMP2 is a natural inhibitor of the matrix metalloproteinases [61].
In another study, decreased methylation at site cg01374129 of the hyaluronan synthase 2 (HAS2) gene (encodes for an ECM constituent) was linked to an increased curvature, suggesting it could serve as a promising biomarker for distinguishing between patients with and without curve progression [62].
Genes associated with spinal development
In a 2-stage GWAS involving around 12,000 Japanese subjects, researchers have discovered that the common variant rs12946942 is significantly linked to severe idiopathic scoliosis [51]. This variant, located on chromosome 17q24.3 near SRY-Box transcription factor 9 (SOX9), has been associated with scoliosis phenotypes [51]. The product of SOX9 expression was implicated in chondrocyte differentiation toward cartilage formation [63]. The results were also replicated in The Chinese population as well [51]. Furthermore, an international meta-analysis with four ethnically diverse cohorts (2272 severe idiopathic scoliosis cases and 13,859 controls) confirmed this association (combined p=7.23 × 10^-13; odds ratio = 1.36, 95% CI = 1.25–1.49) [64]. In silico analyses indicate SOX9 as the likely gene influencing idiopathic scoliosis curve progression in this region [64].
In another study, hypermethylation of the paired like homeodomain 1 (PITX1) gene promoter in the blood cells of idiopathic scoliosis patients was significantly linked to the Cobb angle of the main curve, indicating a connection to the disease progression [65]. Further, four probes were associated with curve severity: cg02477677 (RARA gene), cg12922161 (LOC150622 gene), cg08826461, and cg16382077 [66]. Promoter regions for WNT10A (WNT signaling) and NPY (bone and energy homeostasis) were prioritized based on methylation concordance in bone, suggesting relevance for bone formation and remodeling [66].
Susceptibility & progression genes
Genes that have been found to contribute both to idiopathic scoliosis susceptibility and progression were found to have common biological involvements (Fig. 1). As such, we identified genes pertinent to the musculoskeletal system (CHD7, IGF1, MATN1, LBX1, BNC2, TGFB1, LAPTM4B, COMP, H19, ADIPOQ, HSPG2, DOT1L) and genes related to hormones and neurotransmitters (ESRRA, ESR1, ESR2, MTNR1B, CALM1, CNTNAP2).
Genes attributable to the musculoskeletal system
The Chromodomain Helicase DNA Binding Protein 7 (CHD7) was the first gene to be associated with idiopathic scoliosis susceptibility [67]. Its role in the etiopathogenesis of CHARGE (Coloboma, Heart defects, Atresia of the nasal choanae, developmental Restriction, Genitourinary abnormalities, and Ear malformations) syndrome, characterized by an increased incidence of scoliosis, suggested a shared underlying etiology with idiopathic scoliosis [67,68]. Research conducted on Polish Caucasian females identified a significant association between the rs1017861 polymorphism in CHD7 and idiopathic scoliosis susceptibility [69]. The rs1017861 and rs4738813 polymorphisms in CHD7 were found to be significantly correlated with the severity and progression of spinal curvature as well [69]. Additionally, in the Chinese Han population, the rs121434341 polymorphism in CHD7 was significantly linked to adolescent idiopathic scoliosis [70]. Furthermore, CHD7 expression has also been positively correlated with bone mineral content, indicating a potential role in the abnormal bone mass observed in patients with this condition [70]. Contrasting these findings, another study that genotyped 22 single nucleotide polymorphisms in the CHD7 gene did not find a statistically significant association with familial idiopathic scoliosis, highlighting the complexity and variability of genetic influences in the development of idiopathic scoliosis [71].
Insulin-like growth factor 1 (IGF-1) is essential for bone growth [72]. A study of 506 Chinese girls with idiopathic scoliosis (Cobb angle >20 degrees) and 227 age-matched controls found that IGF-1 polymorphism affects curve severity but not the onset of scoliosis, suggesting IGF-1 may influence disease severity [73]. However, these results were not replicated in a Japanese cohort, where common single nucleotide polymorphisms in genes for matrilin 1 (MATN1), melatonin receptor 1B (MTNR1B), and tryptophan hydroxylase 1 (TPH1) were also found not to be associated with the idiopathic scoliosis [74].
In contrast, a study of 68 Korean idiopathic scoliosis patients and 35 healthy age- and sex-matched adolescents found that rs5742612 in IGF-1 was associated with both susceptibility to and curve severity in idiopathic scoliosis [75]. In this study, the single nucleotide polymorphism rs2449539 in lysosomal-associated transmembrane protein 4 beta (LAPTM4B) was also found associated with both susceptibility to and curve severity in idiopathic scoliosis [75]. Interestingly, the product of LAPTM4B gene was found to be a negative regulator of transforming growth factor (TGF-β1) [76], which was also independently linked to idiopathic scoliosis [77].
More specifically, the TGFB1 gene allele -509T and genotype -509TT were significantly associated with an increased risk of idiopathic scoliosis in both females and males (p<.01) [77]. Logistic regression revealed a recessive genetic association between the C-509T polymorphism of the TGFB1 gene and idiopathic scoliosis [77]. Additionally, sexual dimorphism was observed: in females, the C-509T polymorphism of the TGFB1 gene was linked to both earlier onset and greater curve severity, but this was not the case in males [77].
Previously, a Japanese study found no significant association with idiopathic scoliosis for matrilin 1 (MATN1) gene, involved in the formation of filamentous networks in the extracellular matrices [74,78]. Likewise, an analysis of MATN1 gene polymorphisms in Turkish individuals using real-time PCR on 53 adolescents with idiopathic scoliosis and 54 healthy adults found no significant differences between the scoliosis and control groups [79]. Conversely, research on Chinese patients identified the tagSNP rs1149048 polymorphism in the MATN1 promoter region as being linked to both susceptibility and progression of idiopathic scoliosis [80]. A recent meta-analysis further confirmed a significant association between the rs1149048 polymorphism and idiopathic scoliosis risk, particularly in the Asian population [81].
A previous study on developing zebrafish implicated basonuclin 2 (BNC2) in the etiology of idiopathic scoliosis by observing a curve development dependent on BNC2 expression levels [82]. Later research on a Chinese population of idiopathic scoliosis patients found that BNC2 gene expression levels were significantly higher compared to controls and strongly correlated with scoliosis curve severity [83].
The polymorphisms rs4794665 in C17orf67 gene and rs12459350 in DOT1L gene were linked to idiopathic scoliosis susceptibility, however, only one of the genotyped SNPs were also correlated with the severity of spinal curvature [84].The product of DOT1L expression is a histone methylator and wad suggested to be involved in protecting cartilage homeostasis [85].
A total of 949 idiopathic scoliosis patients and 976 age-matched healthy controls were recruited for the study, where the SNP rs11190870 near the ladybird homebox 1 (LBX1) gene was linked to both the susceptibility to and progression of idiopathic scoliosis [86]. LBX1 gene product was reported to regulate the muscle precursor cell migration [87]. A meta-analysis of 3 genome-wide association studies, which included 79,211 Japanese individuals, confirmed the association of rs11190870 near LINC01514/LBX1 with idiopathic scoliosis [88]. Additionally, research conducted on the northern Chinese Han population identified two SNPs near LBX1 (rs11190870 and rs1322331) that are associated with an increased risk of idiopathic scoliosis [89].
High cartilage oligomeric matrix protein (COMP) promoter methylation was associated with a high Cobb angle, suggesting it may be a valuable predictor of idiopathic scoliosis susceptibility and curve progression [90]. H19 imprinted maternally expressed transcript (H19) and adiponectin, C1Q and collagen domain containing (ADIPOQ) genes showed inconsistent expression, with lower H19 and higher ADIPOQ levels in concave-sided muscle tissues compared to convex-sided ones [91]. This expression pattern correlated positively with the spinal curve and the age of onset, suggesting a putative role for these genes in both susceptibility and scoliosis progression [91]. Moreover, H19 is located near IGF-2 and has been shown to be involved in osteoporosis development [92]. At the same time, the structure of ADIPOQ gene was founding coding for proteins like collagens X and VIII [93].
The coding variant p.Asn786Ser in the heparan sulfate proteoglycan 2 (HSPG2) gene, encoding a protein that binds to and cross-links many extracellular matrix components, was found to be significantly more prevalent in a larger cohort of idiopathic scoliosis cases than in the control group (p=.024). This suggested an association with the idiopathic scoliosis phenotype [94].
Hormonal and neural genes
In 1978, a prospective study of 26,947 students found a 4.5% incidence of adolescent idiopathic scoliosis, with a female-to-male ratio of 1.25:1 overall and increasing with curve severity [10]. Later, a study of 304 girls with adolescent idiopathic scoliosis found that the XbaI (rs9340799) polymorphism in the estrogen receptor gene was linked with curve progression [95,96]. Girls with XX and Xx genotypes had greater Cobb's angles, a higher risk of a 5-degree curve progression, and a greater likelihood of requiring surgery compared to those with the xx genotype [95,96]. These findings implicated estrogen receptor polymorphisms in curve progression and indicated that DNA analysis could be used to predict this progression.
A study comparing 202 scoliosis patients to 174 healthy controls found that the XbaI (rs9340799) polymorphism of the estrogen receptor gene is linked to idiopathic scoliosis susceptibility in females, with the XX genotype and/or X allele being risk indicators [97]. On the other hand, the PvuII polymorphism showed no association with idiopathic scoliosis risk [97]. However, in a cohort of 540 Chinese girls, no association was found between PvuII and XbaI (rs9340799) polymorphisms of the estrogen receptor 1 and scoliosis susceptibility or curve severity [98]. A meta-analysis of 4 studies (1,827 idiopathic scoliosis cases and 1,253 controls) found no significant association between XbaI (rs9340799) and idiopathic scoliosis(OR 1.09, 95% CI 0.96–1.23, p=.17), suggesting that the XbaI (rs9340799) polymorphism is unlikely to be a susceptibility variant for idiopathic scoliosis predisposition but may be linked to idiopathic scoliosis severity, progression, and treatment [99]. Further, patients resistant to scoliosis brace treatment were linked to the GA genotype and G allele of the estrogen related receptor alpha (ESRRA) gene and the AT genotype and A allele of the tryptophan hydroxylase 1 (TPH1) gene, suggesting that ESRRA and TPH1 are potential genetic markers for predicting brace treatment outcomes [100]. TPH1 gene encodes for an enzyme that is involved in serotonin synthesis, which precedes melatonin synthesis [101].
The AluI site polymorphism of the estrogen receptor 2 (ESR2) gene was linked to Cobb angle severity (AA: 31.9°, AG: 43.2°, GG: 38.9°, p=.002) and differed between moderate (<40°) and severe (≥40°) scoliosis (p=.0011) [102]. While the ESR2 polymorphism was not associated with idiopathic scoliosis predisposition in Caucasian females, it may be linked to curve severity [102]. A missense variant in ESR1 (c.868A>G) and a pathogenic variant in ESR2 (c.236T>C) were identified in idiopathic scoliosis patients with Cobb angles of 41° and 45°, respectively [103]. Both variants showed significantly decreased ability to activate downstream genes, suggesting genetic mutations in ESR1/2 can be associated with idiopathic scoliosis risk [103].
It has been previously reported that calmodulin has an affinity for the estrogen receptor, decreasing its estrogen-binding capacity [104]. This led to the hypothesis that calmodulin's effect on curve progression might be due to the loss of estrogen-binding affinity for the receptor [96]. Researhers investigated the correlation between SNPs in calmodulin 1 (CALM1) and ESR1 genes and double curve idiopathic scoliosis in 67 patients and 100 controls [105]. Significant differences in the polymorphic distribution of rs2234693 in the ER1 gene and rs12885713 and rs5871 in the CALM1 gene were found between idiopathic scoliosis patients and controls. The findings suggest that different SNPs in these genes may be associated with specific idiopathic scoliosis subtypes, particularly double curve idiopathic scoliosis [105].
In a study of 146 idiopathic scoliosis patients and 146 controls, 12 SNPs in the CALM1 gene were analyzed [106]. Three SNPs—rs2300496, rs2300500, and rs3231718—were linked to idiopathic scoliosis predisposition, with no differences found in curve severity or genotype distributions, suggesting CALM1 gene variants can be associated with idiopathic scoliosis susceptibility [106]. In a different study of 55 idiopathic scoliosis patients, calmodulin levels correlated with curve progression and stabilization, indicating potential as a marker for identifying stable and progressive curves [107]. Another study found that idiopathic scoliosis patients had an asymmetric distribution of calmodulin in paraspinal muscles, with higher levels on the convex side and lower levels on the concave side [108]. Interestingly, platelet calmodulin did not reflect the muscle protein values [108].
Contactin-associated protein-like 2 (CNTNAP2) is another gene of interest previously linked to idiopathic scoliosis and later confirmed through a genome-wide association study [35]. CNTNAP2 is also of particular interest, as it participates in axon pathfinding and interacts directly with L1 and Robo class proteins [109]. The latter, when mutated, causes horizontal gaze palsy with progressive scoliosis (HGPPS), a rare disease marked by severe scoliosis [110].
Studying a Chinese population of patients with idiopathic scoliosis, researchers identified interleukin 17 receptor C (IL17RC) gene single nucleotide polymorphism rs708567 associated with both susceptibility and curve progression [111].
Pathogenesis of idiopathic scoliosis
Evolution of erect posture and bipedalism
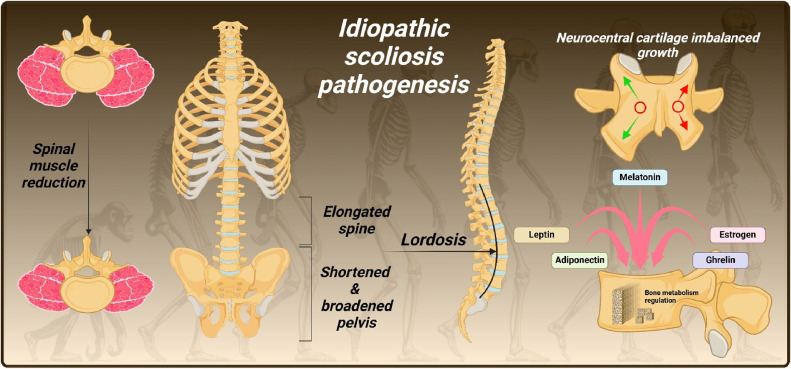
Modern humans possess a highly mobile lower spine compared to other great apes and short-backed primates, due to unique anatomical features [112] [Fig. 2]. The development of lordosis, enabled by the elongation of the spinal column, aligned the spine over the hip joints in an erect stance [112]. It positioned the center of mass of the head, arms, and trunk over the ground contact point without the need to flex both the hip and knee, as do apes [[112], [113], [114]]. In parallel, ilia and sacrum have broadened and shortened, preventing restrictive contact with the lower lumbar vertebrae [112]. The latter developed a progressive widening of their laminae and increased space between their articular processes [115]. The orientation of human facets became more coronal to resist anterior displacement of L5 at the L5/S1 joint [116]. The mass and cross-sectional area of the erector spinae muscles were reduced [117]. These adaptations, coupled with subtle imbalances in the elongated lumbar spine, may contribute to the rotational instability from which scoliosis originates [118,119]. This might remain subclinical until more severe effects manifest in the spine [112].
Fig. 2.
Pathogenesis of idiopathic scoliosis.
The evolutionary drive for bipedality led to significant functional adaptations despite the increased risk of scoliotic deviations and flexion-induced injuries [112]. This suggests that the benefits of upright walking long outweighed potential spinal vulnerabilities. However, over the past 3 million years, primary changes in the human spine and pelvis have focused on lumbar shortening, possibly, among other purposes, to mitigate scoliosis and flexion injuries [112]. Despite this trend towards strengthening the spine, in evolutionary terms these changes are still recent. Consequently, the incomplete adaptation to upright posture and bipedality remains a predisposing factor for the development of scoliotic deformities [120].
Spinal growth and developmental aberrations
Spinal growth is primarily driven by 2 neurocentral joints known as Schmorl's cartilage [121]. The latter are 2 synchondroses that connect each vertebral body to a corresponding semiposterior arch [122]. The asymmetrical development of these cartilages is 1 of the proposed theories behind scoliosis development [120]. This is supported by MRI findings of asymmetrical vertebral pedicles in children with scoliosis and the correlation between intensified joint activity and aggravated idiopathic scoliosis [123,124]. Experimental studies on young pigs show that restricting growth on 1 side of Schmorl's cartilage induces convex scoliosis on the restricted side [125,126]. However, inconsistent results, such as scoliosis on the opposite side of the lesion when electrically sterilized, challenge this theory [127].
Various factors related to abnormal growth and development of the spine contribute to the progression of scoliosis [128]. Some individuals are born with abnormalities in the cartilage of their vertebrae [129,130]. These congenital defects can disrupt normal spinal growth and alignment, contributing to the development and progression of scoliosis [129,130].
Children with scoliosis often experience growth spurts earlier than their peers [128,131]. This rapid growth can exacerbate the spinal curvature because the spine is lengthening faster than it can stabilize. Moreover, during periods of rapid growth, such as puberty, scoliosis tends to worsen. Studies indicate that girls with adolescent idiopathic scoliosis are typically taller and exhibit faster growth rates during puberty than their healthy peers [132,133]. Some researchers have found that during growth phases, the anterior part of the vertebrae can grow more than the posterior part [134]. This uneven growth can cause the vertebrae to become wedged or tilted [135]. The Hueter-Volkmann Law states that compression decelerates both growth whereas traction accelerates [136]. This means that, if the spine starts with a small asymmetry, the differential growth rates will cause this asymmetry to worsen over time.
Anatomical and MRI studies have shown that in structural scoliosis patients, the anterior parts of the spine are longer than the posterior parts, a condition called ‘relative anterior spinal overgrowth’ (RASO) [[137], [138], [139], [140], [141], [142], [143]]. A similar spinal overgrowth was found in both idiopathic scoliosis and neuromuscular scoliosis patients, suggesting RASO is a general feature of scoliosis rather than a specific cause of idiopathic scoliosis [144]. The anterior-to-posterior length difference correlated with the Cobb angle, indicating RASO may contribute to curve progression [144]. However, RASO's role as a primary cause of adolescent idiopathic scoliosis (AIS) is debated and does not apply to all curve types.
Other researchers suggest disproportional growth between the skeletal and neural systems in idiopathic scoliosis, either due to a short spinal cord or rapid spine growth [140,145,146]. This concept was termed asynchronous neuro-osseous growth [140,[145], [146], [147], [148]]. It was found that in severe AIS, the vertebral column is significantly longer without detectable changes in spinal cord length [149,150]. They suggested that anterior spinal overgrowth stretches the spinal cord and cauda equina, leading to hypokyphosis and thoracic spine deformity, causing scoliosis [149,150].
Finally, the neuromuscular theory of idiopathic scoliosis posited that muscular and nerve imbalances contribute to the condition [151]. Animal studies showed that muscle excision or nerve root division could induce scoliosis, highlighting the role of muscle and nerve integrity in spinal stability [152]. Equilibrial dysfunction at the brainstem level was also suggested, with abnormalities in balance more common in scoliosis patients, correlating with curve severity and resolving with maturity [153]. However, subsequent findings of muscle abnormalities, such as dystrophy and disproportions in muscle fiber types, were inconsistent in their locations, leaving the theory subjected to controversy [[154], [155], [156], [157]].
Bone metabolism regulation
The role of endocrine hormones and bone homeostasis in adolescent idiopathic scoliosis (AIS) is a significant area of research [158]. During adolescence, the rapid changes in hormone levels that regulate growth and development might influence idiopathic scoliosis [158].
The androgen receptor (AR) in bone tissue is concentrated in the growth plate, where androgens stimulate cartilage proliferation and endochondral ossification [159,160]. Idiopathic scoliosis patients have been found to have lower androgen levels than non-AIS patients, suggesting a potential link between androgens and idiopathic scoliosis development [160]. However, research on androgens' role in idiopathic scoliosis pathogenesis is limited, with most studies focusing on estrogen.
Estrogen's involvement in idiopathic scoliosis pathogenesis is viewed from 2 perspectives [158]. One theory suggests that abnormal estrogen levels delay menarche in females, postponing bone development and maturity, thereby increasing the risk of spine deformity [158]. Research supports this theory, identifying significantly lower serum estradiol levels in idiopathic scoliosis patients compared to healthy individuals [161,162]. Low levels of estrogen and delayed menarche can lead to decreased bone mineralization and strength, increasing the risk of bone deformity [163]. Studies of female ballet dancers found that those with delayed menarche were more likely to experience idiopathic scoliosis and stress fractures [164], and that dancers were at a significantly higher risk of developing scoliosis than nondancers of the same age [165].
While the mechanism by which low estrogen indirectly causes idiopathic scoliosis by delaying menarche is plausible, it is important to note that idiopathic scoliosis also occurs in females with normal menarche and in males. In female idiopathic scoliosis patients with normal menarche, estrogen's role is more likely via abnormal bone metabolism and development, increasing the risk of malformations [166].
The second theory suggests that abnormal estrogen levels directly affect bone metabolism and remodeling, leading to improper bone growth and a higher likelihood of developing idiopathic scoliosis [163,167,168]. Supporting this, studies have found that adolescent girls with idiopathic scoliosis exhibit lower bone mineral density and higher bone turnover rates compared to healthy controls [169,170]. Additionally, low bone mineral density in the femoral neck correlates with curve progression [171].
Estrogen and its receptors are widespread in the body, playing roles in various developmental processes. Some studies suggest that estrogen receptors (ERs) might be asymmetrically expressed in the paraspinal muscles of idiopathic scoliosis patients [172]. However, further research did not confirm this, finding no significant difference between idiopathic scoliosis patients and controls [173]. The expression levels of melatonin, estrogen, and other receptors in idiopathic scoliosis paraspinal muscle are very low, implying that estrogen and melatonin likely influence idiopathic scoliosis through their regulatory effects on cartilage and bone development.
Melatonin, an indoleamine regulating biological rhythms, is known to influence bone development [174]. In 1959, it was discovered that chickens developed scoliosis after their pineal gland was removed, suggesting melatonin deficiency might cause idiopathic scoliosis [175,176]. This finding was replicated in bipedal rats [119]. However, subsequent studies showed that adolescents with IS had normal melatonin levels [176,177] and that pinealectomized monkeys did not develop scoliosis [178]. Instead, it was proposed that a dysfunction in melatonin signaling, particularly affecting osteoblasts, could be involved [179]. This led to exploring abnormalities in melatonin signaling pathways.
It was found that the melatonin receptor MT2, but not MT1, showed reduced levels in IS patients, and MT2-related gene polymorphisms were linked to IS [[180], [181], [182]]. Melatonin promotes bone density and mass through MT2 receptors and the MAPK pathway, affecting osteoblast proliferation and differentiation [183]. Further research identified a functional abnormality in the melatonin MT2/PKC signaling pathway in idiopathic scoliosis patients' growth plate chondrocytes, potentially leading to abnormal endochondral ossification [184,185]. Melatonin also reduces osteoclastogenesis and increases osteoclast apoptosis by modulating OPG and RANKL levels, promoting bone formation [186,187]. Therefore, altered melatonin levels and MT2 receptor dysfunction might contribute to the development of idiopathic scoliosis by affecting bone development.
Leptin, ghrelin, and adiponectin play significant roles in the development of scoliosis [158]. Leptin, a hormone produced by adipose tissue, is typically lower in individuals with idiopathic scoliosis despite their low BMI, possibly due to higher utilization and reduced secretion [188,189]. This deficit leads to disrupted muscle and bone development, as leptin is crucial for inhibiting muscle degradation and promoting osteoblast activity [158]. Consequently, the asymmetry and postural imbalances characteristic of scoliosis are exacerbated. Adiponectin, also secreted by adipose tissue, is found at higher levels in idiopathic scoliosis patients [190] and exhibits asymmetric expression in paraspinal muscles [91]. It promotes bone resorption by decreasing OPG and increasing RANKL, potentially resulting in lower bone mass and spinal instability [190,191]. Ghrelin, a hormone from the stomach [192], is elevated in idiopathic scoliosis patients [193] and is linked to scoliosis severity [194]. It affects cartilage and bone development through various signaling pathways, such as ERK/STAT3, and may contribute to abnormal bone mass and cartilage development, exacerbating scoliosis [[195], [196], [197]]. Together, these hormones influence muscle, bone, and cartilage development, playing a pivotal role in scoliosis pathogenesis.
Genetics and pathogenesis of adult degenerative scoliosis
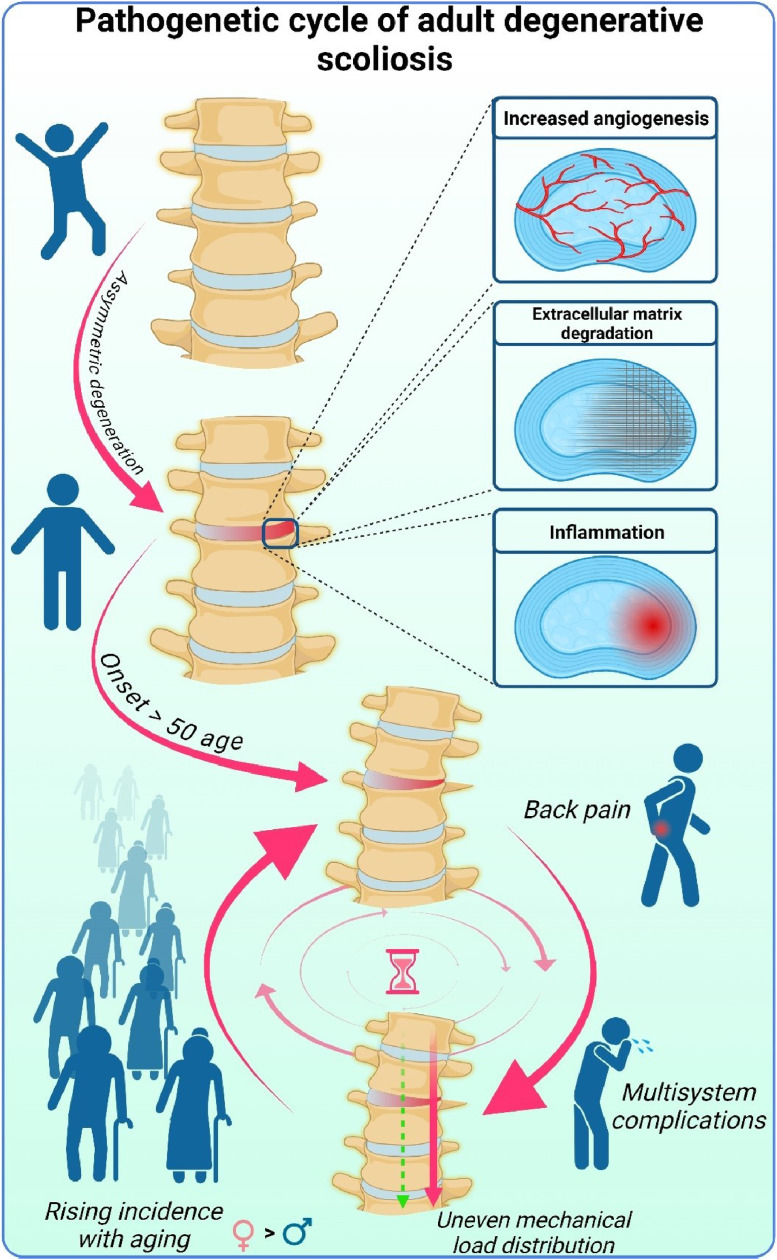
In earlier work, we identified 6 key factors likely contributing to the development and progression of adult degenerative scoliosis: uneven wear of intervertebral discs and facet joints, autophagy-driven angiogenesis and inflammation within the discs, extracellular matrix degradation, bone metabolism abnormalities, muscle loss (sarcopenia), and irregular mechanical stress distribution along the spine [14]. These factors highlight a complex asymmetric degenerative process in the spine with a deviated vertical load axis. This results in disproportional biomechanical pressure, worsening the degeneration and creating a vicious cycle [Fig. 3]. Despite this understanding of its progression, less is known about the origin of adult degenerative scoliosis.
Fig. 3.
Vicious cycle of adult degenerative scoliosis.
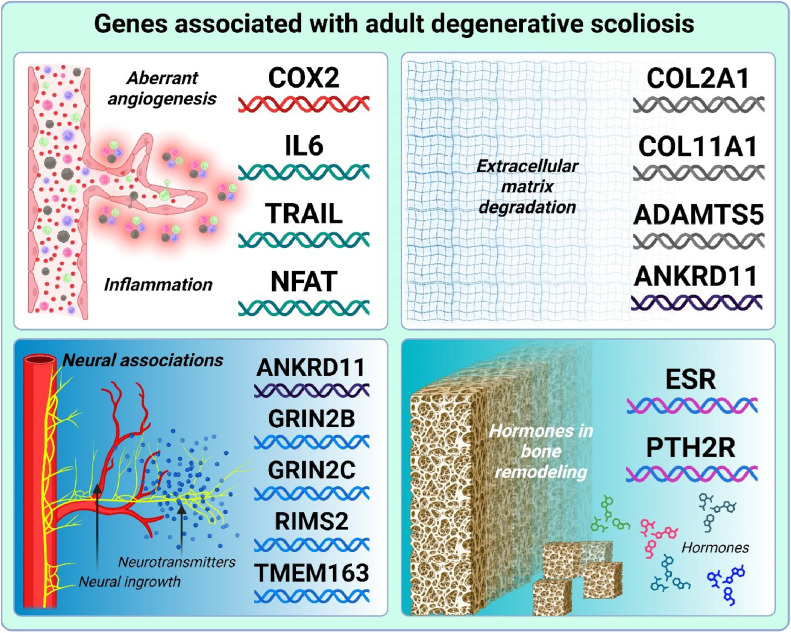
Heritability is 1 key area of research that may help illuminate the manifestation of adult degenerative scoliosis. However, to date fewer genetic factors have been identified in adult degenerative scoliosis compared to other degenerative spine diseases, such as idiopathic scoliosis [198,199]. Nevertheless, several gene groups linked to different pathogenic mechanisms have been reported [Fig. 4].
Fig. 4.
Genes associated with adult degenerative scoliosis.
Angiogenesis and inflammation
One significant group of genes associated with adult degenerative scoliosis pertains to aberrant angiogenesis and inflammation. This group includes cyclooxygenase-2 (COX2), interleukin 6 (IL6), tumor necrosis factor-related apoptosis ligand (TRAIL), and nuclear factor of activated T-cells (NFATC). Previous studies have indicated that COX-2 expression is elevated in degenerative disc disease compared to normal discs, potentially linking it to increased angiogenesis [200,201]. In adult degenerative scoliosis, investigations revealed significantly higher COX-2 levels and lower miR-143 (a COX-2 inhibitor) levels compared to idiopathic scoliosis and control groups further supporting the putative pathogenetic role COX-2 [202].
Another study compared 184 patients with adult degenerative scoliosis to 220 healthy controls, revealing a significant difference in the IL6-572 G/C polymorphism [203]. Additionally, when serum levels of TRAIL and polymorphisms in the TRAIL gene were investigated in patients with intervertebral disc disease, higher serum TRAIL levels in patients with more severe disc degeneration were revealed [204]. The G/C mutation at loci 1525/1595 of the TRAIL gene was also more frequent in patients than in controls [204]. Although no subgroup analysis was conducted for patients with scoliosis, the above evidence suggests TRAIL might also be a contributing factor in adult degenerative scoliosis development [204]. Finally, a genomic study had associated the NFATC gene with adult degenerative scoliosis as well [205]. The product of NFATC gene expression is known to be involved in inducible gene transcription during an immune response [206]. Interestingly, it has also been found to play an important role in osteoclastogenesis and hence, may also be involved in bone remodeling [207].
Aberrant angiogenesis and inflammation have been identified as contributors to the intervertebral disc degeneration [208,209]. Samples from the posterior annulus fibrosus of adult degenerative scoliosis patients showed increased expression of proangiogenic factors like angiogenin and platelet-derived growth factor B (PDGF-B) [210]. Cells from degenerated discs were found expressing TRAIL and death receptors DR4 and DR5, markers of inflammation that correlated with the disc's degenerative state [211]. Together, aberrant angiogenesis and local inflammation promote extracellular matrix degradation, further driving angiogenesis and perpetuating the degenerative cycle [212].
Extracellular matrix degradation
Extracellular matrix (ECM) disruption is a key factor in the degeneration of intervertebral discs and facet joints [14,213]. Type II collagen, an essential ECM component for the nucleus pulposus and facet joints, plays a crucial role in maintaining their structure. Elevated levels of the C-propeptide of type II collagen (CPII), indicating increased collagen synthesis, have been observed in patients with degenerative lumbar scoliosis [213]. A significant association has been found between the SNP rs2276454 in the collagen type II alpha 1 (COL2A1) gene and adult degenerative scoliosis in Korean patients, underscoring collagen's role in bone homeostasis [214]. This association was further confirmed by comparing 51 adult degenerative scoliosis patients to 235 healthy controls [215]. In the Chinese Han population, single nucleotide polymorphisms rs1337185 in COL11A1 and rs162509 in ADAMTS5 have been linked to a higher risk of lumbar disc degeneration [216].
Neural associations
A genomic study identified the gene ankyrin repeat domain containing 11 (ANKRD11) as being associated with adult degenerative scoliosis [205]. It is thought to have a role in the proliferation and development of cortical neural precursors [217] and may regulate bone homeostasis [218]. Angiogenesis in intervertebral disc degeneration is thought to be accompanied by neural ingrowth, which has been suggested as the main reason for lower back pain development [219]. Given the presence of N-methyl-D-aspartate (NMDA) receptors in bone cells, researchers examined the genetic link between cSNPs in NMDA receptor genes and adult degenerative scoliosis [220,221]. Although no significant overall association was found [222], specific cSNPs `in the glutamate ionotropic receptor NMDA type subunit 2C (GRIN2C) gene were linked to larger Cobb angles, and cSNPs in the glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B) gene were associated with greater lateral listhesis within the scoliosis group [221]. However, when the serum proteome profiles of 12 patients with degenerative scoliosis were compared to those of healthy controls, several downregulated proteins were revealed [223]. Among all, isoform 1 of G protein-regulated inducer of neurite outgrowth 1 (GPRIN1) was the most significantly downregulated one [223].
Another study linked an SNP rs10461 in regulating synaptic membrane exocytosis 2 (RIMS2) gene to adult degenerative scoliosis [224]. A genomic study had associated the transmembrane protein 163 (TMEM163) gene, product of which is an integral component of synaptic vesicle membrane, with adult degenerative scoliosis as well [205].
Together, while the precise role of glutamate signaling in degenerative scoliosis remains an intriguing subject of future studies, the existent evidence is supportive of adult degenerative scoliosis having neural associations.
Hormonal influences
Individuals with vertebral compression fractures from osteoporosis, particularly women, face an increased risk of developing scoliotic spine deformities [225]. Women can have up to a 50% lifetime risk of fractures due to bone fragility [226]. Additionally, cartilage is thought to be sensitive to sex hormones [227].
In this context, a notable difference was identified in the Pvu II polymorphism, suggesting that the Pvu II polymorphism may serve as a genetic marker for the prevalence of adult degenerative scoliosis [228]. Additionally, women with degenerative changes in the lumbar spine were found to have a significantly higher frequency of the A-allele of the parathyroid hormone 2 receptor (PTH2R) SNP rs897083 [229]. Although 122 women had adult degenerative scoliosis, no subgroup analysis was performed [229]. Yet, the PTH2R gene variations may contribute to age-related spinal degeneration, potentially leading to adult degenerative scoliosis.
Miscellaneous genes
Proteomic analysis of sera from adult degenerative scoliosis patients identified 11 differentially expressed proteins (Clusterin, CLU cDNA FLJ57622, ALB cDNA FLJ50830, Hypothetical short protein, HLA-A MHC class I antigen [Fragment], ALB 23 kDa protein, Isoform 1 of G protein-regulated inducer of neurite outgrowth 1 [GPRIN1] and Ficolin-3) [230]. Western blot confirmed 2 of these, Clusterin and Ficolin-3, suggesting their potential as biomarkers [230]. Additional analysis of scoliosis patient mesenchymal stem cells revealed differential levels of PIAS2, NDUFA2, and TRIM68, distinct from those in serum [231]. While these proteins may serve as disease biomarkers, further research is needed to validate them across diverse patient populations due to possible environmental and epigenetic influences.
Discussion
Scoliosis affects approximately 2%–3% of the population, translating to an estimated 7–9 million individuals in the United States [232]. Early detection of a progressive curve is crucial for effective treatment of idiopathic scoliosis, as it allows timely intervention. However, significant challenges persist in predicting who will develop scoliosis, understanding the etiological factors, assessing the likelihood of progression, and determining the extent of disease advancement [34]. To this end, genetic testing offers the potential to diagnose idiopathic scoliosis before symptom onset, enabling earlier and more targeted treatments.
Research previously suggested that idiopathic scoliosis may follow autosomal and X-linked dominant inheritance patterns [233]. However, discrepancies in data and the diminishing risk of idiopathic scoliosis across generations indicated a multifactorial inheritance model to be more likely [120]. This model implies a complex interaction between multiple genes and environmental factors, rather than a straightforward hereditary pattern, poising idiopathic scoliosis as a multifaceted disease.
Over time, the methodology for studying the genetics of scoliosis has advanced. Genetic linkage analysis, a widely utilized technique, examines familial cases by analyzing genetic information across generations [234]. This approach identifies genetic markers that consistently co-occur with the disease, pinpointing the chromosome regions likely housing the causative genes. Genetic linkage analysis was the predominant methodology for identifying genetic factors associated with scoliosis. Then followed the emergence of genetic association analysis which focuses on identifying statistical correlations between specific genetic variants—typically single nucleotide polymorphisms (SNPs)—and the presence of diseases in broader populations [1]. This method is particularly useful for complex diseases such as scoliosis, likely influenced by multiple genetic and environmental factors. Each method has its advantages and both have allowed to generate an insight into the genetic factors driving scoliosis development.
Despite the growing interest in the genetics of scoliosis, no specific genetic markers have been identified to date. Research has primarily focused on idiopathic scoliosis, likely because it is the most common form of the condition and typically manifests at a younger age [235]. In contrast, adult degenerative scoliosis generally affects individuals over fifty [13].
Considering different age of scoliosis onset, one may presume the genetic signature that predisposes individuals to scoliosis development is likely different in idiopathic scoliosis vs adult degenerative scoliosis. Supporting this notion, our review identified only the estrogen receptor and collagen genes as common genetic factors in both diseases. In idiopathic scoliosis, abnormal estrogen delays menarche, postponing bone development and maturity [158]. It directly affects bone metabolism and remodeling, leading to improper bone growth and an increased risk of scoliosis [163]. In adult degenerative scoliosis, women can have up to a 50% lifetime risk of fractures due to bone fragility [225,226]. Thus, while estrogen-related genetic changes increase susceptibility to both IS and ADS, they do so via distinct mechanisms.
Another shared feature of idiopathic scoliosis and adult degenerative scoliosis is the development of spinal asymmetry, though the underlying mechanisms differ significantly as well. In idiopathic scoliosis, the most widely accepted mechanism is the asymmetrical activity of the neurocentral cartilage [120]. This uneven growth pattern leads to spinal curvature and has led to therapeutic suggestions, such as targeting the growth of neurocentral cartilage with thoracic pedicle screw instrumentation [236].
Conversely, in adult degenerative scoliosis, asymmetry arises primarily from the uneven degeneration of intervertebral discs [14]. This degeneration is thought to be driven by a combination of aberrant angiogenesis and chronic inflammation, which together perpetuate the degradation of the extracellular matrix [14]. These processes contribute to the uneven wear and tear of the intervertebral discs, resulting in spinal asymmetry. However, unlike in IS, where the sequence of pathogenic events is relatively well theorized, the reason why these degenerative processes become asymmetric in the aging spine leading to adult degenerative scoliosis, remains unclear.
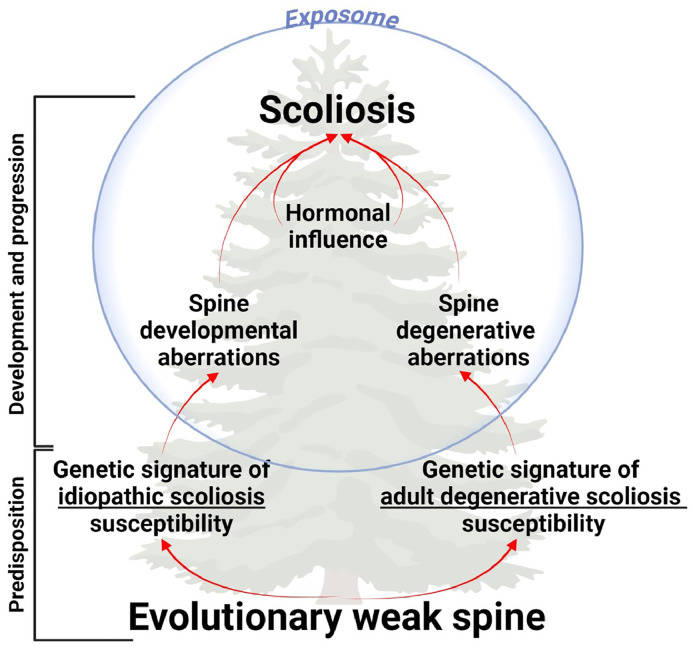
Further, we propose a new tree-like model of scoliosis etiopathogenesis, which depicts multiple layers of disease susceptibility and progression [Fig. 5]. The first layer involves an evolutionarily maladaptive spine, predisposing humans to spinal deformities and serving as a common predisposition for both types of scoliosis. The second layer, consisting of distinct genetic signatures, differentiates the pathogenetic trajectories of idiopathic and adult degenerative scolioses. Influenced by hormonal changes and exposomal factors, these genetic factors lead to aberrations in spinal development and intervertebral disc degeneration, resulting in idiopathic and adult degenerative scolioses, respectively.
Fig. 5.
The etiopathogenetic tree of scoliosis.
The term exposome was coined by Christopher Wild in 2005 to represent a compilation of all environmental, lifestyle, behavioral and social factors that may contribute to disease development and/or progression [237]. Studies on monozygotic twins have previously highlighted the significant role of environmental factors in idiopathic scoliosis [238]. However, research on the role of exposomal factors in idiopathic scoliosis has not been gaining momentum. It has been suggested to be due to an excessive emphasis on the genetic aspect of the disease.
Among the new evidence is recognition of epigenetic regulations as bridges between the exposome and the inner pathogenetic mechanisms. It has been shown to reflect the early life environmental influences [239]. Continued research in this direction may help shedding more light on the origin of the process that eventually lead to idiopathic scoliosis development.
Studies on the exposomal factors for adult degenerative scoliosis have been more prevalent in identifying the risk factors for the disease. As such, bone mineral density <−1.85 g/cm2, body mass index >25.57 kg/m2, and sagittal vertical axis >3.98 cm were suggested to be potential risk factors for degenerative scoliosis [240]. A body mass index [BMI] over 25 kg/m² is linked to a higher risk of degenerative lumbar scoliosis [241]. Excess weight is associated with more frequent falls, sarcopenia, and the production of proinflammatory cytokines like IL-6 and TNF-α, which contribute to increased osteoclastogenesis and bone loss [[242], [243], [244]]. Conversely, a high BMI is also associated with higher bone mineral density (BMD), indicating stronger bones [245]. This paradox can be partially explained by mechanical loading and strain, as well as estrogen production by adipocytes, which enhances bone formation and reduces resorption [246]. The net effect of these conflicting influences of obesity on degenerative scoliosis remains unclear. Therefore, further research is needed to understand the precise roles of physical activity and obesity in the development and progression of adult degenerative scoliosis.
Understanding these distinct pathogenetic mechanisms is crucial, as it could inform more targeted treatments for each type of scoliosis. In idiopathic scoliosis, interventions might focus on modulating the growth and development of spinal structures during critical periods, whereas in ADS, therapies could aim at mitigating the degenerative processes and inflammation that contribute to disc asymmetry. Despite the differences, both conditions underscore the complexity of spinal asymmetry and the need for continued research to fully elucidate these mechanisms.
Conclusion
Despite major advancements and success in therapy of scoliosis, the disease continues to affect large populations both among children and adults. In the case of adult degenerative scoliosis, the global trend toward aging is alarming, since it portends a rise in disease prevalence. Given these considerations, it is imperative to refine predictive and preventive strategies to enhance the diagnosis and treatment of scoliosis. To do so, more studies are needed to better understand the etiology and pathogenesis of scoliosis.
Summary
This comprehensive review explores the genetic factors and multifaceted pathogenesis of idiopathic and adult degenerative scoliosis, proposing a new model for scoliosis etiopathogenesis and highlighting future research directions.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: EP: Nothing to disclose. JF: Nothing to disclose. CSA: Nothing to disclose. MSL: Nothing to disclose. TRK: Nothing to disclose. NSD: Nothing to disclose. NEET: Nothing to disclose.
References
- 1.Choi JH, Oh EG, Lee HJ. Comparisons of postural habits, body image, and peer attachment for adolescents with idiopathic scoliosis and healthy adolescents. J Korean Acad Child Health Nurs. 2011;17(3):167–173. [Google Scholar]
- 2.Kontodimopoulos N, Damianou K, Stamatopoulou E, Kalampokis A. Loukos I. Children's and parents' perspectives of health-related quality of life in newly diagnosed adolescent idiopathic scoliosis. J Orthop. 2018;15(2):319–323. doi: 10.1016/j.jor.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoelen TA, Willems PC, Arts JJ, van Mastrigt G, Evers S. The economic and societal burden associated with adolescent idiopathic scoliosis: a burden-of-disease study protocol. N Am Spine Soc J. 2023;14 doi: 10.1016/j.xnsj.2023.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Tetteroo D, Arts JJC, Markopoulos P, Ito K. Quality of life of adolescent idiopathic scoliosis patients under brace treatment: a brief communication of literature review. Qual Life Res. 2021;30(3):703–711. doi: 10.1007/s11136-020-02671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piątek E, Zawadzka D, Ostrowska B. Correlation between clinical condition of scoliosis and perception of one's body image by girls with adolescent idiopathic scoliosis. Physiother Quarter. 2018;26(3):34–38. [Google Scholar]
- 6.Gallant JN, Morgan CD, Stoklosa JB, Gannon SR, Shannon CN, Bonfield CM. Psychosocial difficulties in adolescent idiopathic scoliosis: body image, eating behaviors, and Mood disorders. World Neurosurg. 2018;116:421–432. doi: 10.1016/j.wneu.2018.05.104. e1. [DOI] [PubMed] [Google Scholar]
- 7.Xiong B, Sevastik JA, Hedlund R, Sevastik B. Radiographic changes at the coronal plane in early scoliosis. Spine (Phila Pa 1976) 1994;19(2):159–164. doi: 10.1097/00007632-199401001-00008. [DOI] [PubMed] [Google Scholar]
- 8.Negrini S, Donzelli S, Aulisa AG, et al. 2016 SOSORT guidelines: orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018;13:3. doi: 10.1186/s13013-017-0145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menger RP, Sin AH. StatPearls Publishing; Treasure Island, FL: 2023. Adolescent Idiopathic Scoliosis. [Google Scholar]
- 10.Rogala EJ, Drummond DS, Gurr J. Scoliosis: incidence and natural history. a prospective epidemiological study. J Bone Joint Surg Am. 1978;60(2):173–176. [PubMed] [Google Scholar]
- 11.Herring JA. Elsevier Health Sciences; Texas: 2020. Tachdjian’s Pediatric Orthopaedics: From the Texas Scottish Rite Hospital for Children: 2-Volume Set. [Google Scholar]
- 12.Little DG, Song KM, Katz D, Herring JA. Relationship of peak height velocity to other maturity indicators in idiopathic scoliosis in girls. J Bone Joint Surg Am. 2000;82(5):685–693. doi: 10.2106/00004623-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 2005;30(9):1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 14.Petrosyan E, Fares J, Lesniak MS, Koski TR, El Tecle NE. Biological principles of adult degenerative scoliosis. Trends Mol Med. 2023;29(9):740–752. doi: 10.1016/j.molmed.2023.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J ortho (Belle Mead, NJ) 2003;32(2):77–82. discussion. [PubMed] [Google Scholar]
- 16.Benner B, Ehni G. Degenerative lumbar scoliosis. Spine (Phila Pa 1976) 1979;4(6):548–552. [PubMed] [Google Scholar]
- 17.Ascani E, Bartolozzi P, Logroscino CA, Marchetti PG, Ponte A, Savini R, et al. Natural history of untreated idiopathic scoliosis after skeletal maturity. Spine (Phila Pa 1976) 1986;11(8):784–789. doi: 10.1097/00007632-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Ploumis A, Transfledt EE, Denis F. Degenerative lumbar scoliosis associated with spinal stenosis. Spine J. 2007;7(4):428–436. doi: 10.1016/j.spinee.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Schwab FJ, Lafage V, Farcy JP, Bridwell KH, Glassman S, Shainline MR. Predicting outcome and complications in the surgical treatment of adult scoliosis. Spine (Phila Pa 1976) 2008;33(20):2243–2247. doi: 10.1097/BRS.0b013e31817d1d4e. [DOI] [PubMed] [Google Scholar]
- 20.Duramaz A, Yilmaz S, Ziroglu N, Bursal Duramaz B, Kara T. The effect of deformity correction on psychiatric condition of the adolescent with adolescent idiopathic scoliosis. Eur Spine J. 2018;27(9):2233–2240. doi: 10.1007/s00586-018-5639-4. [DOI] [PubMed] [Google Scholar]
- 21.Al-Mohrej OA, Aldakhil SS, Al-Rabiah MA, Al-Rabiah AM. Surgical treatment of adolescent idiopathic scoliosis: complications. Ann Med Surg (Lond) 2020;52:19–23. doi: 10.1016/j.amsu.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin CT, Pugely AJ, Gao Y, Mendoza-Lattes SA, Ilgenfritz RM, Callaghan JJ, et al. Increasing hospital charges for adolescent idiopathic scoliosis in the United States. Spine (Phila Pa 1976) 2014;39(20):1676–1682. doi: 10.1097/BRS.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 23.Glassman SD, Berven S, Kostuik J, Dimar JR, Horton WC, Bridwell K. Nonsurgical resource utilization in adult spinal deformity. Spine (Phila Pa 1976) 2006;31(8):941–947. doi: 10.1097/01.brs.0000209318.32148.8b. [DOI] [PubMed] [Google Scholar]
- 24.Glassman SD, Carreon LY, Shaffrey CI, Polly DW, Ondra SL, Berven SH, et al. The costs and benefits of nonoperative management for adult scoliosis. Spine (Phila Pa 1976) 2010;35(5):578–582. doi: 10.1097/BRS.0b013e3181b0f2f8. [DOI] [PubMed] [Google Scholar]
- 25.Burwell RG, James NJ, Johnson F, Webb JK, Wilson YG. Standardised trunk asymmetry scores. a study of back contour in healthy school children. J Bone Joint Surg Br. 1983;65(4):452–463. doi: 10.1302/0301-620X.65B4.6874719. [DOI] [PubMed] [Google Scholar]
- 26.Brooks HL, Azen SP, Gerberg E, Brooks R, Chan L. Scoliosis: a prospective epidemiological study. J Bone Joint Surg Am. 1975;57(7):968–972. [PubMed] [Google Scholar]
- 27.Kotwal S, Pumberger M, Hughes A, Girardi F. Degenerative scoliosis: a review. HSS J. 2011;7(3):257–264. doi: 10.1007/s11420-011-9204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giampietro PF, Blank RD, Raggio CL, Merchant S, Jacobsen FS, Faciszewski T, et al. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res. 2003;1(2):125–136. doi: 10.3121/cmr.1.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.10 ed. Oxford University Press; UK: 2020. Concise Medical Dictionary. [Google Scholar]
- 30.Kouwenhoven J-WM, Castelein RM. The pathogenesis of adolescent idiopathic scoliosis: review of the literature. Spine. 2008;33(26):2898–2908. doi: 10.1097/BRS.0b013e3181891751. [DOI] [PubMed] [Google Scholar]
- 31.Staub HA. Eine skoliotikerfamilie: ein beitrag zur frage der congenitalen skoliose und der heredität der skoliosen: breslauer genossensch. Chir. 1922;43(1) [Google Scholar]
- 32.Kesling KL, Reinker KA. Scoliosis in twins. a meta-analysis of the literature and report of six cases. Spine (Phila Pa 1976) 1997;22(17):2009–2014. doi: 10.1097/00007632-199709010-00014. [DOI] [PubMed] [Google Scholar]
- 33.Andersen MO, Thomsen K, Kyvik KO. Adolescent idiopathic scoliosis in twins: a population-based survey. Spine (Phila Pa 1976) 2007;32(8):927–930. doi: 10.1097/01.brs.0000259865.08984.00. [DOI] [PubMed] [Google Scholar]
- 34.Wise CA, Gao X, Shoemaker S, Gordon D, Herring JA. Understanding genetic factors in idiopathic scoliosis, a complex disease of childhood. Curr Genomics. 2008;9(1):51–59. doi: 10.2174/138920208783884874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S, Gao X, Londono D, Devroy SE, Mauldin KN, Frankel JT, et al. Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum Mol Genet. 2011;20(7):1456–1466. doi: 10.1093/hmg/ddq571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holm J, Hillenbrand R, Steuber V, Bartsch U, Moos M, Lubbert H, et al. Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur J Neurosci. 1996;8(8):1613–1629. doi: 10.1111/j.1460-9568.1996.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen S, Mantei N, Dong L, Schachner M. Prevention of neuronal cell death by neural adhesion molecules L1 and CHL1. J Neurobiol. 1999;38(3):428–439. doi: 10.1002/(sici)1097-4695(19990215)38:3<428::aid-neu10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Dong L, Chen S, Richter M, Schachner M. Single-chain variable fragment antibodies against the neural adhesion molecule CHL1 (close homolog of L1) enhance neurite outgrowth. J Neurosci Res. 2002;69(4):437–447. doi: 10.1002/jnr.10250. [DOI] [PubMed] [Google Scholar]
- 39.Haller G, Alvarado D, McCall K, Yang P, Cruchaga C, Harms M, et al. A polygenic burden of rare variants across extracellular matrix genes among individuals with adolescent idiopathic scoliosis. Hum Mol Genet. 2016;25(1):202–209. doi: 10.1093/hmg/ddv463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kou I, Takahashi Y, Johnson TA, Takahashi A, Guo L, Dai J, et al. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45(6):676–679. doi: 10.1038/ng.2639. [DOI] [PubMed] [Google Scholar]
- 41.Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, et al. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci. 2013;33(46):17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun P, He L, Jia K, Yue Z, Li S, Jin Y, et al. Regulation of body length and bone mass by Gpr126/Adgrg6. Sci Adv. 2020;6(12):eaaz0368. doi: 10.1126/sciadv.aaz0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma S, Londono D, Eckalbar WL, Gao X, Zhang D, Mauldin K, et al. A PAX1 enhancer locus is associated with susceptibility to idiopathic scoliosis in females. Nat Commun. 2015;6:6452. doi: 10.1038/ncomms7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120(5):1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- 45.Patten SA, Margaritte-Jeannin P, Bernard JC, Alix E, Labalme A, Besson A, et al. Functional variants of POC5 identified in patients with idiopathic scoliosis. J Clin Invest. 2015;125(3):1124–1128. doi: 10.1172/JCI77262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathieu H, Patten SA, Aragon-Martin JA, Ocaka L, Simpson M, Child A, et al. Genetic variant of TTLL11 gene and subsequent ciliary defects are associated with idiopathic scoliosis in a 5-generation UK family. Sci Rep. 2021;11(1):11026. doi: 10.1038/s41598-021-90155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Backer CB, Gutzman JH, Pearson CG, Cheeseman IM. CSAP localizes to polyglutamylated microtubules and promotes proper cilia function and zebrafish development. Mol Biol Cell. 2012;23(11):2122–2130. doi: 10.1091/mbc.E11-11-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azimzadeh J, Hergert P, Delouvee A, Euteneuer U, Formstecher E, Khodjakov A, et al. hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J Cell Biol. 2009;185(1):101–114. doi: 10.1083/jcb.200808082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu Y, Mao SH, Qian BP, Jiang J, Qiu XS, Zhao Q, et al. A promoter polymorphism of neurotrophin 3 gene is associated with curve severity and bracing effectiveness in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2012;37(2):127–133. doi: 10.1097/BRS.0b013e31823e5890. [DOI] [PubMed] [Google Scholar]
- 50.Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- 51.Miyake A, Kou I, Takahashi Y, Johnson TA, Ogura Y, Dai J, et al. Identification of a susceptibility locus for severe adolescent idiopathic scoliosis on chromosome 17q24.3. PLoS One. 2013;8(9):e72802. doi: 10.1371/journal.pone.0072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raab-Graham KF, Radeke CM, Vandenberg CA. Molecular cloning and expression of a human heart inward rectifier potassium channel. Neuroreport. 1994;5(18):2501–2505. doi: 10.1097/00001756-199412000-00024. [DOI] [PubMed] [Google Scholar]
- 53.Ashen MD, O'Rourke B, Kluge KA, Johns DC, Tomaselli GF. Inward rectifier K+ channel from human heart and brain: cloning and stable expression in a human cell line. Am J Physiol. 1995;268(1 Pt 2):H506–H511. doi: 10.1152/ajpheart.1995.268.1.H506. [DOI] [PubMed] [Google Scholar]
- 54.Shi B, Mao S, Xu L, Li Y, Sun X, Liu Z, et al. Quantitation analysis of PCDH10 methylation in adolescent idiopathic scoliosis using pyrosequencing study. Spine (Phila Pa 1976) 2020;45(7):E373–E3E8. doi: 10.1097/BRS.0000000000003292. [DOI] [PubMed] [Google Scholar]
- 55.Zhen Y, Pavez M, Li X. The role of Pcdh10 in neurological disease and cancer. J Cancer Res Clin Oncol. 2023;149(10):8153–8164. doi: 10.1007/s00432-023-04743-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller NH, Mims B, Child A, Milewicz DM, Sponseller P, Blanton SH. Genetic analysis of structural elastic fiber and collagen genes in familial adolescent idiopathic scoliosis. J Orthop Res. 1996;14(6):994–999. doi: 10.1002/jor.1100140621. [DOI] [PubMed] [Google Scholar]
- 57.Buchan JG, Alvarado DM, Haller GE, Cruchaga C, Harms MB, Zhang T, et al. Rare variants in FBN1 and FBN2 are associated with severe adolescent idiopathic scoliosis. Hum Mol Genet. 2014;23(19):5271–5282. doi: 10.1093/hmg/ddu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang J, Qian B, Mao S, Zhao Q, Qiu X, Liu Z, et al. A promoter polymorphism of tissue inhibitor of metalloproteinase-2 gene is associated with severity of thoracic adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2012;37(1):41–47. doi: 10.1097/BRS.0b013e31820e71e3. [DOI] [PubMed] [Google Scholar]
- 59.Ogura Y, Takahashi Y, Kou I, Nakajima M, Kono K, Kawakami N, et al. A replication study for association of 5 single nucleotide polymorphisms with curve progression of adolescent idiopathic scoliosis in Japanese patients. Spine (Phila Pa 1976) 2013;38(7):571–575. doi: 10.1097/BRS.0b013e3182761535. [DOI] [PubMed] [Google Scholar]
- 60.Andrusiewicz M, Harasymczuk P, Janusz P, Biecek P, Zbikowska A, Kotwicka M, et al. TIMP2 polymorphisms association with curve initiation and progression of thoracic idiopathic scoliosis in the caucasian females. J Orthop Res. 2019;37(10):2217–2225. doi: 10.1002/jor.24380. [DOI] [PubMed] [Google Scholar]
- 61.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1-2):267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 62.Meng Y, Lin T, Liang S, Gao R, Jiang H, Shao W, et al. Value of DNA methylation in predicting curve progression in patients with adolescent idiopathic scoliosis. E Bio Med. 2018;36:489–496. doi: 10.1016/j.ebiom.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lefebvre V, Dvir-Ginzberg M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect Tissue Res. 2017;58(1):2–14. doi: 10.1080/03008207.2016.1183667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda K, Kou I, Otomo N, Grauers A, Fan YH, Ogura Y, et al. A multiethnic meta-analysis defined the association of rs12946942 with severe adolescent idiopathic scoliosis. J Hum Genet. 2019;64(5):493–498. doi: 10.1038/s10038-019-0575-7. [DOI] [PubMed] [Google Scholar]
- 65.Shi B, Xu L, Mao S, Xu L, Liu Z, Sun X, et al. Abnormal PITX1 gene methylation in adolescent idiopathic scoliosis: a pilot study. BMC Musculoskelet Disord. 2018;19(1):138. doi: 10.1186/s12891-018-2054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carry PM, Terhune EA, Trahan GD, Vanderlinden LA, Wethey CI, Ebrahimi P, et al. Severity of idiopathic scoliosis is associated with differential methylation: an epigenome-wide association study of monozygotic twins with idiopathic scoliosis. Genes (Basel) 2021;12(8) doi: 10.3390/genes12081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao X, Gordon D, Zhang D, Browne R, Helms C, Gillum J, et al. CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. Am J Hum Genet. 2007;80(5):957–965. doi: 10.1086/513571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chetty M, Roberts TS, Elmubarak M, Bezuidenhout H, Smit L, Urban M. CHARGE syndrome: genetic aspects and dental challenges, a review and case presentation. Head Face Med. 2020;16(1):10. doi: 10.1186/s13005-020-00224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borysiak K, Janusz P, Andrusiewicz M, Chmielewska M, Kozinoga M, Kotwicki T, et al. CHD7 gene polymorphisms in female patients with idiopathic scoliosis. BMC Musculoskelet Disord. 2020;21(1):18. doi: 10.1186/s12891-019-3031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu Z, Dai Z, Yuwen W, Liu Z, Qiu Y, Cheng JC, et al. Genetic variants of CHD7 are associated with adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2021;46(11):E618–EE24. doi: 10.1097/BRS.0000000000003857. [DOI] [PubMed] [Google Scholar]
- 71.Tilley MK, Justice CM, Swindle K, Marosy B, Wilson AF, Miller NH. CHD7 gene polymorphisms and familial idiopathic scoliosis. Spine (Phila Pa 1976) 2013;38(22):E1432–E1436. doi: 10.1097/BRS.0b013e3182a51781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawai M, Rosen CJ. The insulin-like growth factor system in bone: basic and clinical implications. Endocrinol Metab Clin North Am. 2012;41(2):323–333. doi: 10.1016/j.ecl.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yeung HY, Tang NL, Lee KM, Ng BK, Hung VW, Kwok R, et al. Genetic association study of insulin-like growth factor-I (IGF-I) gene with curve severity and osteopenia in adolescent idiopathic scoliosis. Stud Health Technol Inform. 2006;123:18–24. [PubMed] [Google Scholar]
- 74.Takahashi Y, Matsumoto M, Karasugi T, Watanabe K, Chiba K, Kawakami N, et al. Lack of association between adolescent idiopathic scoliosis and previously reported single nucleotide polymorphisms in MATN1, MTNR1B, TPH1, and IGF1 in a Japanese population. J Orthop Res. 2011;29(7):1055–1058. doi: 10.1002/jor.21347. [DOI] [PubMed] [Google Scholar]
- 75.Moon ES, Kim HS, Sharma V, Park JO, Lee HM, Moon SH, et al. Analysis of single nucleotide polymorphism in adolescent idiopathic scoliosis in Korea: for personalized treatment. Yonsei Med J. 2013;54(2):500–509. doi: 10.3349/ymj.2013.54.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huygens C, Lienart S, Dedobbeleer O, Stockis J, Gauthy E, Coulie PG, et al. Lysosomal-associated transmembrane protein 4B (LAPTM4B) decreases transforming growth factor beta1 (TGF-beta1) production in human regulatory t cells. J Biol Chem. 2015;290(33):20105–20116. doi: 10.1074/jbc.M115.655340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ryzhkov II, Borzilov EE, Churnosov MI, Ataman AV, Dedkov AA, Polonikov AV. Transforming growth factor beta 1 is a novel susceptibility gene for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2013;38(12):E699–E704. doi: 10.1097/BRS.0b013e31828de9e1. [DOI] [PubMed] [Google Scholar]
- 78.Li P, Fleischhauer L, Nicolae C, Prein C, Farkas Z, Saller MM, et al. Mice lacking the matrilin family of extracellular matrix proteins develop mild skeletal abnormalities and are susceptible to age-associated osteoarthritis. Int J Mol Sci. 2020;21(2) doi: 10.3390/ijms21020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yilmaz H, Zateri C, Uludag A, Bakar C, Kosar S, Ozdemir O. Single-nucleotide polymorphism in Turkish patients with adolescent idiopathic scoliosis: curve progression is not related with MATN-1, LCT C/T-13910, and VDR BsmI. J Orthop Res. 2012;30(9):1459–1463. doi: 10.1002/jor.22075. [DOI] [PubMed] [Google Scholar]
- 80.Chen Z, Tang NL, Cao X, Qiao D, Yi L, Cheng JC, et al. Promoter polymorphism of matrilin-1 gene predisposes to adolescent idiopathic scoliosis in a Chinese population. Eur J Hum Genet. 2009;17(4):525–532. doi: 10.1038/ejhg.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang H, Zhao S, Zhao Z, Tang L, Guo Q, Liu S, et al. The association of rs1149048 polymorphism in matrilin-1(MATN1) gene with adolescent idiopathic scoliosis susceptibility: a meta-analysis. Mol Biol Rep. 2014;41(4):2543–2549. doi: 10.1007/s11033-014-3112-y. [DOI] [PubMed] [Google Scholar]
- 82.Ogura Y, Kou I, Miura S, Takahashi A, Xu L, Takeda K, et al. A functional SNP in BNC2 is associated with adolescent idiopathic scoliosis. Am J Hum Genet. 2015;97(2):337–342. doi: 10.1016/j.ajhg.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu L, Xia C, Qin X, Sun W, Tang NL, Qiu Y, et al. Genetic variant of BNC2 gene is functionally associated with adolescent idiopathic scoliosis in Chinese population. Mol Genet Genomics. 2017;292(4):789–794. doi: 10.1007/s00438-017-1315-3. [DOI] [PubMed] [Google Scholar]
- 84.Mao S, Xu L, Zhu Z, Qian B, Qiao J, Yi L, et al. Association between genetic determinants of peak height velocity during puberty and predisposition to adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2013;38(12):1034–1039. doi: 10.1097/BRS.0b013e318287fcfd. [DOI] [PubMed] [Google Scholar]
- 85.Monteagudo S, Cornelis FMF, Aznar-Lopez C, Yibmantasiri P, Guns LA, Carmeliet P, et al. DOT1L safeguards cartilage homeostasis and protects against osteoarthritis. Nat Commun. 2017;8:15889. doi: 10.1038/ncomms15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang H, Qiu X, Dai J, Yan H, Zhu Z, Qian B, et al. Association of rs11190870 near LBX1 with adolescent idiopathic scoliosis susceptibility in a Han Chinese population. Eur Spine J. 2013;22(2):282–286. doi: 10.1007/s00586-012-2532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brohmann H, Jagla K, Birchmeier C. The role of Lbx1 in migration of muscle precursor cells. Development. 2000;127(2):437–445. doi: 10.1242/dev.127.2.437. [DOI] [PubMed] [Google Scholar]
- 88.Kou I, Otomo N, Takeda K, Momozawa Y, Lu HF, Kubo M, et al. Genome-wide association study identifies 14 previously unreported susceptibility loci for adolescent idiopathic scoliosis in Japanese. Nat Commun. 2019;10(1):3685. doi: 10.1038/s41467-019-11596-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu S, Wu N, Zuo Y, Zhou Y, Liu J, Liu Z, et al. Genetic polymorphism of LBX1 is associated with adolescent idiopathic scoliosis in northern chinese han population. Spine (Phila Pa 1976) 2017;42(15):1125–1129. doi: 10.1097/BRS.0000000000002111. [DOI] [PubMed] [Google Scholar]
- 90.Mao SH, Qian BP, Shi B, Zhu ZZ, Qiu Y. Quantitative evaluation of the relationship between COMP promoter methylation and the susceptibility and curve progression of adolescent idiopathic scoliosis. Eur Spine J. 2018;27(2):272–277. doi: 10.1007/s00586-017-5309-y. [DOI] [PubMed] [Google Scholar]
- 91.Jiang H, Yang F, Lin T, Shao W, Meng Y, Ma J, et al. Asymmetric expression of H19 and ADIPOQ in concave/convex paravertebral muscles is associated with severe adolescent idiopathic scoliosis. Mol Med. 2018;24(1):48. doi: 10.1186/s10020-018-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S, Liu D, Zhou Z, Qin S. Role of long non-coding RNA H19 in the development of osteoporosis. Mol Med. 2021;27(1):122. doi: 10.1186/s10020-021-00386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54(130):1–13. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 94.Baschal EE, Wethey CI, Swindle K, Baschal RM, Gowan K, Tang NL, et al. Exome sequencing identifies a rare HSPG2 variant associated with familial idiopathic scoliosis. G3 (Bethesda) 2014;5(2):167–174. doi: 10.1534/g3.114.015669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inoue M, Minami S, Nakata Y, Takaso M, Otsuka Y, Kitahara H, et al. Prediction of curve progression in idiopathic scoliosis from gene polymorphic analysis. Stud Health Technol Inform. 2002;91:90–96. [PubMed] [Google Scholar]
- 96.Inoue M, Minami S, Nakata Y, Kitahara H, Otsuka Y, Isobe K, et al. Association between estrogen receptor gene polymorphisms and curve severity of idiopathic scoliosis. Spine (Phila Pa 1976) 2002;27(21):2357–2362. doi: 10.1097/00007632-200211010-00009. [DOI] [PubMed] [Google Scholar]
- 97.Wu J, Qiu Y, Zhang L, Sun Q, Qiu X, He Y. Association of estrogen receptor gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2006;31(10):1131–1136. doi: 10.1097/01.brs.0000216603.91330.6f. [DOI] [PubMed] [Google Scholar]
- 98.Tang NL, Yeung HY, Lee KM, Hung VW, Cheung CS, Ng BK, et al. A relook into the association of the estrogen receptor [alpha] gene (PvuII, XbaI) and adolescent idiopathic scoliosis: a study of 540 Chinese cases. Spine (Phila Pa 1976) 2006;31(21):2463–2468. doi: 10.1097/01.brs.0000239179.81596.2b. [DOI] [PubMed] [Google Scholar]
- 99.Chen S, Zhao L, Roffey DM, Phan P, Wai EK. Association between the ESR1 -351A>G single nucleotide polymorphism (rs9340799) and adolescent idiopathic scoliosis: a systematic review and meta-analysis. Eur Spine J. 2014;23(12):2586–2593. doi: 10.1007/s00586-014-3481-x. [DOI] [PubMed] [Google Scholar]
- 100.Xu L, Qiu X, Sun X, Mao S, Liu Z, Qiao J, et al. Potential genetic markers predicting the outcome of brace treatment in patients with adolescent idiopathic scoliosis. Eur Spine J. 2011;20(10):1757–1764. doi: 10.1007/s00586-011-1874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C, Jiang X, Liu G, Wassie T, Girmay S. An ancient mutation in the TPH1 gene is consistent with the changes in mammalian reproductive rhythm. Int J Mol Sci. 2019;20(23) doi: 10.3390/ijms20236065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kotwicki T, Janusz P, Andrusiewicz M, Chmielewska M, Kotwicka M. Estrogen receptor 2 gene polymorphism in idiopathic scoliosis. Spine (Phila Pa 1976) 2014;39(26):E1599–E1607. doi: 10.1097/BRS.0000000000000643. [DOI] [PubMed] [Google Scholar]
- 103.Wang L, Zhang Y, Zhao S, Dong X, Li X, You Y, et al. Estrogen receptors (ESRs) mutations in adolescent idiopathic scoliosis: a cross-sectional study. Med Sci Monit. 2020;26 doi: 10.12659/MSM.921611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bouhoute A, Leclercq G. Calmodulin decreases the estrogen binding capacity of the estrogen receptor. Biochem Biophys Res Commun. 1996;227(3):651–657. doi: 10.1006/bbrc.1996.1564. [DOI] [PubMed] [Google Scholar]
- 105.Zhao D, Qiu GX, Wang YP, Zhang JG, Shen JX, Wu ZH. Association between adolescent idiopathic scoliosis with double curve and polymorphisms of calmodulin1 gene/estrogen receptor-alpha gene. Orthop Surg. 2009;1(3):222–230. doi: 10.1111/j.1757-7861.2009.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y, Gu Z, Qiu G. The association study of calmodulin 1 gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Biomed Res Int. 2014;2014 doi: 10.1155/2014/168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lowe T, Lawellin D, Smith D, Price C, Haher T, Merola A, et al. Platelet calmodulin levels in adolescent idiopathic scoliosis: do the levels correlate with curve progression and severity? Spine (Phila Pa 1976) 2002;27(7):768–775. doi: 10.1097/00007632-200204010-00016. [DOI] [PubMed] [Google Scholar]
- 108.Acaroglu E, Akel I, Alanay A, Yazici M, Marcucio R. Comparison of the melatonin and calmodulin in paravertebral muscle and platelets of patients with or without adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2009;34(18):E659–E663. doi: 10.1097/BRS.0b013e3181a3c7a2. [DOI] [PubMed] [Google Scholar]
- 109.Banerjee S, Blauth K, Peters K, Rogers SL, Fanning AS, Bhat MA. Drosophila neurexin IV interacts with Roundabout and is required for repulsive midline axon guidance. J Neurosci. 2010;30(16):5653–5667. doi: 10.1523/JNEUROSCI.6187-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304(5676):1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou S, Qiu XS, Zhu ZZ, Wu WF, Liu Z, Qiu Y. A single-nucleotide polymorphism rs708567 in the IL-17RC gene is associated with a susceptibility to and the curve severity of adolescent idiopathic scoliosis in a Chinese Han population: a case-control study. BMC Musculoskelet Disord. 2012;13:181. doi: 10.1186/1471-2474-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lovejoy CO. The natural history of human gait and posture. Part 1. Spine and pelvis. Gait Posture. 2005;21(1):95–112. doi: 10.1016/j.gaitpost.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Jenkins FA., Jr Chimpanzee bipedalism: cineradiographic analysis and implications for the evolution of gait. Science. 1972;178(4063):877–879. doi: 10.1126/science.178.4063.877. [DOI] [PubMed] [Google Scholar]
- 114.Elftman H, Manter J. Chimpanzee and human feet in bipedal walking. Am J Phys Anthropol. 1935;20(1):69–79. [Google Scholar]
- 115.Ohlsson C. The lumbar and lumbosacral diarthodial joints. J Anat. 1933;67:127–167. [Google Scholar]
- 116.Walker A, Leakey RE. The nariokotome Homo erectus skeleton. Harvard University Press; USA: 1993. [Google Scholar]
- 117.Benton R. Morphological evidence for adaptations within the epaxial region of the primates. Baboon Med Res. 1967;2:201. [Google Scholar]
- 118.Machida M, Murai I, Miyashita Y, Dubousset J, Yamada T, Kimura J. Pathogenesis of idiopathic scoliosis: experimental study in rats. Spine. 1999;24(19):1985. doi: 10.1097/00007632-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 119.Machida M, Saito M, Dubousset J, Yamada T, Kimura J, Shibasaki K. Pathological mechanism of idiopathic scoliosis: experimental scoliosis in pinealectomized rats. Eur Spine J. 2005;14:843–848. doi: 10.1007/s00586-004-0806-1. [DOI] [PubMed] [Google Scholar]
- 120.de Seze M, Cugy E. Pathogenesis of idiopathic scoliosis: a review. Ann Phys Rehabil Med. 2012;55(2):128–138. doi: 10.1016/j.rehab.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 121.Kyere KA, Than KD, Wang AC, Rahman SU, Valdivia-Valdivia JM, La Marca F, et al. Schmorl's nodes. Eur Spine J. 2012;21(11):2115–2121. doi: 10.1007/s00586-012-2325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Knutsson F. Growth and differentiation of the postnatal vertebra. Acta radiol. 1961;55(6):401–408. [PubMed] [Google Scholar]
- 123.Chu WC, Rasalkar DD, Cheng JC. Asynchronous neuro-osseous growth in adolescent idiopathic scoliosis—MRI-based research. Pediat radiol. 2011;41:1100–1111. doi: 10.1007/s00247-010-1778-4. [DOI] [PubMed] [Google Scholar]
- 124.Vital J, Beguiristain J, Algara C, Villas C, Lavignolle B, Grenier N, et al. The neurocentral vertebral cartilage: anatomy, physiology and physiopathology. Surg Radiol Anat. 1989;11(4):323–328. doi: 10.1007/BF02098705. [DOI] [PubMed] [Google Scholar]
- 125.Beguiristain J, De Salis J, Oriaifo A, Canadell J. Experimental scoliosis by epiphysiodesis in pigs. Int orthop. 1980;3:317–321. doi: 10.1007/BF00266028. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Sucato DJ. Unilateral pedicle screw epiphysiodesis of the neurocentral synchondrosis: production of idiopathic-like scoliosis in an immature animal model. JBJS. 2008;90(11):2460–2469. doi: 10.2106/JBJS.G.01493. [DOI] [PubMed] [Google Scholar]
- 127.Caballero A, Barrios C, Burgos J, Hevia E, Correa C. Vertebral growth modulation by hemicircumferential electrocoagulation: an experimental study in pigs. Eur Spine J. 2011;20:367–375. doi: 10.1007/s00586-011-1909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheung CSK, Lee WTK, Tse YK, Tang SP, Lee KM, Guo X, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine. 2003;28(18):2152–2157. doi: 10.1097/01.BRS.0000084265.15201.D5. [DOI] [PubMed] [Google Scholar]
- 129.Fleming A, Keynes RJ, Tannahill D. The role of the notochord in vertebral column formation. J Anat. 2001;199(1-2):177–180. doi: 10.1046/j.1469-7580.2001.19910177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goto S, Uhthoff HK. Notochord and spinal malformations. Acta Orthopaedica Scandinavica. 1986;57(2):149–153. doi: 10.3109/17453678609000890. [DOI] [PubMed] [Google Scholar]
- 131.Lonstein JE, Carlson JM. The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg Am. 1984;66(7):1061–1071. [PubMed] [Google Scholar]
- 132.Willner S. A study of growth in girls with adolescent idiopathic structural scoliosis. Clin Orthop Relat Res. 1974;101:129–135. [PubMed] [Google Scholar]
- 133.Chazono M, Soshi S, Kida Y, Hashimoto K, Inoue T, Nakamura Y, et al. Height velocity curves in female patients with idiopathic scoliosis. Stud Health Technol Inform. 2012;176:202–205. [PubMed] [Google Scholar]
- 134.Zhu F, Qiu Y, Yeung HY, Lee KM, CHENG JCY. Histomorphometric study of the spinal growth plates in idiopathic scoliosis and congenital scoliosis. Pediatr Int. 2006;48(6):591–598. doi: 10.1111/j.1442-200X.2006.02277.x. [DOI] [PubMed] [Google Scholar]
- 135.Dickson RA, Lawton JO, Archer IA. Butt WP. The pathogenesis of idiopathic scoliosis. Biplanar spinal asymmetry. J Bone Joint Surg Br. 1984;66(1):8–15. doi: 10.1302/0301-620X.66B1.6693483. [DOI] [PubMed] [Google Scholar]
- 136.Mau H. [Specification of corresponding growth laws of Hueter-Volkmann and Pauwels (growth deformities) and their relation to deformities caused by loading] Z Orthop Ihre Grenzgeb. 1984;122(3):293–298. doi: 10.1055/s-2008-1044629. [DOI] [PubMed] [Google Scholar]
- 137.Millner PA, Dickson RA. Idiopathic scoliosis: biomechanics and biology. Eur Spine J. 1996;5(6):362–373. doi: 10.1007/BF00301963. [DOI] [PubMed] [Google Scholar]
- 138.Roaf R. Vertebral growth and its mechanical control. J Bone Joint Surg Br. 1960;42-B:40–59. doi: 10.1302/0301-620X.42B1.40. [DOI] [PubMed] [Google Scholar]
- 139.Deane G, Duthie RB. A new projectional look at articulated scoliotic spines. Acta Orthop Scand. 1973;44(4):351–365. doi: 10.3109/17453677308989071. [DOI] [PubMed] [Google Scholar]
- 140.Porter RW. Idiopathic scoliosis: the relation between the vertebral canal and the vertebral bodies. Spine (Phila Pa 1976) 2000;25(11):1360–1366. doi: 10.1097/00007632-200006010-00007. [DOI] [PubMed] [Google Scholar]
- 141.Guo X, Chau WW, Chan YL, Cheng JC. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis. Results of disproportionate endochondral-membranous bone growth. J Bone Joint Surg Br. 2003;85(7):1026–1031. doi: 10.1302/0301-620x.85b7.14046. [DOI] [PubMed] [Google Scholar]
- 142.Guo X, Chau WW, Chan YL, Cheng JC, Burwell RG, Dangerfield PH. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis–result of disproportionate endochondral-membranous bone growth? Summary of an electronic focus group debate of the IBSE. Eur Spine J. 2005;14(9):862–873. doi: 10.1007/s00586-005-1002-7. [DOI] [PubMed] [Google Scholar]
- 143.Birchall D, Hughes D, Gregson B, Williamson B. Demonstration of vertebral and disc mechanical torsion in adolescent idiopathic scoliosis using three-dimensional MR imaging. Eur Spine J. 2005;14(2):123–129. doi: 10.1007/s00586-004-0705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brink RC, Schlosser TPC, Colo D, Vavruch L, van Stralen M, Vincken KL, et al. Anterior spinal overgrowth is the result of the scoliotic mechanism and is located in the disc. Spine (Phila Pa 1976) 2017;42(11):818–822. doi: 10.1097/BRS.0000000000001919. [DOI] [PubMed] [Google Scholar]
- 145.Roth M. Idiopathic scoliosis from the point of view of the neuroradiologist. Neuroradiol. 1981;21(3):133–138. doi: 10.1007/BF00339521. [DOI] [PubMed] [Google Scholar]
- 146.Roth M. Idiopathic scoliosis caused by a short spinal cord. Acta Radiol Diagn (Stockh) 1968;7(3):257–271. doi: 10.1177/028418516800700308. [DOI] [PubMed] [Google Scholar]
- 147.Porter RW. The pathogenesis of idiopathic scoliosis: uncoupled neuro-osseous growth? Eur Spine J. 2001;10(6):473–481. doi: 10.1007/s005860100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Burwell RG, Dangerfield PH, Freeman BJ. Concepts on the pathogenesis of adolescent idiopathic scoliosis. Bone growth and mass, vertebral column, spinal cord, brain, skull, extra-spinal left-right skeletal length asymmetries, disproportions and molecular pathogenesis. Stud Health Technol Inform. 2008;135:3–52. [PubMed] [Google Scholar]
- 149.Chu WC, Man GC, Lam WW, Yeung BH, Chau WW, Ng BK, et al. Morphological and functional electrophysiological evidence of relative spinal cord tethering in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2008;33(6):673–680. doi: 10.1097/BRS.0b013e318166aa58. [DOI] [PubMed] [Google Scholar]
- 150.Chu WC, Lam WW, Chan YL, Ng BK, Lam TP, Lee KM, et al. Relative shortening and functional tethering of spinal cord in adolescent idiopathic scoliosis?: study with multiplanar reformat magnetic resonance imaging and somatosensory evoked potential. Spine (Phila Pa 1976) 2006;31(1):E19–E25. doi: 10.1097/01.brs.0000193892.20764.51. [DOI] [PubMed] [Google Scholar]
- 151.Riddle HF, Roaf R. Muscle imbalance in the causation of scoliosis. Lancet. 1955;268(6877):1245–1247. doi: 10.1016/s0140-6736(55)91020-5. [DOI] [PubMed] [Google Scholar]
- 152.SCHWARTZMANN JR, MILES M. Experimental production of scoliosis in rats and mice. JBJS. 1945;27(1):59–69. [Google Scholar]
- 153.Yamada K, Ikata T, Yamamoto H, Nakagawa Y, Tanaka H. Equilibrium function in scoliosis and active corrective plaster jacket for the treatment. Tokushima J Exp Med. 1969;16(1):1–7. [PubMed] [Google Scholar]
- 154.Hirano S. Electron microscopic studies on back muscles in scoliosis. Nihon Seikeigeka Gakkai Zasshi. 1972;46(1):47–62. [PubMed] [Google Scholar]
- 155.Fidler M, Jowett R, Troup JZP, Zorab P. In: Scoliosis and Muscle. Zorab London PA, editor. William Heinemann Medical Books Ltd; 1974. Histochemical study of the function of multifidus in scoliosis; pp. 184–192. Edited by. Edited by. [Google Scholar]
- 156.Fidler MW, Jowett RL. Muscle imbalance in the aetiology of scoliosis. J Bone Joint Surg Br. 1976;58(2):200–201. doi: 10.1302/0301-620X.58B2.932082. [DOI] [PubMed] [Google Scholar]
- 157.Spencer G, Zorab P. Spinal muscle in scoliosis: Comparison of normal and scoliotic rabbits. J Neurol Sci. 1976;30(2-3):405–410. doi: 10.1016/0022-510x(76)90143-x. [DOI] [PubMed] [Google Scholar]
- 158.Liang ZT, Guo CF, Li J, Zhang HQ. The role of endocrine hormones in the pathogenesis of adolescent idiopathic scoliosis. FASEB J. 2021;35(9):e21839. doi: 10.1096/fj.202100759R. [DOI] [PubMed] [Google Scholar]
- 159.Chen JF, Lin PW, Tsai YR, Yang YC, Kang HY. Androgens and androgen receptor actions on bone health and disease: from androgen deficiency to androgen therapy. Cells. 2019;8(11):1318. doi: 10.3390/cells8111318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abu E, Horner A, Kusec V, Triffitt J, Compston J. The localization of androgen receptors in human bone. J Clin Endocrinol Metabol. 1997;82(10):3493–3497. doi: 10.1210/jcem.82.10.4319. [DOI] [PubMed] [Google Scholar]
- 161.Esposito T, Uccello R, Caliendo R, Di Martino G, Carnevale UG, Cuomo S, et al. Estrogen receptor polymorphism, estrogen content and idiopathic scoliosis in human: a possible genetic linkage. J Ster Biochem Mol Bio. 2009;116(1-2):56–60. doi: 10.1016/j.jsbmb.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 162.Kulis A, Goździalska A, Drąg J, Jaśkiewicz J, Knapik-Czajka M, Lipik E, et al. Participation of sex hormones in multifactorial pathogenesis of adolescent idiopathic scoliosis. Int Orthop. 2015;39:1227–1236. doi: 10.1007/s00264-015-2742-6. [DOI] [PubMed] [Google Scholar]
- 163.Leboeuf D, Letellier K, Alos N, Edery P, Moldovan F. Do estrogens impact adolescent idiopathic scoliosis? Trnds Endocrinol Metabol. 2009;20(4):147–152. doi: 10.1016/j.tem.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 164.Warren MP, Gunn JB, Hamilton LH, Warren LF, Hamilton WG. Scoliosis and fractures in young ballet dancers. New Eng J Med. 1986;314(21):1348–1353. doi: 10.1056/NEJM198605223142104. [DOI] [PubMed] [Google Scholar]
- 165.Longworth B, Fary R, Hopper D. Prevalence and predictors of adolescent idiopathic scoliosis in adolescent ballet dancers. Arch phy med rehab. 2014;95(9):1725–1730. doi: 10.1016/j.apmr.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 166.Letellier K, Azeddine B, Parent S, Labelle H, Rompré PH, Moreau A, et al. Estrogen cross-talk with the melatonin signaling pathway in human osteoblasts derived from adolescent idiopathic scoliosis patients. J Pineal Res. 2008;45(4):383–393. doi: 10.1111/j.1600-079X.2008.00603.x. [DOI] [PubMed] [Google Scholar]
- 167.Sanders JO, Browne RH, McConnell SJ, Margraf SA, Cooney TE, Finegold DN. Maturity assessment and curve progression in girls with idiopathic scoliosis. JBJS. 2007;89(1):64–73. doi: 10.2106/JBJS.F.00067. [DOI] [PubMed] [Google Scholar]
- 168.Grivas TB, Vasiliadis E, Mouzakis V, Mihas C, Koufopoulos G. Association between adolescent idiopathic scoliosis prevalence and age at menarche in different geographic latitudes. Scoliosis. 2006;1:1–12. doi: 10.1186/1748-7161-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li X-F, Li H, Liu Z-D, Dai L-Y. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17:1431–1440. doi: 10.1007/s00586-008-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cheung CS, Lee WT, Tse YK, Lee KM, Guo X, Qin L, et al. Generalized osteopenia in adolescent idiopathic scoliosis–association with abnormal pubertal growth, bone turnover, and calcium intake? Spine (Phila Pa 1976) 2006;31(3):330–338. doi: 10.1097/01.brs.0000197410.92525.10. [DOI] [PubMed] [Google Scholar]
- 171.Hung VWY, Qin L, Cheung CSK, Lam TP, Ng BKW, Tse YK, et al. Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87(12):2709–2716. doi: 10.2106/JBJS.D.02782. [DOI] [PubMed] [Google Scholar]
- 172.Rusin B, Kotwicki T, Głodek A, Andrusiewicz M, Urbaniak P, Kotwicka M. Research into Spinal Deformities. Vol. 8. IOS Press; Netherlands: 2012. Estrogen receptor 2 expression in back muscles of girls with idiopathic scoliosis–relation to radiological parameters; pp. 59–62. [PubMed] [Google Scholar]
- 173.Zamecnik J, Krskova L, Hacek J, Stetkarova I, Krbec M. Etiopathogenesis of adolescent idiopathic scoliosis: Expression of melatonin receptors 1A/1B, calmodulin and estrogen receptor 2 in deep paravertebral muscles revisited. Mol Med Rep. 2016;14(6):5719–5724. doi: 10.3892/mmr.2016.5927. [DOI] [PubMed] [Google Scholar]
- 174.Malakoti F, Zare F, Zarezadeh R, Raei Sadigh A, Sadeghpour A, Majidinia M, et al. The role of melatonin in bone regeneration: a review of involved signaling pathways. Biochimie. 2022;202:56–70. doi: 10.1016/j.biochi.2022.08.008. [DOI] [PubMed] [Google Scholar]
- 175.MJ T. Vertebral column deformities following epiphysectomy in the chick [in French] CR Hebd Seances Acad Sci. 1959;248:1238–1240. [PubMed] [Google Scholar]
- 176.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J. Role of melatonin deficiency in the development of scoliosis in pinealectomised chickens. J Bone Joint Surg Br Vol. 1995;77(1):134–138. [PubMed] [Google Scholar]
- 177.Bagnall KM, Raso VJ, Hill DL, Moreau M, Mahood JK, Jiang H, et al. Melatonin levels in idiopathic scoliosis: diurnal and nocturnal serum melatonin levels in girls with adolescent idiopathic scoliosis. Spine. 1996;21(17):1974–1978. doi: 10.1097/00007632-199609010-00006. [DOI] [PubMed] [Google Scholar]
- 178.Cheung KM, Wang T, Poon AM, Carl A, Tranmer B, Hu Y, et al. The effect of pinealectomy on scoliosis development in young nonhuman primates. Spine. 2005;30(18):2009–2013. doi: 10.1097/01.brs.0000179087.38730.5d. [DOI] [PubMed] [Google Scholar]
- 179.Da W, Tao L, Wen K, Tao Z, Wang S, Zhu Y. Protective role of melatonin against postmenopausal bone loss via enhancement of citrate secretion from osteoblasts. Front Pharmacol. 2020;11:667. doi: 10.3389/fphar.2020.00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li Y, Zhou J, Wu Y, Lu T, Yuan M, Cui Y, et al. Association of osteoporosis with genetic variants of circadian genes in Chinese geriatrics. Osteoporos Int. 2016;27(4):1485–1492. doi: 10.1007/s00198-015-3391-8. [DOI] [PubMed] [Google Scholar]
- 181.Qiu XS, Tang NL, Yeung HY, Lee KM, Hung VW, Ng BK, et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2007;32(16):1748–1753. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]
- 182.Yang P, Liu H, Lin J, Yang H. The association of rs4753426 polymorphism in the melatonin receptor 1B (MTNR1B) gene and susceptibility to adolescent idiopathic scoliosis: a systematic review and meta-analysis. Pain Phys. 2015;18(5):419–431. [PubMed] [Google Scholar]
- 183.Li J, Li N, Chen Y, Hui S, Fan J, Ye B, et al. SPRY4 is responsible for pathogenesis of adolescent idiopathic scoliosis by contributing to osteogenic differentiation and melatonin response of bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2019;10(11):805. doi: 10.1038/s41419-019-1949-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Azeddine B, Letellier K, Wang da S, Moldovan F, Moreau A. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:45–52. doi: 10.1097/BLO.0b013e31811f39fa. [DOI] [PubMed] [Google Scholar]
- 185.Han Y, Kim YM, Kim HS, Lee KY. Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Sci Rep. 2017;7(1):5716. doi: 10.1038/s41598-017-06304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu H, Liu Z, Man CW, Guo J, Han X, Hu Z, et al. The effect of exogenous melatonin on reducing scoliotic curvature and improving bone quality in melatonin-deficient C57BL/6J mice. Sci Rep. 2019;9(1):6202. doi: 10.1038/s41598-019-42467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Histing T, Anton C, Scheuer C, Garcia P, Holstein JH, Klein M, et al. Melatonin impairs fracture healing by suppressing RANKL-mediated bone remodeling. J Surg Res. 2012;173(1):83–90. doi: 10.1016/j.jss.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 188.Qiu Y, Sun X, Qiu X, Li W, Zhu Z, Zhu F, et al. Decreased circulating leptin level and its association with body and bone mass in girls with adolescent idiopathic scoliosis. Spine. 2007;32(24):2703–2710. doi: 10.1097/BRS.0b013e31815a59e5. [DOI] [PubMed] [Google Scholar]
- 189.Tam EM, Liu Z, Lam T-P, Ting T, Cheung G, Ng BK, et al. Lower muscle mass and body fat in adolescent idiopathic scoliosis are associated with abnormal leptin bioavailability. Spine. 2016;41(11):940–946. doi: 10.1097/BRS.0000000000001376. [DOI] [PubMed] [Google Scholar]
- 190.Zhang HQ, Wang LJ, Liu SH, Li J, Xiao LG, Yang GT. Adiponectin regulates bone mass in AIS osteopenia via RANKL/OPG and IL6 pathway. J Transl Med. 2019;17(1):64. doi: 10.1186/s12967-019-1805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Martinez-Llorens J, Ramirez M, Colomina MJ, Bago J, Molina A, Caceres E, et al. Muscle dysfunction and exercise limitation in adolescent idiopathic scoliosis. Eur Respir J. 2010;36(2):393–400. doi: 10.1183/09031936.00025509. [DOI] [PubMed] [Google Scholar]
- 192.Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo) 2013;2013 doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sales de Gauzy J, Gennero I, Delrous O, Salles JP, Lepage B. Accadbled F. Fasting total ghrelin levels are increased in patients with adolescent idiopathic scoliosis. Scoliosis. 2015;10:33. doi: 10.1186/s13013-015-0054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu HG, Zhang HQ, Zhou ZH, Wang YJ. High ghrelin level predicts the curve progression of adolescent idiopathic scoliosis girls. Biomed Res Int. 2018;2018 doi: 10.1155/2018/9784083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gomez R, Lago F, Gomez-Reino JJ, Dieguez C, Gualillo O. Expression and modulation of ghrelin O-acyltransferase in cultured chondrocytes. Arthritis Rheum. 2009;60(6):1704–1709. doi: 10.1002/art.24522. [DOI] [PubMed] [Google Scholar]
- 196.Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. 2013;16(6):619–624. doi: 10.1097/MCO.0b013e328365b9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liang ZT, Li J, Rong R, Wang YJ, Xiao LG, Yang GT, et al. Ghrelin up-regulates cartilage-specific genes via the ERK/STAT3 pathway in chondrocytes of patients with adolescent idiopathic scoliosis. Biochem Biophys Res Commun. 2019;518(2):259–265. doi: 10.1016/j.bbrc.2019.08.044. [DOI] [PubMed] [Google Scholar]
- 198.Walker CT, Bonney PA, Martirosyan NL, Theodore N. Genetics underlying an individualized approach to adult spinal disorders. Front Surg. 2016;3:61. doi: 10.3389/fsurg.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Akbik OS, Ban VS, MacAllister MC, Aoun SG, Bagley CA. Genetic and serum markers in adult degenerative scoliosis: a literature review. Spine Deform. 2022;10(3):479–488. doi: 10.1007/s43390-021-00451-y. [DOI] [PubMed] [Google Scholar]
- 200.David G, Ciurea AV, Iencean SM, Mohan A. Angiogenesis in the degeneration of the lumbar intervertebral disc. J Med Life. 2010;3(2):154–161. [PMC free article] [PubMed] [Google Scholar]
- 201.Miyamoto H, Saura R, Doita M, Kurosaka M, Mizuno K. The role of cyclooxygenase-2 in lumbar disc herniation. Spine (Phila Pa 1976) 2002;27(22):2477–2483. doi: 10.1097/00007632-200211150-00011. [DOI] [PubMed] [Google Scholar]
- 202.Zheng J, Yang Y, Zhao K, Wang R. Low expression of microRNA-143 is related to degenerative scoliosis possibly by regulation of cyclooxygenase-2 expression. Int J Clin Exp Med. 2015;8(3):4140–4145. [PMC free article] [PubMed] [Google Scholar]
- 203.Lee JS, Shin JK, Goh TS. Interleukin 6 gene polymorphism in patients with degenerative lumbar scoliosis: a cohort study. Eur Spine J. 2018;27(3):607–612. doi: 10.1007/s00586-017-5074-y. [DOI] [PubMed] [Google Scholar]
- 204.Xu S, Liang T, Li S. Correlation between polymorphism of TRAIL gene and condition of intervertebral disc degeneration. Med Sci Monit. 2015;21:2282–2287. doi: 10.12659/MSM.894157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shin J-H, Ha K-Y, Jung S-H, Chung Y-J. Genetic predisposition in degenerative lumbar scoliosis due to the copy number variation. Spine. 2011;36(21):1782–1793. doi: 10.1097/BRS.0b013e318221a65f. [DOI] [PubMed] [Google Scholar]
- 206.Chuvpilo S, Avots A, Berberich-Siebelt F, Glockner J, Fischer C, Kerstan A, et al. Multiple NF-ATc isoforms with individual transcriptional properties are synthesized in T lymphocytes. J Immunol. 1999;162(12):7294–7301. [PubMed] [Google Scholar]
- 207.Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279(25):26475–26480. doi: 10.1074/jbc.M313973200. [DOI] [PubMed] [Google Scholar]
- 208.Navone SE, Campanella R, Guarnaccia L, Ouellet JA, Locatelli M, Cordiglieri C, et al. Inflammatory interactions between degenerated intervertebral discs and microglia: Implication of sphingosine-1-phosphate signaling. J Orthop Res. 2021;39(7):1479–1495. doi: 10.1002/jor.24827. [DOI] [PubMed] [Google Scholar]
- 209.Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12(104) doi: 10.1098/rsif.2015.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Koerner JD, Markova DZ, Yadla S, Mendelis J, Hilibrand A, Vaccaro AR, et al. Differential gene expression in anterior and posterior annulus fibrosus. Spine (Phila Pa 1976) 2014;39(23):1917–1923. doi: 10.1097/BRS.0000000000000590. [DOI] [PubMed] [Google Scholar]
- 211.Bertram H, Nerlich A, Omlor G, Geiger F, Zimmermann G, Fellenberg J. Expression of TRAIL and the death receptors DR4 and DR5 correlates with progression of degeneration in human intervertebral disks. Mod Pathol. 2009;22(7):895–905. doi: 10.1038/modpathol.2009.39. [DOI] [PubMed] [Google Scholar]
- 212.Bridgen DT, Fearing BV, Jing L, Sanchez-Adams J, Cohan MC, Guilak F, et al. Regulation of human nucleus pulposus cells by peptide-coupled substrates. Acta Biomater. 2017;55:100–108. doi: 10.1016/j.actbio.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hosogane N, Watanabe K, Tsuji T, Miyamoto T, Ishii K, Niki Y, et al. Serum cartilage metabolites as biomarkers of degenerative lumbar scoliosis. J Orthop Res. 2012;30(8):1249–1253. doi: 10.1002/jor.22067. [DOI] [PubMed] [Google Scholar]
- 214.Hwang DW, Kim KT, Lee SH, Kim JY, Kim DH. Association of COL2A1 gene polymorphism with degenerative lumbar scoliosis. Clin Orthop Surg. 2014;6(4):379. doi: 10.4055/cios.2014.6.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hwang DW, Kim KT, Lee SH, Kim JY, Kim DH. Association of COL2A1 gene polymorphism with degenerative lumbar scoliosis. Clin Orthop Surg. 2014;6(4):379–384. doi: 10.4055/cios.2014.6.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Jiang H, Yang Q, Jiang J, Zhan X, Xiao Z. Association between COL11A1 (rs1337185) and ADAMTS5 (rs162509) gene polymorphisms and lumbar spine pathologies in Chinese Han population: an observational study. BMJ open. 2017;7(5) doi: 10.1136/bmjopen-2016-015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gallagher D, Voronova A, Zander MA, Cancino GI, Bramall A, Krause MP, et al. Ankrd11 is a chromatin regulator involved in autism that is essential for neural development. Dev Cell. 2015;32(1):31–42. doi: 10.1016/j.devcel.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 218.Barbaric I, Perry MJ, Dear TN, Rodrigues Da Costa A, Salopek D, Marusic A, et al. An ENU-induced mutation in the Ankrd11 gene results in an osteopenia-like phenotype in the mouse mutant Yoda. Physiol Genomics. 2008;32(3):311–321. doi: 10.1152/physiolgenomics.00116.2007. [DOI] [PubMed] [Google Scholar]
- 219.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197(3):286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 220.Merle B, Itzstein C, Delmas PD, Chenu C. NMDA glutamate receptors are expressed by osteoclast precursors and involved in the regulation of osteoclastogenesis. J Cell Biochem. 2003;90(2):424–436. doi: 10.1002/jcb.10625. [DOI] [PubMed] [Google Scholar]
- 221.Kim KT, Kim J, Han YJ, Kim JH, Lee JS, Chung JH. Assessment of NMDA receptor genes (GRIN2A, GRIN2B and GRIN2C) as candidate genes in the development of degenerative lumbar scoliosis. Exp Ther Med. 2013;5(3):977–981. doi: 10.3892/etm.2013.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kim K-T, Kim J, Han YJ, Kim JH, Lee JS, Chung J-H. Assessment of NMDA receptor genes (GRIN2A, GRIN2B and GRIN2C) as candidate genes in the development of degenerative lumbar scoliosis. Exp Ther Med. 2013;5(3):977–981. doi: 10.3892/etm.2013.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhu Y, Han S, Zhao H, Liang J, Zhai J, Wu Z, et al. Comparative analysis of serum proteomes of degenerative scoliosis. J Orthop Res. 2011;29(12):1896–1903. doi: 10.1002/jor.21466. [DOI] [PubMed] [Google Scholar]
- 224.Kim KT, Lee JS, Lee BW, Seok H, Jeon HS, Kim JH, et al. Association between regulating synaptic membrane exocytosis 2 gene polymorphisms and degenerative lumbar scoliosis. Biomed Rep. 2013;1(4):619–623. doi: 10.3892/br.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Randell A, Sambrook PN, Nguyen TV, Lapsley H, Jones G, Kelly PJ, et al. Direct clinical and welfare costs of osteoporotic fractures in elderly men and women. Osteoporos Int. 1995;5(6):427–432. doi: 10.1007/BF01626603. [DOI] [PubMed] [Google Scholar]
- 226.Ponnusamy KE, Iyer S, Gupta G, Khanna AJ. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J. 2011;11(1):54–63. doi: 10.1016/j.spinee.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 227.Martin-Millan M, Castaneda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine. 2013;80(4):368–373. doi: 10.1016/j.jbspin.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 228.Park YS, Suh KT, Shin JK, Lee JS. Estrogen receptor gene polymorphism in patients with degenerative lumbar scoliosis. Br J Neurosurg. 2017;31(1):63–66. doi: 10.1080/02688697.2016.1206186. [DOI] [PubMed] [Google Scholar]
- 229.Akesson K, Tenne M, Gerdhem P, Luthman H, McGuigan FE. Variation in the PTH2R gene is associated with age-related degenerative changes in the lumbar spine. J Bone Miner Metab. 2015;33(1):9–15. doi: 10.1007/s00774-013-0550-x. [DOI] [PubMed] [Google Scholar]
- 230.Zhu Y, Han S, Zhao H, Liang J, Zhai J, Wu Z, et al. Comparative analysis of serum proteomes of degenerative scoliosis. J Orthop Res. 2011;29(12):1896–1903. doi: 10.1002/jor.21466. [DOI] [PubMed] [Google Scholar]
- 231.Han S, Zhu Y, Wu Z, Zhang J, Qiu G. The differently expressed proteins in MSCs of degenerative scoliosis. J Orthop Sci. 2013;18:885–892. doi: 10.1007/s00776-013-0444-8. [DOI] [PubMed] [Google Scholar]
- 232.National Scoliois Foundation [Available from: https://www.scoliosis.org/info.php], (accessed June 15, 2024).
- 233.Wang WJ, Yeung HY, Chu WC, Tang NL, Lee KM, Qiu Y, et al. Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop. 2011;31(1 Suppl):S14–S27. doi: 10.1097/BPO.0b013e3181f73c12. [DOI] [PubMed] [Google Scholar]
- 234.Hodge SE. Linkage analysis versus association analysis: distinguishing between two models that explain disease-marker associations. Am J Hum Genet. 1993;53(2):367–384. [PMC free article] [PubMed] [Google Scholar]
- 235.Konieczny MR, Senyurt H, Krauspe R. Epidemiology of adolescent idiopathic scoliosis. J Child Orthop. 2013;7(1):3–9. doi: 10.1007/s11832-012-0457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lonner BS, Auerbach JD, Estreicher MB, Kean KE. Thoracic pedicle screw instrumentation: the learning curve and evolution in technique in the treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2009;34(20):2158–2164. doi: 10.1097/BRS.0b013e3181b4f7e8. [DOI] [PubMed] [Google Scholar]
- 237.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 238.Aulia TN, Djufri D, Gatam L, Yaman A. Etiopathogenesis of adolescent idiopathic scoliosis (AIS): Role of genetic and environmental factors. Narra J. 2023;3(3):e217. doi: 10.52225/narra.v3i3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Szyf M. The early life social environment and DNA methylation: DNA methylation mediating the long-term impact of social environments early in life. Epigenetics. 2011;6(8):971–978. doi: 10.4161/epi.6.8.16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang C, Chang H, Gao X, Xu J, Meng X. Risk factors of degenerative lumbar scoliosis in patients with lumbar spinal canal stenosis. Medicine (Baltimore) 2019;98(38):e17177. doi: 10.1097/MD.0000000000017177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, Luukkonen R, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 2005;29(8):903–908. doi: 10.1038/sj.ijo.0802974. [DOI] [PubMed] [Google Scholar]
- 242.Gkastaris K, Goulis DG, Potoupnis M, Anastasilakis AD, Kapetanos G. Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact. 2020;20(3):372–381. [PMC free article] [PubMed] [Google Scholar]
- 243.Hita-Contreras F, Martinez-Amat A, Cruz-Diaz D, Perez-Lopez FR. Osteosarcopenic obesity and fall prevention strategies. Maturitas. 2015;80(2):126–132. doi: 10.1016/j.maturitas.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 244.Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kim YS, Han JJ, Lee J, Choi HS, Kim JH, Lee T. The correlation between bone mineral density/trabecular bone score and body mass index, height, and weight. Osteoporos Sarcopenia. 2017;3(2):98–103. doi: 10.1016/j.afos.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Hum Reprod Update. 2017;23(3):300–321. doi: 10.1093/humupd/dmw045. [DOI] [PMC free article] [PubMed] [Google Scholar]