Abstract
Despite significant and coordinated efforts to combat schistosomiasis, such as providing clean water, sanitation, hygiene, and snail control, these strategies still fall short, as regions previously thought to be disease-free have shown active schistosomiasis transmission. Therefore, it is necessary to implement integrated control methods, emphasizing vaccine development for sustainable control of schistosomiasis. Vaccination has significantly contributed to global healthcare and has been the most economically friendly method for avoiding pathogenic infections. Over the years, different vaccine candidates for schistosomiasis have been investigated with varying degrees of success in clinical trials with many not proceeding past the early clinical phase. Recently, proteins have been mentioned as targets for drug discovery and vaccine development, especially those with multiple functions in schistosomes. Moonlighting proteins are a class of proteins that can perform several functions besides their known functions. This multifunctional property is believed to have been expressed through evolution, where the polypeptide chain gained the ability to perform other tasks without undergoing any structural changes. Since proteins have gained more traction as drug targets, multifunctional proteins have thus become attractive for discovering and developing novel drugs since the drug can target more than one function. Moonlighting proteins are promising drug and vaccine candidates for diseases such as schistosomiasis, since they aid in disease promotion in the human host. This manuscript elucidates vital moonlighting proteins used by schistosomes to drive their life cycle and to ensure their survival in the human host, which can be used to develop anti-schistosomal therapeutics and vaccinomics.
Keywords: Drug discovery, GAPDH, Hsp70, enolase, moonlighting proteins, schistosomiasis, triosephosphate isomerase, vaccinomics
Introduction
Schistosomiasis, a neglected tropical disease (NTD), is sub-Saharan Africa’s second most prevalent parasitic disease [1]. It is a major global zoonosis with adverse medical and financial effects as it affects over 240 million people, with 800 million at risk of infection [2]. It is also responsible for more than 500,000 people dying annually [3,4]. The disease is endemic in more than 76 countries, with countries such as Nigeria, Tanzania, Venezuela, and Brazil experiencing the heaviest disease burden [5]. In humans, the disease is predominantly caused by three species of parasitic flatworms of the genus Schistosoma: S. haematobium, S. japonicum, and S. mansoni [6,7]. The disease is more common in poor rural areas where dwellers use schistosome-infested freshwaters in their daily lives, such as washing clothes, cooking, and bathing [8]. It is also more prevalent in school-aged children and leads to impaired cognition, undernourishment, and anemia [8,9]. Although schistosomiasis has a low mortality rate, it leads to a reduction in the quality of life by over 70 million disability-adjusted life years (DALYs) annually [10], which results in severe morbidity and mortality [11].
Infection in humans begins when cercariae penetrate human host skin, which causes a macular rash that can be seen within 24 hours of infection [12,13]. The cercariae then mature and enter the venous circulation, feeding on blood while migrating to the liver, where they develop into adult worms that mate and produce 500 to 3500 eggs daily [5]. These eggs can then migrate to different organs in the body, predisposing the host to various diseases such as HIV and cancer due to tissue scarring and inflammation [14,15]. The parasites can survive in the human body for over 40 years without any disturbance because they are coated with and express multiple proteins that play more than one key role in the life cycle of these worms; these proteins are called moonlighting proteins. This review discusses the moonlighting proteins expressed in Schistosoma worms throughout their life cycle, which can serve as new therapeutic agents and vaccine candidates for schistosomiasis.
Drug discovery and vaccine development in NTDs
Healthcare systems constantly require new drugs to treat diseases in various endemic areas. The pharmaceutical sector continually works to bring new medicines to the market through the challenging processes of drug discovery and development. The discovery process includes several steps, including selecting the target and its validation, identifying hits, generating and optimizing leads, and identifying candidates for further development [16]. Drug development includes improving chemical synthesis and formulation, performing animal toxicology studies, conducting clinical trials, and ultimately receiving regulatory approval [16].
Twenty diseases are categorized as NTDs, and these collectively affect over two billion individuals globally, accounting for approximately 11% of the global disease burden and causing over 35000 deaths daily [17,18]. These illnesses are primarily found in tropical and subtropical regions and are caused by bacteria, viruses, fungi, parasites, and toxins. NTDs significantly infect vulnerable individuals with poverty, social exclusion, location, or unusual way of life, causing considerable suffering, disability, and death [19]. Unfortunately, there is inadequate progress in developing new treatments for these diseases due to insufficient funding and being ignored by pharmaceutical companies in favor of the “big three diseases”: tuberculosis, malaria, and HIV/AIDS [17]. The World Health Organization (WHO) vouched that it will eliminate five diseases (leprosy, sleeping sickness, blinding trachoma, Guinea worm disease, and lymphatic filariasis) and control five diseases (schistosomiasis, helminthiases, visceral leishmaniasis, onchocerciasis, and Chagas disease) by the year 2020 [18]. Regrettably, the lack of safe, inexpensive, and effective drugs has played a role in hindering this goal. In the new 2030 WHO roadmap, the organization aims to eliminate five of the most prevalent NTDs (trachoma, soil-transmitted helminthiases [STHs], schistosomiasis, lymphatic filariasis, and onchocerciasis) [20].
Over the years, only a few new drugs have been developed that are solely dedicated to NTDs. From 1975 to 2011, among the 2243 new drugs that were approved, only 18 (0.8%) were directed towards NTDs [18]. Recently, drug repurposing has gained much traction for eradicating NTDs, especially drugs initially used for disease control in animals. Oxantel pamoate, a veterinary medicine, has demonstrated high efficacy against human trichuriasis (whipworm) [21,22]. It has recently shown high clinical efficacy against hookworm infections when combined with albendazole and pyrantel pamoate. Similarly, two other veterinary medications, emodepside, and moxidectin, are presently being tested in human clinical trials for onchocerciasis, possibly having action against several human helminths [23]. With combined efforts from governments and pharmaceutical companies, progress can be made in developing novel drugs and combining therapies to combat NTDs.
Past and present drugs used for the treatment of schistosomiasis
Over the years, multiple drugs have been developed to treat schistosomiasis, even though their use had drawbacks (Table 1). One of these drugs was metrifonate, previously known as trichlorfon, which is a compound used to treat urinary schistosomiasis. Bayer discovered the drug as an insecticide, but indirect use in humans led to its discovery as highly effective against S. haematobium [24]. Metrifonate has no activity against the other Schistosoma species due to its incomplete metabolism as an effective acetylcholinesterase inhibitor and organophosphate [25]. Studies by Doehring and colleagues [26] suggest that metrifonate may also be effective against S. mansoni strains but that the position of the worms may make them resistant to the medication. The differences in cholinoceptors between the various schistosome species may also contribute to the distinct ways metrifonate affects different schistosome species [24]. Despite this, Shekhar [27] concluded it was the best for treating urinary schistosomiasis; however, the administration of multiple doses during treatment made the drug lose public approval and acceptance [28,29].
Table 1.
Mechanism of action and limitations of past and present schistosomiasis treatments
| Drug | Parasite | Study type | Mechanism | Drawbacks |
|---|---|---|---|---|
| Praziquantel | S. mansoni, S. haematobium, and S. japonicum | Human | Disruption of Ca2+ homeostasis, thus altering the worm’s tegument. | Failure to kill juvenile worms and prevent reinfection. |
| Metrifonate | S. haematobium | Hamster | Inhibition of acetylcholinesterase. | It is only effective on S. haematobium species; a higher drug dosage is required. The drug was also less effective compared to PZQ. |
| OXA | S. mansoni | Mice | Alkylates schistosome DNA once it is transformed into its active state. | Ineffective against S. haematobium and S. japonicum, but S. mansoni strains were resistant to the drug. |
| Artemisinin | S. mansoni, S. haematobium, and S. japonicum | Human | Modifies the glycogen contents, which brings about morphological changes in the worm. | Less effective against all strains of adult worms. |
| Mefloquine | S. mansoni | Mice | Interferes with the glycolytic pathway. | Requires higher dosage to decrease worm burden. |
Additionally, the drug was less effective compared to Praziquantel (PZQ), the current gold treatment for all schistosome infections, and its exact mechanism of action against S. haematobium strains still needs to be elucidated [30]. However, James and colleagues [31] suggested that the worm is transported to the lungs, where metrifonate hinders its development by inhibiting acetylcholinesterase, an enzyme found in the muscles and nerves of the parasite. This disrupts schistosome nerve signals, which induces muscular spasms [32].
Oxamniquine (OXA), unquestionably not a novel medication, was used to treat millions of S. mansoni-infected individuals long before PZQ was made available. The primary drawback of the drug is that it is ineffective against S. haematobium and S. japonicum strains, which has deterred its use outside South America since these other regions are only mostly affected by S. mansoni strains [33,34]. This is because sulfotransferase, which is exclusively present in S. mansoni strains, converts this drug into its active form [35], which results in an electrophilic reactant that dissociates non-enzymatically and alkylates schistosome DNA once it is transformed to its active state (sulfate ester) [36]. This causes nucleic acid creation to be inhibited, protein synthesis to be disrupted, and the parasite to be delayed in death and destruction [37,38]. Male worms are significantly more vulnerable to OXA than female worms, and like PZQ, OXA is more effective against adult worms than juvenile stages [34]. The drug is also deemed safe, with limited and mild side effects, such as dizziness [39]. Although this drug has been used for over two decades, some resistant S. mansoni strains have been reported [40], which may be caused by a single autosomal recessive gene encoding a sulfotransferase [41]. PZQ has, however, replaced OXA due to its effectiveness and cost efficiency [42].
Since the repurposing of drugs has become an alternative for disease control, artemisinin, a sesquiterpene lactone usually used to treat malaria due to the active pharmacophore it contains, has recently become a drug of interest. Semi-synthetic derivatives of artemisinin, such as artemether (ART) and artesunate (AS), have proven able to treat human schistosomiasis [43]. Unlike PZQ and OXA, ART and AS are very active against juvenile worms while less effective against adult worms and their invasive stages [44]. Adult female worms are also somewhat more vulnerable to ART than male worms compared to OXA. Artemisinin’s specific mode of action against schistosomes is currently unknown. However, data suggest that it modifies the glycogen content of the organisms, bringing about morphologic changes akin to those brought on by PZQ [29]. Clinical trials have shown that, compared to PZQ, artemisinin derivatives alone have low cure rates; however, recent studies have indicated that using artemisinin in conjunction with PZQ can raise the cure rates for schistosomiasis [45,46]. This is due to the artemisinin’s ability to kill juvenile worms, while PZQ kills adult worms, preventing reinfection [47]. Although the use of PZQ combined with artemisinin is of great interest for the treatment of schistosomiasis, the risk that artemisinin may encourage the development of drug-resistant plasmodia in malarial co-endemic areas limits its use against schistosomiasis [48].
Mefloquine, an antimalarial agent, has been suggested as a candidate schistosomicide [49,50]. An investigation into the effect of mefloquine on multidrug resistance in vertebrates, anti-helminthic resistance, and the presence of two relevant genes in S. mansoni has led to the hypothesis that mefloquine may have anti-schistosomal activity. Van Nassauw and colleagues [50] investigated this hypothesis by administering 150 mg/kg to mice infected with adult S. mansoni worms. The results showed that while the drug dramatically decreased the number of eggs, it did not affect worm burden. Additional in vivo research showed that a single dose of 200 mg/kg of mefloquine exhibits potent action against S. mansoni, reducing the overall worm load by 72.3% [51].
On the other hand, infected mice receiving oral administration of a higher dose at 400 mg/kg experienced worm burden reductions of 86.7% and 95.1% for immature and mature female worms respectively [49,52]. Mefloquine causes extensive and severe morphologic, histopathological, and ultrastructural damage to adult and juvenile schistosomes, with the worm tegument, musculature, gut, and vitelline glands of female worms as the critical sites attacked by the drug [53-56]. In an early clinical trial, mefloquine and AS showed higher cure rates against S. haematobium and S. mansoni infections than the combination therapy using mefloquine and PZQ [57,58]. Additionally, several mefloquine-related arylmethanols showed potential effects against schistosomes in vivo, which is a valuable clue for developing new anti-schistosomal drugs [51].
In the 1970s, PZQ was introduced as a chemotherapeutic drug to treat schistosomiasis. The drug was initially used to treat veterinary cestodes but later met the WHO’s requirements for treating human schistosomiasis [43]. PZQ is mass-administered in endemic areas because it is cost-effective and readily available [28]. It is ingested with food and drink to minimize gastrointestinal side effects and acts within an hour of ingestion. However, its mechanism of action against the adult worm is unknown [59-61]. Based on the stage within the worm’s life cycle, the drug has bimodal activity with two evident phases against schistosomes [62]. The drug is less effective during the first few days of infection, with decreased efficacy as the disease progresses three to 21 days post-infection [62]. However, when egg production begins, approximately 42 days post-infection, the worm becomes more prone to disruption by the drug [54]. Although PZQ is currently the only drug for treating schistosomiasis, its exact mode of action and molecular targets remain unknown, and the difference in efficacy against juvenile and mature worms remains to be established [63]. However, it has been widely and severally postulated that PZQ causes tetanic contractions and disruption of tegumental vacuoles by altering the membrane permeability of adult worms [59]. The initial effects of the drug include intense muscle contractions and later paralysis caused by the disruption of calcium ion (Ca2+) homeostasis, which leads to an increase in Ca2+ influx [41]. This causes the worms to detach from the veins and be transported to the liver, where they are eliminated [62]. Since PZQ affects different developmental stages of schistosomes, it causes contraction of the middle region when exposed to miracidia, giving it a dumbbell-like structure. In contrast, exposure of the drug to cercariae leads to morphological and biological changes in the tegument [62]. Even though the sporocysts survive when exposed to PZQ, they are susceptible to tegumental damage [64].
Although the drug has a cure rate of 60% to 90%, recent studies have revealed the emergence of PZQ-resistant schistosome strains in certain parts of Egypt and Kenya [60,65] due to drug pressure and the incapability of the drug to kill juvenile worms [62,63]. It is, therefore, crucial to investigate new possible drug targets that will serve as the basis for developing new anti-schistosomal drugs. Over the years, enzymes have become attractive therapeutic agents because of their biochemical roles in sustaining life [66]. These enzymes are moonlighting proteins, which play various roles in disease progression.
Vaccines under clinical trials for schistosomiasis
Vaccines have historically been proven to be the most effective way of controlling diseases caused by pathogens [67]. Vaccination resistance is so uncommon that vaccines have been seen as key to fighting pathogen resistance [68]. Smallpox, for example, was eradicated because no viral strains that could spread between vaccinated people ever arose [69]. Another benefit of vaccination is that it increases immunity against multiple targets on a pathogen compared to drugs [68]. Upon vaccination, the innate immune system will detect whether it is a threat by recognizing tiny subregions that stimulate the immunological recognition of epitopes [70]. This stimulus will then elicit responses from multiple components of the innate immune system, which will opsonize or adhere to the agent, assisting in its engulfment by antigen-presenting cells like macrophages or monocytes [70]. These antigen-presenting cells will subsequently process the antigens from the pathogenic agent, which will then attach the processed antigen and the major histocompatibility complex (MHC) protein to their surface [70]. Should the antigen be viral, it will bind to the MHC I protein and be delivered to a CD8 cell by the antigen-presenting cell, which aids in the control of intracellular infections, thereby initiating cell-mediated immunity [71]. Suppose it is a bacterial or parasite antigen; it will be associated with the MHC II protein and be delivered by the antigen-presenting cell to a CD4 cell, triggering an antibody-mediated response and assisting B-cells in controlling extracellular infections [71]. These CD4 cells are classified into two types: Type 1 T helper (Th1) and Type 2 T helper (Th2) cells, where the former aid in the promotion of cell-mediated immunity and the latter aid in the promotion of antibody-mediated immunity [72].
For schistosomal infections, the Th1 response, with elevated levels of tumor necrosis factor (TNF-α) and interferon-gamma (IFN-γ), relates to the acute phase of disease as a response to the invading pathogen [61]. On the other hand, continuous exposure to cercariae-infested waters causes the generation of other pro-inflammatory cytokines [73]. This cytokine pattern may explain the altered state of acute schistosomiasis [74]. If the disease is ignored for an extended period, it can progress to a chronic infection caused by eggs in various locations in the human body and the activation of the Th2 response, which is mediated by Interleukin (IL)-4 and IL-13 cytokines [73]. During this stage, inflammatory cells are gradually eliminated and replaced by fibrosis [75]. Since the adult worms are covered with host antigens, they can persist in the bloodstream for years without being detected by the immune system [14].
Initiatives such as managing snail intermediate hosts and water, sanitation, and hygiene programs have had little to no effect on controlling schistosomiasis [76]. These flaws show that control measures could be more effective at stopping transmission by necessitating the adoption of new control strategies. Even though these hygiene programs have been implemented to reduce pathogen infections, vaccination will still play a crucial role in global health [76]. Although there is no vaccine against human schistosomiasis, recent research has shown that developing and administering vaccines will more effectively encourage long-term protection against the disease [77]. The schistosome life cycle is very complex, which makes it particularly difficult to produce viable vaccine targets, but it also offers a chance to do so. The fact that schistosomes do not increase in the human host may be of the utmost significance, making even a small reduction in worm load brought on by a vaccine highly beneficial to managing schistosomiasis [78].
The type of schistosomiasis vaccine candidates that should be considered for development and what qualities make a successful vaccination drive have been the subject of various experts’ recommendations over the past few decades. However, it has been agreed that an effective prophylactic vaccine should reduce adult worm burden by 75% in immunized individuals. It should also minimize egg excretion rates by close to 75%, according to the Preferred Product Characteristics (PPC) [79,80]. It would also be advantageous to have a vaccine that reduces egg production but leaves non-pathogenic worms intact to maintain natural immunity [80]. Although many antigens promise to protect against schistosomiasis, few have made it to human clinical trials. This is mainly because most identified antigens have been tested only in mice, which may not accurately reflect their action in the human body. For example, in naive mice, over 68% of S. mansoni cercariae fail to develop into adult worms [81]. Choosing suitable animal models for creating a schistosomiasis vaccine is thus crucial. It has been suggested that promising vaccine candidates should be studied in non-human primates, such as baboons, which are natural hosts of schistosomes. Cercarial infections in baboons lead to nearly 90% maturation into adult worms, as in humans [81].
Creating a vaccine to combat schistosomiasis is a challenging task, which is made more difficult by the production of host Immunoglobulin E (IgE), which can cause allergic reactions and increase the risk of granulomas and fibrosis due to egg-induced responses [82]. Amidst these challenges, there are reasons to be optimistic about developing schistosomiasis vaccines. Several antigens have been identified and tested against one or more primary schistosome species over the past three decades [82]. However, only three recombinant antigens have progressed to various stages of human clinical trials (Table 2). These include S. mansoni tetraspanin (Sm-TSP-2), a 9-kilodalton (kDa) surface antigen [83], S. mansoni 14-kDa fatty acid-binding protein (Sm14), and S. haematobium 28-kD glutathione S-transferase (Sh28GST) [3,84]. Due to its efficacy, the fourth antigen, the major subunit of S. mansoni calpain (Sm-p80), has been cleared for human clinical trials [85].
Table 2.
Current schistosomiasis vaccine candidates in clinical trials
| Vaccine Candidate | Function | Targeted species | Experimental models | Vaccine efficacy | Resulting immune response | Clinical trial | Status of the vaccine | References |
|---|---|---|---|---|---|---|---|---|
| Sm-TSP-2/Alhydrogel® | Tegument stability and parasite development | S. mansoni | Mice | Reduction of 25%-58% worm burden and 27%-56% reduction in tissue eggs | Vaccinated individuals generated sufficient IgG antibodies | Phases 1a & 1b completed | Phase 1 trials to determine the toxicity and immunogenicity of the vaccine in healthy adults. | [91,119] |
| Sm14/GLA-SE | Absorption and transportation of fatty acids from the host | S. mansoni | Mice and rabbits | A 65.9% and 93.2% decrease in worm burden in rabbits and mice respectively | Triggered the Th1 and Th2 cytokines and produced a substantial amount of Sm14-specific IgG, with no IgE detected | Phases 1 and 2a completed | The vaccine produced durable immunogenicity, which led to the planning of Phase 2b and Phase 3 trials. | [94,96] |
| Sh28GST/Alhydrogel (Bilharvax®) | Fatty acid metabolism and prostaglandin D2 production; might aid in parasite immune evasion in immunized people | S. haematobium | Rodents, primates, and cattle | A 40-60% reduction of the worm burden and a significant decrease in female worms | Highly immunogenic in adults, however, immunized children lacked IgG3 and IgA antibodies, which are linked to acquired immunity | Phases 1, 2 & 3 completed | Failed to achieve protection against urinary schistosomiasis. | [111,120] |
| Sm-p80/GLA-SE | Surface membrane biogenesis and renewal to escape the host immune response | S. mansoni | Mice and baboons | A 93.45% reduction in adult female worms and an 89.95% decrease in tissue egg load | Produced Sm-p80-specific total IgG and IgG subtypes with an increase in Th1 cytokines IFN-γ, IL-2, and TNF-α | Phase 1 initiated | Clinical trials are still ongoing. | [114,117,118] |
Sm-TSP-2
Schistosomes contain TSPs, proteins on the surface membrane, and scaffold [86], which play a vital role in regulating the functions of other membrane proteins and in tegument formation and trafficking [87]. Sm-TSP-1 and Sm-TSP-2 are the two primary types of TSPs produced by S. mansoni [88]. The four transmembrane domains of schistosome TSPs are connected by extracellular loops easily visible to the host’s immune system. Tran and colleagues [88] focused on the Sm-TSP-2 antigen because it is significantly identified by IgG1 and IgG3 from putatively resistant individuals, unlike Sm-TSP-1 [89]. These antibodies are observed in normal immunological responses in healthy persons. In studies using either Sm-TSP-2 or a combination of Sm-TSP-2 and 5B (an immunogenic region of the Na-APR-1 hookworm vaccine), significant levels of protection against S. mansoni infections were observed [89,90]. These vaccines were formulated with alum/CpG, which led to a 25% to 58% reduction in worm burdens and a 27% to 56% reduction in tissue egg burdens, respectively; these vaccines also induced humoral immune responses [89,90]. In another study, mice inoculated with an Sm-TSP-2/Sm29 chimera led to a decrease of 51% in adult worms and generated Th1-type immune responses [90]. In addition, sera from chronically infected patients living in regions where S. mansoni and hookworm co-exist did not contain Sm-TSP-2-specific or Sm-TSP-2/5B chimeric IgE antibodies [90].
The Sm-TSP-2/Alhydrogel® vaccine was started in Phase 1a clinical trials in healthy adults from an S. mansoni non-endemic location in 2014. The study’s findings demonstrated no adverse vaccination-related effects and that the vaccine was secure and well-tolerated. After the second booster, the vaccination produced a dose-dependent peak in Sm-TSP-2-specific IgG [91]. The dose-escalation Phase 1b research evaluated the immunogenicity, safety, and tolerability of Sm-TSP-2/Alhydrogel® with or without AP 10-701 in random healthy persons exposed to S. mansoni infections from an endemic area in Brazil (https://clinicaltrials.gov/ct2/show/NCT03110757). The study was completed in 2019, but the results have not yet been published. Phase 1 and 2b trials are currently being conducted to investigate the dose-escalation safety, immunogenicity, and effectiveness of Sm-TSP-2/Alhydrogel® with or without AP 10-701 in healthy Ugandan adults. At the same time, the impact of the vaccine on S. haematobium-infected individuals is also being evaluated (https://clinicaltrials.gov/ct2/show/NCT03910972).
Sm14
Since schistosomes lack oxygen-dependent mechanisms to synthesize sterols and fatty acids, the parasite must rely solely on its host to synthesize these organic molecules [92]. The parasite, therefore, utilizes fatty acid-binding proteins (FABPs) to take up, transport, and compartmentalize these fatty acids derived from the host [92]. The first FABP homolog discovered in helminths was the 14 kDa S. mansoni (Sm14) antigen [84], which led to other FABPs in several helminth species. Brito and colleagues [93] discovered that Schistosoma-resistant individuals in an endemic area of Brazil have a solid Th1-mediated immune response to the Sm14. The recombinant Sm14 (rSm14) efficacy studies assessed in New Zealand on white rabbits and Swiss mice showed that following an S. mansoni cercarial challenge decreases in worm burdens of 65.9% and 93.2% were observed, respectively [94]. Sm14 also stimulates immune cross-protection against infection by Fasciola hepatica in mice [94] and sheep [95]. The Phase 1 clinical trial was conducted to test the safety and immunogenicity of Sm14 formulated in glucopyranosyl lipid A adjuvant in an oil-in-water emulsion (GLA-SE) (Sm14/GLA-SE). The vaccine was evaluated in male volunteers from a non-schistosome endemic area in Brazil. The results showed high tolerability of the vaccine and specific humoral immune responses with no IgE antibody production [96]. The results from the Phase 2a clinical trials of healthy adult males from an S. mansoni and S. haematobium endemic area showed that the Sm14/GLA-SE vaccine is safe (https://clinicaltrials.gov/ct2/show/NCT03041766). This led to the assessment of Sm14 Phase 2b-Sn in school-aged children living in the same endemic area; this was completed in 2019, but the results of this study have not been published as yet (https://clinicaltrials.gov/ct2/show/study/NCT03799510).
Sh28GST
The GSTs are a family of immunogenic and pharmacologically active enzymes found in schistosomes [97]. Two isoforms of GST exist in schistosomes: 26 kDa GST and 28 kDa GST. These isoforms differ in localization and substrates to which they bind [98]. The 26 kDa isoform is linked to lipid peroxidation, while the 28 kDa isoform plays a role in the inactivation of exogenous xenobiotics with minimal involvement in lipid peroxidation [98,99]. These proteins are highly expressed throughout the life cycle of schistosomes, showing greater expression in eggs and male worms [100]. GSTs are responsible for the catalysis of glutathione conjugation to hydrophobic ligands [100]. This group of enzymes also serves various functions, including detoxification, catalysis of nucleophilic conjugations to endogenous and xenobiotic electrophilic toxins, minimization of lipid peroxidation, the prevention of toxin accumulation, repair of oxidized macromolecules by reactive oxygen species, and regeneration of S-thiolated proteins due to oxidative stress [3,101,102].
Living organisms are constantly in contact with non-nutritional foreign chemicals; these xenobiotics can harm an organism due to their toxic effects. Organisms must, therefore, withstand the harm posed by these xenobiotic substances [102]. This is achieved by using GST to facilitate a nucleophilic attack on an endogenous/exogenous electrophile by adding glutathione (GSH) via the S-linker to the toxic compound [103,104]. Since this is a detoxifying enzyme, GST in schistosomes allows the worm to function without host-related disturbances [101]. Moreover, schistosomes must have GST because it works similarly to mammalian GST and allows the parasite to protect itself against oxidative stress caused by the host [100]. Furthermore, schistosomal GSTs are thought to trigger the passive detoxification of anti-schistosomal medications and hematin [105]. According to McTigue and colleagues [106], the structural differences observed in the xenobiotic binding site between S. japonicum GST (SjGST) and human GST can form the foundation for designing novel drugs by inhibiting this site to combat drug-resistant schistosomes. Schistosomal GSTs have also been linked to vaccination therapy for the illness, in addition to being pharmacological targets. Sh28GST, a 28-kDa antigen, has been used as a vaccine and has shown reduced parasite egg production and transmission [107,108].
Phase 1 clinical studies assessed the safety, tolerability, and immunogenicity of recombinant Sh28GST (rSh28GST) adsorbed to Alhydrogel (Bilharvax®) in adult males. Alhydrogel is an aluminum hydroxide wet gel suspension that stimulates a Th2 response by increasing antigen attraction and absorption by antigen-presenting cells (APCs) [109]. It can also activate innate immune pathways activated by pattern recognition receptors (PRRs) [109]. The vaccine was regarded safe and elicited a robust Th2-biased response [110]. According to the same investigators, the rSh28GST and PZQ co-administration was similarly well tolerated in healthy individuals during a Phase 2 clinical trial [111]. These findings prompted a Phase 3 trial to assess the safety, immunogenicity, and protective effectiveness of Bilharvax® in PZQ-treated infected Senegalese school-aged children; however, the authors found unsatisfactory effectiveness [111]. They hypothesized that the lack of efficacy was partly due to repeated PZQ treatment interference and the vaccine-administration protocol adopted, which preferred blocking IgG4 synthesis over generating protective IgG3 antibodies [111].
Sm-p80
Calpain is a cysteine protease activated by calcium that is highly expressed on the tegument of schistosomes and throughout the parasite’s life cycle [112]. This protein comprises two subunits: a large catalytic and a small proteolytic unit [113]. The large subunit is responsible for tegument biogenesis and turnover, a crucial process for helminths to evade the host’s immune system [85]. This large subunit, Sm-p80, was discovered as a vaccine candidate and put through preclinical testing because of its crucial role in host evasion and promoting schistosome survival in the host. The Sm-p80-based vaccine provided significant prophylactic, therapeutic, anti-pathologic, cross-species, and transmission-blocking protection in vaccinated S. mansoni-infected animal models, according to several vaccine efficacy studies using the Sm-p80 antigen [80,112,114-116]. In a study by Zhang and colleagues [117], S. mansoni-infected baboons vaccinated with Sm-p80/GLA-SE had a total reduction of 93.45% in female adult worms, as well as a decrease of 89.95% in tissue egg load, thereby reducing the chances of developing chronic intestinal schistosomiasis. It was also found that the hatching of eggs in feces also decreased significantly [117]. Sera from schistosome-infected individuals showed no expression of Sm-p80-specific IgE antibodies [114], reducing the possibility of an allergic reaction after human Sm-p80 immunization. Currently, Phase 1 clinical trials of Sm-p80 formulated in GLA-SE (SchistoShield®) are being tested on adult humans in the United States of America, followed by a dose-escalation study in African adults with a planned future dose-escalation in school-age children [118].
Moonlighting proteins and schistosomiasis
Moonlighting proteins, first discovered in the 1980s, were commonly known as gene-sharing proteins [121,122]. This class of proteins comprises a single multifunctional protein that forms part of various biochemical mechanisms [123]. According to Karkowska-Kuleta and Kozik [124], their functions are based on their localization and substrate concentration. Moonlighting proteins often perform unrelated functions, not caused by gene fusion or splitting the tasks into other protein domains, but emerge from an unintentional interaction with a new partner, usually another protein, or occasionally DNA or RNA [123,125]. However, this class of proteins should not be confused with pleiotropic effects, which are the inactivation of a protein’s function that leads to the activation of another function [122,126]. Moonlighting proteins are independent of their tasks since they differ mechanistically [127]. Over 270 moonlighting proteins have been identified across the phylogenetic tree [126], of which most are in the cytosol as they are involved in various processes. Examples include cytokines, cytoskeletal components, chaperones, and DNA compactors [123]. They may, however, perform different biologic actions at multiple sites within or outside the cell. Certain cell types may even perform completely distinct functions. The monomeric or oligomeric state and the concentrations of the substrate/ligand can also influence the nature of some proteins’ biological activity [127,128]. Recent studies have shown that some moonlighting proteins restore balance in the body when an individual is diseased [66,127]. However, some of these proteins play a role in the development and progression of diseases [129]. These proteins can negatively impact the body’s antibody response and tissue growth [129]. In Staphylococcus aureus, moonlighting enzymes improve the bacterium’s attachment to host cells and its binding to some host matrix proteins. In some situations, moonlighting has emerged as a virulence mechanism [130]. Another example of moonlighting proteins in disease progression is found in Mycobacterium tuberculosis, where moonlighting activities lead to ciprofloxacin27 antibiotic resistance. Glutamate racemase is an enzyme that contributes to cell wall formation; however, the enzyme also inhibits DNA gyrase, which results in antibiotic resistance [131].
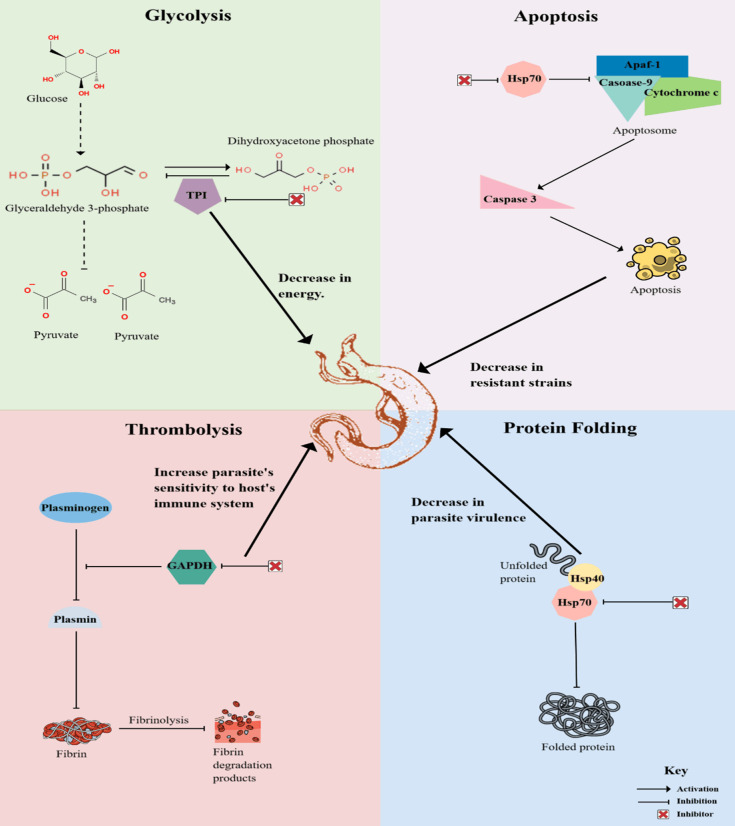
MultitaskProtDB-II, a multifunctional protein database, showed that 78% of moonlighting proteins are related to human diseases, of which 48% of the analyzed moonlighting proteins are targets of current drugs [132]. This makes moonlighting proteins attractive drug targets since more than one function can be targeted [128]. In schistosomes, multiple proteins that elicit these moonlighting functions have been identified, which are necessary for the growth and survival of the parasite in the human body, as shown in Figure 1. These proteins should be studied further to understand how they contribute to the survival of the worms for over 40 years in the human host. It is also essential to gain more information on whether these proteins play a role in the emerging schistosomal resistance to PZQ [65], as seen in the case of M. tuberculosis.
Figure 1.
Model depicting the effects of inhibiting specific moonlighting proteins for developing novel drugs to treat schistosomiasis. Glycolysis plays a crucial role in the survival of schistosomes by providing them with energy to sustain their life cycle within the human host. Inhibiting TPI will thus decrease energy for the worms since the other downstream processes of the pathway will also be blocked. Once PZQ has been administered, it is expected that schistosomes will undergo apoptosis; however, Hsp70 blocks this pathway, which means that the inhibition of this protein will lead to a decrease in PZQ-resistant strains. Schistosome worms coat themselves with GAPDH to undergo thrombolysis and dissolve the blood clots that form around the worm. The inhibition of this protein will, therefore, increase the sensitivity of the worms to the host’s immune cells so as to undergo degradation. Protein folding is essential in parasite survival for the worms to keep replicating. The inhibition of Hsp70 will lead to degraded schistosome proteins, thus decreasing the parasite’s virulence.
Triosephosphate isomerase (TPI)
TPI is a homodimeric enzyme in the cytosol [133]. Each monomer forms a TPI barrel fold comprising eight α-helices surrounding eight parallel β-stands that are hydrogen-bonded to each other [134]. The dimer is stabilized by Cys15 at the dimer’s interface [135]. TPI is the most active catalytic enzyme that plays a crucial role in the Embden-Meyerhof pathway [135]. It is a 26 kDa protein that catalyzes the co-enzyme-independent interconversion of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (GAP) in the fifth step of glycolysis [136]. However, only GAP can proceed in the pathway to produce adenosine triphosphate (ATP) and the second pyruvate molecule necessary for anaerobic and aerobic metabolism, especially in organisms such as schistosomes [134,137]. This catalytic process also inhibits the accumulation of DHAP, which can be detrimental to cells since it leads to high concentrations of toxic methylglyoxal [138]. According to Roland and colleagues [137], TPI exhibits catalytic and non-catalytic activity and is expressed differently across various cancer types. It also plays a role in regulating the cell cycle, acts as an autoantigen that helps evade the immune response, and even serves as a virulence factor in certain animals [139]. In schistosomes, this protein is a plasminogen binder that induces thrombolysis [140]; thus promoting the survival of the worm in the human host. Since TPI is located on the tegument of the worms, it elicits both glycolytic activity and antibody response. According to Da’Dara and colleagues [141], TPI is a promising vaccine candidate against schistosomiasis.
A phylogenetic study showed that TPIs from parasitic flatworms contain a three-amino acid motif SXD/E (X represents Ala, Ile, or Lys) that is absent from TPIs within hosts or non-parasitic flatworms [142]. This further supports the claim that TPI is a potential target for developing schistosomiasis vaccines. Some immunoglobulins are thought to be able to distinguish between parasite and human enzymes due to minute variations in the surface structure, such as the SXD/E motif [143,144]. However, the crystal structure of S. mansoni TPI (SmTPI) revealed four linear epitopes that are more likely to elicit a more robust immune response than the one found in the SXD/E motif [145]. The kinetic characteristics of the SmTPI show that it is slightly distinct from the human TPI. This implies that it would be challenging to find compounds that might distinguish between the active sites of human and parasite TPI enzymes [144]. However, it might be possible to take advantage of the minor variations in the three-dimensional (3D) structures between TPI from the parasite and the host [145], such as creating inhibitors unique to the parasite’s TPI but nonreactive to the host’s TPI. Developing small molecule inhibitors that specifically interfere with SmTPI’s ability to form homodimers or otherwise interfere with this enzyme’s enzymatic activity might thus be conceivable, especially since TPI is only active in its dimeric form [146,147]. Because the catalytic site lies at the dimeric interface, a disruption in the topology of the interface, even if it does not result in dimer disintegration, may be sufficient to block TPI function. Ferraro and colleagues [134] discovered several compounds that block dimerization and may block F. hepatica TPI from binding to its substrates. Inactivation of the TPI with these compounds led to a decrease in juvenile and adult worms of F. hepatica [134]. These inactivator compounds also decreased schistosomula and adult worm burdens of S. mansoni blood flukes [134]. This shows the potential of TPI as a possible drug target for treating schistosomiasis. Thiol-conjugating agents may also react with the exposed cysteines on the SmTPI and reduce the enzyme’s catalytic activity [145]. Chemical alteration of the C222 residue of the TPI from Giardia lamblia in the non-catalytic region resulted in decreased affinity for substrates by thiol-reactive chemicals [148,149]. There was also a comparable region of the C221 of Opisthorchis viverrini TPI that had a similar environment to that of G. lamblia TPI discovered by Son and colleagues [150], which led to the hypothesis that an inhibitory strategy based on thiol-reactive compounds is also conceivable in the case of O. viverrini TPI.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
GAPDH is a tetramer composed of four identical 37 kDa subunits [65]. Each monomer contains two domains: an NAD-binding domain and a catalytic domain. This glycolytic enzyme converts D-glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate in the presence of NAD+ and an inorganic phosphate [65]. The NAD+ generates NADH, which transports electrons to the electron transport chain to produce energy, ultimately required for the parasite’s survival [151]. The production of 1,3-Bisphosphoglycerate by GAPDH plays a significant role in ATP production under anaerobic conditions since it is a high-energy molecule [151]. GAPDH has also been identified as a moonlighting protein since it facilitates iron uptake in schistosomes, which is necessary for growth and development [1]. According to Sotillo and colleagues [152], since GAPDH is located on the tegument of schistosomes and is highly expressed throughout the worm’s life cycle, the protein aids in evading the host’s immune system. GAPDH aids in the worm’s survival by promoting the production of plasmin from plasminogen, which is vital for dissolving blood clots that form around the worms [1]; thus enabling the parasite to cross barriers, migrate, and infect human host cells [153]. This plasminogen-binding activity was also discovered in the parasitic nematode Haemonchus contortus [154]. The protein is also associated with other processes, including nutrient uptake, osmoregulation, signal transduction, excretion, protein-protein interactions, and interactions with the host’s hemostatic systems [155,156]. These multitasking functions have been identified in other parasites such as Dirofilaria immitis, where GAPDH is located on the worm’s surface, and as excretory/secretory antigens [157]. Steisslinger and colleagues [158] assessed the efficacy of the Onchocerca volvulus - GAPDH vaccine candidate in immunized mice and found a significant adult worm load reduction of up to 57% reduction and up to 95% microfilaraemia reduction. This further supports the use of GAPDH as a vaccine candidate due to its moonlighting activities in the human host.
Due to the protein’s multifunctionality and extracellular location, Zinsser and colleagues [151] suggested GAPDH as a vaccination candidate; however, the highly conserved character of the protein still makes it difficult to distinguish between the host’s and the parasite’s protein [151]. For instance, the NAD+ location in S. mansoni is like that of humans, but S. mansoni GAPDH contains Arg116 [65]. This slight difference may be exploited to develop new anti-schistosomal vaccines. According to Bourguignon and colleagues [159], various parasites’ GAPDH have demonstrated that compounds that block GAPDH enzyme function reduce parasite survival since GAPDH is located on the parasite’s tegument. A study by Argiro and colleagues [160] identified many B and T epitopic areas on schistosome GAPDH. One of these determinants is extremely antigenic in human schistosome infection and is linked to resistance to reinfection while significantly boosting the immune system in mice and rats. It is divergent from the equivalent human gene but well-conserved throughout schistosome species. Due to these factors, Argiro and colleagues [160] concluded that this peptide ought to be given substantial consideration as a potential anti-schistosome subunit vaccine ingredient.
Enolase
Enolase is an essential metalloenzyme in the glycolytic pathway, where schistosomes obtain most of their energy [161]. It belongs to a class of surface proteins delivered to the cell surface through an unidentified process despite lacking the conventional surface transport mechanism [161]. It catalyzes the reversible conversion of 2-phosphoglycerate (2-PGA) to phosphoenolpyruvate (PEP), which is essential for cellular function and energy production [162], with the forward reaction being glycolysis and the reverse reaction being gluconeogenesis. As a result, depending on whether it should modify its metabolism to complete the catabolic or anabolic process, the organism must be able to optimize the characteristics of this enzyme to enable flux in either direction [162]. Nonetheless, enolase has been discovered to perform other non-glycolytic functions beneficial to parasites [162]. In some circumstances, organisms have several enolase isoforms with specific isoforms assigned to these moonlighting activities [163]. In protozoan parasitic worms, enolase is involved in movement, adherence, invasion, differentiation, and growth [164]. These functions have also been described in parasites, such as Trypanosoma spp. [162]; Plasmodium spp., where it has been proposed that cell surface enolase plays a role in tissue invasion [165]; Trichomonas vaginalis, where the protein activates plasminogen to plasmin [166]; and G. lamblia, where enolase plays a role in regulating the worm’s excystation [167].
Enolase is highly expressed in schistosomula, adult worms, and eggs and plays a vital role in the growth and migration of these worms in the human host [161]. Since this protein is located on the tegument of schistosomes, it promotes parasite survival in the human host [161,168]. Even though Schistosoma worms activate the human host’s immune response, they can still grow and reproduce because enolase acts as a plasminogen-binding receptor [169], which enables the parasite to evade the human host (see Figure 1). It achieves this by activating the human plasminogen in the presence of a tissue plasminogen activator (tPA), which converts the plasminogen to plasmin, which dissolves the blood clots around the worms [168,170]. These moonlighting functions of enolase make it an attractive drug target for the development of new anti-schistosomal drugs or vaccines. According to Manneck and colleagues [171], the inhibition of glycolysis creates an unfavorable environment for the parasite. Since enolase plays a crucial role in the invasiveness and virulence of the parasite [162], this further proves its importance.
Mefloquine, an antimalarial drug, inhibits enolase in S. mansoni schistosomula by interfering with glycolysis and weakening the parasite via an unknown mechanism [171]. The SmEnolase contains an active site, a magnesium ion (Mg2+) binding site, and plasminogen-binding motifs that are highly conserved, with Mg2+ playing a crucial role in the catalytic activity of enolase [162,169]. It was suggested that since mefloquine contains three fluorine residues, it may inhibit enolase at the active site by combining magnesium and phosphate with fluoride residues to produce a complex destabilizing the enzyme [171-173]. Understanding this drug target may facilitate the development of new anti-schistosomal medicines [174].
Heat shock protein 70 (Hsp70)
Schistosomes undergo various stresses, such as changes in temperature, which can lead to protein unfolding and the formation of protein aggregates [175]. This loss of protein homeostasis will subsequently result in the expression of HSPs. HSPs are proteins that act as a quality control system in identifying misfolded proteins and determining their fate [175]. Hsp70 is activated for heat stress management and plays a role in other stresses such as starvation, hypoxia, and toxins [176]. Schistosomes experience diverse strains throughout their developmental stages, such as cercariae transitioning from cooler to warmer temperatures as they mature into adult worms [177]. Although schistosomes experience harsh environments, they can still withstand them due to the expression of Hsp70 under stressful conditions [178]. Hsp70 is highly expressed in schistosomes throughout their developmental stages [179]. Schistosomes usually undergo oxidative stress caused by exposure to host portal serum, which leads to misfolded proteins [180]. As a chaperone, Hsp70 correctly folds the client protein to its nativity. However, if it remains misfolded, the protein is degraded, which inhibits parasite development in the host and disease transmission [180]. This is also true in Plasmodium falciparum, where Hsp70 ensures the correct folding of the exported proteins for host cell remodeling to promote parasite virulence activities [181]. Hsp70 is also involved in cercarial penetration of the human host skin [178], aiding cercarial invasion, and is thus implicated in cercarial transformation [2]. Although PZQ induces apoptosis in schistosomes, Hsp70 acts as an anti-apoptotic agent because it blocks cell death [182]. It also inhibits caspase activity by interacting with vital apoptotic proteins, which block the apoptosis pathway [182], thus promoting parasite survival and increasing drug resistance in schistosomes [183].
Since Hsp70 performs essential functions in proteostasis, it is a newly discovered target for numerous diseases. However, competitive suppression of Hsp70’s enzymatic activity has proven difficult, and in some circumstances, there might be other effective strategies to reroute the Hsp70 function [184]. Another method is to prevent Hsp70 from interacting with crucial co-chaperones, including J proteins, nucleotide exchange factors (NEFs), and proteins with tetratricopeptide repeat (TPR) domains [185-187]. Typically, these co-chaperones bind Hsp70 and direct its numerous, varied cellular actions. It has been demonstrated that complexes between Hsp70 and co-chaperones perform crucial roles in parasites’ survival [188]. Inhibiting Hsp90, for instance, activates heat shock response in cancer cells, characterized by elevated expression of Hsp70 as a compensatory strategy [189]. Therefore, a promising technique may be to prevent protein-protein interactions between Hsp70 and its co-chaperones or target allosteric regions that disrupt these connections [184].
Conclusion
The use of moonlighting proteins for drug discovery and vaccinomics has opened a new avenue to developing new anti-schistosomal therapeutics. By taking advantage of their multifunctional properties, moonlighting proteins provide opportunities for decreasing the global burden of schistosomiasis and ultimately solving the issue of drug resistance in schistosomes. These multifunctional proteins can also provide more insight into the life cycle of schistosomes and their survival in human hosts, accelerating future identification and the development of novel drugs and vaccines to treat schistosomiasis.
Disclosure of conflict of interest
None.
References
- 1.Pirovich DB, Da’dara AA, Skelly PJ. Schistosoma mansoni glyceraldehyde-3-phosphate dehydrogenase enhances formation of the blood-clot lysis protein plasmin. Biol Open. 2020;9:bio050385. doi: 10.1242/bio.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida K, Jolly ER. Hsp70 may be a molecular regulator of schistosome host invasion. PLoS Negl Trop Dis. 2016;10:e0004986. doi: 10.1371/journal.pntd.0004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KA, Angelucci F, Bellelli A, Hervé M, Fontaine J, Tsernoglou D, Capron A, Trottein F, Brunori M. Crystal structure of the 28 kDa glutathione S-transferase from Schistosoma haematobium. Biochemistry. 2003;42:10084–10094. doi: 10.1021/bi034449r. [DOI] [PubMed] [Google Scholar]
- 4.Masamba P, Weber BW, Sewell BT, Kappo AP. Crystallization and preliminary structural determination of the universal stress G4LZI3 protein from Schistosoma mansoni. Inform Med Unlocked. 2022;32:101057. [Google Scholar]
- 5.Masamba P, Adenowo AF, Oyinloye BE, Kappo AP. Universal stress proteins as new targets for environmental and therapeutic interventions of schistosomiasis. Int J Environ Res Public Health. 2016;13:972. doi: 10.3390/ijerph13100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergquist R, Utzinger J, Keiser J. Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect Dis Poverty. 2017;6:74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirovich DB, Da’dara AA, Skelly PJ. Schistosoma mansoni phosphoglycerate mutase: a glycolytic ectoenzyme with thrombolytic potential. Parasite. 2022;29:41. doi: 10.1051/parasite/2022042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19:196–205. doi: 10.1016/j.bjid.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lothe A, Zulu N, Øyhus AO, Kjetland EF, Taylor M. Treating schistosomiasis among South African high school pupils in an endemic area, a qualitative study. BMC Infect Dis. 2018;18:239. doi: 10.1186/s12879-018-3102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Boni L, Msimang V, De Voux A, Frean J. Trends in the prevalence of microscopically-confirmed schistosomiasis in the South African public health sector, 2011-2018. PLoS Negl Trop Dis. 2021;15:e0009669. doi: 10.1371/journal.pntd.0009669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Li SZ, Zhang LJ, Bergquist R, Dang H, Wang Q, Lv S, Wang TP, Lin DD, Liu JB, Ren GH, Yang K, Liu Y, Dong Y, Zhang SQ, Zhou XN. Surveillance-based evidence: elimination of schistosomiasis as a public health problem in the Peoples’ Republic of China. Infect Dis Poverty. 2020;9:63. doi: 10.1186/s40249-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 13.Lambertucci JR. Acute schistosomiasis mansoni: revisited and reconsidered. Mem Inst Oswaldo Cruz. 2010;105:422–435. doi: 10.1590/s0074-02762010000400012. [DOI] [PubMed] [Google Scholar]
- 14.Verjee MA. Schistosomiasis: still a cause of significant morbidity and mortality. Res Rep Trop Med. 2019;10:153–163. doi: 10.2147/RRTM.S204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengu MD, Dorsamy V, Moodley J. Schistosomiasis infections in South African pregnant women: a review. S Afr J Infect Dis. 2020;35:a171. doi: 10.4102/sajid.v35i1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha S, Vohora D. Drug Discovery and Development. In: Pharmaceutical Medicine and Translational Clinical Research. Elsevier; 2018. pp. 19–32. [Google Scholar]
- 17.Ferreira LLG, de Moraes J, Andricopulo AD. Approaches to advance drug discovery for neglected tropical diseases. Drug Discov Today. 2022;27:2278–2287. doi: 10.1016/j.drudis.2022.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Weng HB, Chen HX, Wang MW. Innovation in neglected tropical disease drug discovery and development. Infect Dis Poverty. 2018;7:67. doi: 10.1186/s40249-018-0444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feasey N, Wansbrough-Jones M, Mabey DC, Solomon AW. Neglected tropical diseases. Br Med Bull. 2010;93:179–200. doi: 10.1093/bmb/ldp046. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. (2017). Crossing the billion: lymphatic filariasis, onchocerciasis, schistosomiasis, soil-transmitted helminthiases and trachoma: preventive chemotherapy for neglected tropical diseases. World Health Organization. https://iris.who.int/handle/10665/255498.
- 21.De Rycker M, Baragaña B, Duce SL, Gilbert IH. Challenges and recent progress in drug discovery for tropical diseases. Nature. 2018;559:498–506. doi: 10.1038/s41586-018-0327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moser W, Ali SM, Ame SM, Speich B, Puchkov M, Huwyler J, Albonico M, Hattendorf J, Keiser J. Efficacy and safety of oxantel pamoate in school-aged children infected with Trichuris trichiura on Pemba Island, Tanzania: a parallel, randomized, controlled, dose-ranging study. Lancet Infect Dis. 2016;16:53–60. doi: 10.1016/S1473-3099(15)00271-6. [DOI] [PubMed] [Google Scholar]
- 23.Awadzi K, Opoku NO, Attah SK, Lazdins-Helds J, Kuesel AC. A randomized, single-ascending-dose, ivermectin-controlled, double-blind study of moxidectin in onchocerca volvulus infection. PLoS Negl Trop Dis. 2014;8:e2953. doi: 10.1371/journal.pntd.0002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis A, Bailey DR. Metrifonate in urinary schistosomiasis. Bull World Health Organ. 1969;41:209–224. [PMC free article] [PubMed] [Google Scholar]
- 25.Borrmann S, Szlezák N, Faucher JF, Matsiegui PB, Neubauer R, Binder RK, Lell B, Kremsner PG. Artesunate and praziquantel for the treatment of Schistosoma haematobium infections: a double-blind, randomized, placebo-controlled study. J Infect Dis. 2001;184:1363–1366. doi: 10.1086/324004. [DOI] [PubMed] [Google Scholar]
- 26.Doehring E, Poggensee U, Feldmeier H. The effect of metrifonate in mixed Schistosoma haematobium and Schistosoma mansoni infections in humans. Am J Trop Med Hyg. 1986;35:323–329. doi: 10.4269/ajtmh.1986.35.323. [DOI] [PubMed] [Google Scholar]
- 27.Shekhar KC. Schistosomiasis drug therapy and treatment considerations. Drugs. 1991;42:379–405. doi: 10.2165/00003495-199142030-00004. [DOI] [PubMed] [Google Scholar]
- 28.Aruleba RT, Adekiya TA, Oyinloye BE, Masamba P, Mbatha LS, Pretorius A, Kappo AP. PZQ therapy: how close are we in the development of effective alternative anti-schistosomal drugs? Infect Disord Drug Targets. 2019;19:337–349. doi: 10.2174/1871526519666181231153139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utzinger J, Xiao S, Keiser J, Chen M, Zheng J, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human Schistosome parasites. Curr Med Chem. 2001;8:1841–60. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- 30.Danso-Appiah A, Utzinger J, Liu J, Olliaro P. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev. 2008:CD000053. doi: 10.1002/14651858.CD000053.pub2. [DOI] [PubMed] [Google Scholar]
- 31.James C, Webbe G, Preston JM. A comparison of the susceptibility to metrifonate of Schistosoma haematobium, S. mattheei and S. mansoni in hamsters. Ann Trop Med Parasitol. 1972;66:467–474. doi: 10.1080/00034983.1972.11686848. [DOI] [PubMed] [Google Scholar]
- 32.Kramer CV, Zhang F, Sinclair D, Olliaro PL. Drugs for treating urinary schistosomiasis. Cochrane Database Syst Rev. 2014;2014:CD000053. doi: 10.1002/14651858.CD000053.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cioli D, Pica-Mattoccia L, Archer S. Antischistosomal drugs: past, present … and future? Pharmacol Ther. 1995;68:35–85. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 34.Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES. Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology. 2001;123(Suppl):S211–228. doi: 10.1017/s0031182001007703. [DOI] [PubMed] [Google Scholar]
- 35.Pica-Mattoccia L, Carlini D, Guidi A, Cimica V, Vigorosi F, Cioli D. The schistosome enzyme that activates oxamniquine has the characteristics of a sulfotransferase. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):307–312. doi: 10.1590/s0074-02762006000900048. [DOI] [PubMed] [Google Scholar]
- 36.Utzinger J, Keiser J, Shuhua X, Tanner M, Singer BH. Combination chemotherapy of schistosomiasis in laboratory studies and clinical trials. Antimicrob Agents Chemother. 2003;47:1487–1495. doi: 10.1128/AAC.47.5.1487-1495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trainor-Moss S, Mutapi F. Schistosomiasis therapeutics: whats in the pipeline? Expert Rev Clin Pharmacol. 2016;9:157–160. doi: 10.1586/17512433.2015.1102051. [DOI] [PubMed] [Google Scholar]
- 38.Harder A. Chemotherapeutic approaches to schistosomes: current knowledge and outlook. Parasitol Res. 2002;88:395–397. doi: 10.1007/s00436-001-0588-x. [DOI] [PubMed] [Google Scholar]
- 39.Foster R. A review of clinical experience with oxamniquine. Trans R Soc Trop Med Hyg. 1987;81:55–59. doi: 10.1016/0035-9203(87)90282-3. [DOI] [PubMed] [Google Scholar]
- 40.Saconato H, Atallah Á. Interventions for treating schistosomiasis mansoni. Cochrane Database Syst Rev. 2000:CD000528. doi: 10.1002/14651858.CD000528. [DOI] [PubMed] [Google Scholar]
- 41.El Ridi RA, Tallima HA. Novel therapeutic and prevention approaches for schistosomiasis: review. J Adv Res. 2013;4:467–478. doi: 10.1016/j.jare.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86:161–183. doi: 10.1016/s0001-706x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 43.Siqueira LDP, Fontes DAF, Aguilera CSB, Timóteo TRR, Ângelos MA, Silva LCPBB, de Melo CG, Rolim LA, da Silva RMF, Neto PJR. Schistosomiasis: drugs used and treatment strategies. Acta Trop. 2017;176:179–187. doi: 10.1016/j.actatropica.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Utzinger J, Chollet J, Tu Z, Xiao S, Tanner M. Comparative study of the effects of artemether and artesunate on juvenile and adult Schistosoma mansoni in experimentally infected mice. Trans R Soc Trop Med Hyg. 2002;96:318–323. doi: 10.1016/s0035-9203(02)90110-0. [DOI] [PubMed] [Google Scholar]
- 45.Pérez del Villar L, Burguillo FJ, López-Abán J, Muro A. Systematic review and meta-analysis of artemisinin based therapies for the treatment and prevention of Schistosomiasis. PLoS One. 2012;7:e45867. doi: 10.1371/journal.pone.0045867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao S, Tanner M, N’Goran EK, Utzinger J, Chollet J, Bergquist R, Chen M, Zheng J. Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia. Acta Trop. 2002;82:175–181. doi: 10.1016/s0001-706x(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 47.Xiao SH, Booth M, Tanner M. The prophylactic effects of artemether against Schistosoma japonicum infections. Parasitol Today. 2000;16:122–126. doi: 10.1016/s0169-4758(99)01601-4. [DOI] [PubMed] [Google Scholar]
- 48.Cioli D, Pica-Mattoccia L, Basso A, Guidi A. Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol. 2014;195:23–29. doi: 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Xiao SH, Chollet J, Utzinger J, Mei JY, Jiao PY, Keiser J, Tanner M. Effect of single-dose oral mefloquine on the morphology of adult Schistosoma japonicum in mice. Parasitol Res. 2009;105:853–861. doi: 10.1007/s00436-009-1471-4. [DOI] [PubMed] [Google Scholar]
- 50.Van Nassauw L, Toovey S, Van Op den bosch J, Timmermans JP, Vercruysse J. Schistosomicidal activity of the antimalarial drug, mefloquine, in Schistosoma mansoni-infected mice. Travel Med Infect Dis. 2008;6:253–258. doi: 10.1016/j.tmaid.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Ingram K, Ellis W, Keiser J. Antischistosomal activities of mefloquine-related arylmethanols. Antimicrob Agents Chemother. 2012;56:3207–3215. doi: 10.1128/AAC.06177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. Mefloquine - an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3:e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao SH. Mefloquine, a new type of compound against schistosomes and other helminthes in experimental studies. Parasitol Res. 2013;112:3723–3740. doi: 10.1007/s00436-013-3559-0. [DOI] [PubMed] [Google Scholar]
- 54.Xiao SH, Xue J, Shen B. Transmission electron microscopic observation on ultrastructural alterations in Schistosoma japonicum caused by mefloquine. Parasitol Res. 2010;106:1179–1187. doi: 10.1007/s00436-010-1782-5. [DOI] [PubMed] [Google Scholar]
- 55.Xiao SH, Zhang CW. Further observation on histopathological alterations of adult Schistosoma japonicum harbored in mice following treatment with mefloquine at a smaller single dose. Parasitol Res. 2010;107:773–781. doi: 10.1007/s00436-010-1928-5. [DOI] [PubMed] [Google Scholar]
- 56.Xiao SH, Mei JY, Jiao PY. The in vitro effect of mefloquine and praziquantel against juvenile and adult Schistosoma japonicum. Parasitol Res. 2009;106:237–246. doi: 10.1007/s00436-009-1656-x. [DOI] [PubMed] [Google Scholar]
- 57.Keiser J, Vargas M, Doenhoff MJ. Activity of artemether and mefloquine against juvenile and adult Schistosoma mansoni in athymic and immunocompetent NMRI mice. Am J Trop Med Hyg. 2010;82:112–114. doi: 10.4269/ajtmh.2010.09-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keiser J, Manneck T, Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J Antimicrob Chemother. 2011;66:1791–1797. doi: 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
- 59.Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magaisa K, Taylor M, Kjetland EF, Naidoo PJ. A review of the control of schistosomiasis in South Africa. S Afr J Sci. 2015;111:1–6. [Google Scholar]
- 61.Masamba P, Munsamy G, Kappo AP. Closing the gap: an atomistic structural and functional perspective of S. mansoni universal stress G4LZI3 protein in complex with phenolic compounds. Curr Drug Discov Technol. 2021;18:e01102020186453. doi: 10.2174/1570163817666201001122436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abou-El-Naga IF. Schistosoma mansoni sarco/endoplasmic reticulum Ca2+ ATPases (SERCA): role in reduced sensitivity to praziquantel. J Bioenerg Biomembr. 2020;52:397–408. doi: 10.1007/s10863-020-09843-7. [DOI] [PubMed] [Google Scholar]
- 63.Aragon AD, Imani RA, Blackburn VR, Cupit PM, Melman SD, Goronga T, Webb T, Loker ES, Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol Biochem Parasitol. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie Y, Li Y, Wu Y, Liu C, Li X, Li X, Fan X. Synthesis of fluorescent derivatives of praziquantel: cell-imaging and interaction with Schistosoma japonicum cercariae. Org Biomol Chem. 2013;11:5989–5993. doi: 10.1039/c3ob41348a. [DOI] [PubMed] [Google Scholar]
- 65.Boreiko S, Silva M, Iulek J. Structure determination and analyses of the GAPDH from the parasite Schistosoma mansoni, the first one from a platyhelminth. Biochimie. 2021;184:18–25. doi: 10.1016/j.biochi.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 66.Kunamneni A, Ogaugwu C, Goli D. Enzymes as therapeutic agents. In: Enzymes in Human and Animal Nutrition. Elsevier; 2018. pp. 301–312. [Google Scholar]
- 67.Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130433. doi: 10.1098/rstb.2013.0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kennedy DA, Read AF. Why does drug resistance readily evolve but vaccine resistance does not? Proc Biol Sci. 2017;284:20162562. doi: 10.1098/rspb.2016.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fenner F. The Florey lecture, 1983. Biological control, as exemplified by smallpox eradication and myxomatosis. Proc R Soc Lond B Biol Sci. 1983;218:259–85. doi: 10.1098/rspb.1983.0039. [DOI] [PubMed] [Google Scholar]
- 70.Clem AS. Fundamentals of vaccine immunology. J Glob Infect Dis. 2011;3:73–8. doi: 10.4103/0974-777X.77299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montero BK, Wasimuddin, Schwensow N, Gillingham MAF, Ratovonamana YR, Rakotondranary SJ, Corman V, Drosten C, Ganzhorn JU, Sommer S. Evidence of MHC class I and II influencing viral and helminth infection via the microbiome in a non-human primate. PLoS Pathog. 2021;17:e1009675. doi: 10.1371/journal.ppat.1009675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masamba P, Kappo AP. Immunological and biochemical interplay between cytokines, oxidative stress and Schistosomiasis. Int J Mol Sci. 2021;22:7216. doi: 10.3390/ijms22137216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jauréguiberry S, Paris L, Caumes E. Acute schistosomiasis, a diagnostic and therapeutic challenge. Clin Microbiol Infect. 2010;16:225–231. doi: 10.1111/j.1469-0691.2009.03131.x. [DOI] [PubMed] [Google Scholar]
- 75.Barsoum RS, Esmat G, El-Baz T. Human schistosomiasis: clinical perspective: review. J Adv Res. 2013;4:433–444. doi: 10.1016/j.jare.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Molehin AJ. Current understanding of immunity against schistosomiasis: impact on vaccine and drug development. Res Rep Trop Med. 2020;11:119–128. doi: 10.2147/RRTM.S274518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fonseca CT, Oliveira SC, Alves CC. Eliminating Schistosomes through vaccination: what are the best immune weapons? Front Immunol. 2015;6:95. doi: 10.3389/fimmu.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hewitson JP, Maizels RM. Vaccination against helminth parasite infections. Expert Rev Vaccines. 2014;13:473–487. doi: 10.1586/14760584.2014.893195. [DOI] [PubMed] [Google Scholar]
- 79.Molehin AJ, Rojo JU, Siddiqui SZ, Gray SA, Carter D, Siddiqui AA. Development of a schistosomiasis vaccine. Expert Rev Vaccines. 2016;15:619–627. doi: 10.1586/14760584.2016.1131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siddiqui AA, Siddiqui SZ. Sm-p80-based schistosomiasis vaccine: preparation for human clinical trials. Trends Parasitol. 2017;33:194–201. doi: 10.1016/j.pt.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson RA, Li XH, Castro-Borges W. Do schistosome vaccine trials in mice have an intrinsic flaw that generates spurious protection data? Parasit Vectors. 2016;9:89. doi: 10.1186/s13071-016-1369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molehin AJ. Schistosomiasis vaccine development: update on human clinical trials. J Biomed Sci. 2020;27:28. doi: 10.1186/s12929-020-0621-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smyth D, McManus DP, Smout MJ, Laha T, Zhang W, Loukas A. Isolation of cDNAs encoding secreted and transmembrane proteins from Schistosoma mansoni by a signal sequence trap method. Infect Immun. 2003;71:2548–2554. doi: 10.1128/IAI.71.5.2548-2554.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moser D, Tendler M, Griffiths G, Klinkert MQ. A 14-kDa Schistosoma mansoni polypeptide is homologous to a gene family of fatty acid-binding proteins. J Biol Chem. 1991;266:8447–8454. [PubMed] [Google Scholar]
- 85.Siddiqui AA, Zhou Y, Podesta RB, Karcz SR, Tognon CE, Strejan GH, Dekaban GA, Clarke MW. Characterization of Ca2+-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181:37–44. doi: 10.1016/0925-4439(93)90087-h. [DOI] [PubMed] [Google Scholar]
- 86.Braschi S, Curwen RS, Ashton PD, Verjovski-Almeida S, Wilson A. The tegument surface membranes of the human blood parasite Schistosoma mansoni: a proteomic analysis after differential extraction. Proteomics. 2006;6:1471–1482. doi: 10.1002/pmic.200500368. [DOI] [PubMed] [Google Scholar]
- 87.Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127:3641–3648. doi: 10.1242/jcs.154906. [DOI] [PubMed] [Google Scholar]
- 88.Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, Don TA, McManus DP, Correa-Oliveira R, Loukas A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 89.Pearson MS, Pickering DA, McSorley HJ, Bethony JM, Tribolet L, Dougall AM, Hotez PJ, Loukas A. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis. 2012;6:e1564. doi: 10.1371/journal.pntd.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pinheiro CS, Ribeiro AP, Cardoso FC, Martins VP, Figueiredo BC, Assis NR, Morais SB, Caliari MV, Loukas A, Oliveira SC. A multivalent chimeric vaccine composed of Schistosoma mansoni SmTSP-2 and Sm29 was able to induce protection against infection in mice. Parasite Immunol. 2014;36:303–312. doi: 10.1111/pim.12118. [DOI] [PubMed] [Google Scholar]
- 91.Keitel WA, Potter GE, Diemert D, Bethony J, El Sahly HM, Kennedy JK, Patel SM, Plieskatt JL, Jones W, Deye G, Bottazzi ME, Hotez PJ, Atmar RL. A phase 1 study of the safety, reactogenicity, and immunogenicity of a Schistosoma mansoni vaccine with or without glucopyranosyl lipid A aqueous formulation (GLA-AF) in healthy adults from a non-endemic area. Vaccine. 2019;37:6500–6509. doi: 10.1016/j.vaccine.2019.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tendler M, Simpson AJ. The biotechnology-value chain: development of Sm14 as a schistosomiasis vaccine. Acta Trop. 2008;108:263–266. doi: 10.1016/j.actatropica.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 93.Brito CF, Caldas IR, Coura Filho P, Correa-Oliveira R, Oliveira SC. CD4+ T cells of schistosomiasis naturally resistant individuals living in an endemic area produce interferon-γ and tumour necrosis factor-α in response to the recombinant 14KDA Schistosoma mansoni fatty acid-binding protein. Scand J Immunol. 2000;51:595–601. doi: 10.1046/j.1365-3083.2000.00710.x. [DOI] [PubMed] [Google Scholar]
- 94.Tendler M, Brito CA, Vilar MM, Serra-Freire N, Diogo CM, Almeida MS, Delbem AC, Da Silva JF, Savino W, Garratt RC, Katz N, Simpson AS. A Schistosoma mansoni fatty acid-binding protein, Sm14, is the potential basis of a dual-purpose anti-helminth vaccine. Proc Natl Acad Sci U S A. 1996;93:269–273. doi: 10.1073/pnas.93.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Almeida MS, Torloni H, Lee-Ho P, Vilar MM, Thaumaturgo N, Simpson AJ, Tendler M. Vaccination against Fasciola hepatica infection using a Schistosoma mansoni defined recombinant antigen, Sm14. Parasite Immunol. 2003;25:135–137. doi: 10.1046/j.1365-3024.2003.00619.x. [DOI] [PubMed] [Google Scholar]
- 96.Santini-Oliveira M, Coler RN, Parra J, Veloso V, Jayashankar L, Pinto PM, Ciol MA, Bergquist R, Reed SG, Tendler M. Schistosomiasis vaccine candidate Sm14/GLA-SE: phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine. 2016;34:586–594. doi: 10.1016/j.vaccine.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 97.Driss V, El Nady M, Delbeke M, Rousseaux C, Dubuquoy C, Sarazin A, Gatault S, Dendooven A, Riveau G, Colombel JF, Desreumaux P, Dubuquoy L, Capron M. The schistosome glutathione S-transferase P28GST, a unique helminth protein, prevents intestinal inflammation in experimental colitis through a Th2-type response with mucosal eosinophils. Mucosal Immunol. 2016;9:322–335. doi: 10.1038/mi.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pooe K, Thulo M, Makumbe H, Akumadu B, Otun O, Aloke C, Achilonu I. Biophysical description of Bromosulfophthalein interaction with the 28-kDa glutathione transferase from Schistosoma japonicum. Mol Biochem Parasitol. 2022;252:111524. doi: 10.1016/j.molbiopara.2022.111524. [DOI] [PubMed] [Google Scholar]
- 99.Walker J, Crowley P, Moreman AD, Barrett J. Biochemical properties of cloned glutathione S-transferases from Schistosoma mansoni and Schistosoma japonicum. Mol Biochem Parasitol. 1993;61:255–264. doi: 10.1016/0166-6851(93)90071-5. [DOI] [PubMed] [Google Scholar]
- 100.Tang CL, Zhou HH, Zhu YW, Huang J, Wang GB. Glutathione S-transferase influences the fecundity of Schistosoma japonicum. Acta Trop. 2019;191:8–12. doi: 10.1016/j.actatropica.2018.12.027. [DOI] [PubMed] [Google Scholar]
- 101.Xiao SH, You JQ, Gao HF, Mei JY, Jiao PY, Chollet J, Tanner M, Utzinger J. Schistosoma japonicum: effect of artemether on glutathione S-transferase and superoxide dismutase. Exp Parasitol. 2002;102:38–45. doi: 10.1016/s0014-4894(02)00145-5. [DOI] [PubMed] [Google Scholar]
- 102.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Angelucci F, Baiocco P, Brunori M, Gourlay L, Morea V, Bellelli A. Insights into the catalytic mechanism of glutathione S-transferase: the lesson from Schistosoma haematobium. Structure. 2005;13:1241–1246. doi: 10.1016/j.str.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 104.Padi N, Akumadu BO, Faerch O, Aloke C, Meyer V, Achilonu I. Engineering a Pseudo-26-kDa Schistosoma glutathione transferase from bovis/haematobium for structure, kinetics, and ligandin studies. Biomolecules. 2021;11:1844. doi: 10.3390/biom11121844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scott JC, McManus DP. Molecular cloning and enzymatic expression of the 28-kDa glutathione S-transferase of Schistosoma japonicum: evidence for sequence variation but lack of consistent vaccine efficacy in the murine host. Parasitol Int. 2000;49:289–300. doi: 10.1016/s1383-5769(00)00058-1. [DOI] [PubMed] [Google Scholar]
- 106.McTigue MA, Williams DR, Tainer JA. Crystal structures of a schistosomal drug and vaccine target: glutathione S-transferase from Schistosoma japonica and its complex with the leading antischistosomal drug praziquantel. J Mol Biol. 1995;246:21–27. doi: 10.1006/jmbi.1994.0061. [DOI] [PubMed] [Google Scholar]
- 107.Mahajan S, Atkins WM. The chemistry and biology of inhibitors and pro-drugs targeted to glutathione S-transferases. Cell Mol Life Sci. 2005;62:1221–1233. doi: 10.1007/s00018-005-4524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu X, Lemaire C, Grzych JM, Pierce RJ, Raccurt M, Mullier F, Zerimech F, Decavel JP, Peyrol S, Liu J, Fontaine J, Lafitte S, Capron A, Cesbron JY. Expression of a Schistosoma mansoni 28-kilodalton glutathione S-transferase in the livers of transgenic mice and its effect on parasite infection. Infect Immun. 1997;65:3867–74. doi: 10.1128/iai.65.9.3867-3874.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shardlow E, Mold M, Exley C. Unraveling the enigma: elucidating the relationship between the physicochemical properties of aluminium-based adjuvants and their immunological mechanisms of action. Allergy Asthma Clin Immunol. 2018;14:80. doi: 10.1186/s13223-018-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Riveau G, Deplanque D, Remoué F, Schacht AM, Vodougnon H, Capron M, Thiry M, Martial J, Libersa C, Capron A. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis. 2012;6:e1704. doi: 10.1371/journal.pntd.0001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Riveau G, Schacht AM, Dompnier JP, Deplanque D, Seck M, Waucquier N, Senghor S, Delcroix-Genete D, Hermann E, Idris-Khodja N, Levy-Marchal C, Capron M, Capron A. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: a phase 3 randomized, controlled trial in Senegalese children. PLoS Negl Trop Dis. 2018;12:e0006968. doi: 10.1371/journal.pntd.0006968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Molehin AJ, Sennoune SR, Zhang W, Rojo JU, Siddiqui AJ, Herrera KA, Johnson L, Sudduth J, May J, Siddiqui AA. Cross-species prophylactic efficacy of Sm-p80-based vaccine and intracellular localization of Sm-p80/Sm-p80 ortholog proteins during development in Schistosoma mansoni, Schistosoma japonicum, and Schistosoma haematobium. Parasitol Res. 2017;116:3175–3188. doi: 10.1007/s00436-017-5634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–S18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, White GL, Carey DW, Mwinzi PN, Ganley-Leal L, Kennedy RC, Siddiqui AA. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis. 2011;204:1437–1449. doi: 10.1093/infdis/jir545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karmakar S, Zhang W, Ahmad G, Torben W, Alam MU, Le L, Damian RT, Wolf RF, White GL, Carey DW, Carter D, Reed SG, Siddiqui AA. Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons. Vaccine. 2014;32:1296–1303. doi: 10.1016/j.vaccine.2013.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang W, Le L, Ahmad G, Molehin AJ, Siddiqui AJ, Torben W, Karmakar S, Rojo JU, Sennoune S, Lazarus S, Khatoon S, Freeborn J, Sudduth J, Rezk AF, Carey D, Wolf RF, Papin JF, Damian R, Gray SA, Marks F, Carter D, Siddiqui AA. Fifteen years of Sm-p80-based vaccine trials in nonhuman primates: antibodies from vaccinated baboons confer protection in vivo and in vitro from schistosoma mansoni and identification of putative correlative markers of protection. Front Immunol. 2020;11:1246. doi: 10.3389/fimmu.2020.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang W, Molehin AJ, Rojo JU, Sudduth J, Ganapathy PK, Kim E, Siddiqui AJ, Freeborn J, Sennoune SR, May J, Lazarus S, Nguyen C, Redman WK, Ahmad G, Torben W, Karmakar S, Le L, Kottapalli KR, Kottapalli P, Wolf RF, Papin JF, Carey D, Gray SA, Bergthold JD, Damian RT, Mayer BT, Marks F, Reed SG, Carter D, Siddiqui AA. Sm-p80-based schistosomiasis vaccine: Double-blind preclinical trial in baboons demonstrates comprehensive prophylactic and parasite transmission-blocking efficacy. Ann N Y Acad Sci. 2018;1425:38–51. doi: 10.1111/nyas.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Panzner U, Excler JL, Kim JH, Marks F, Carter D, Siddiqui AA. Recent advances and methodological considerations on vaccine candidates for human schistosomiasis. Front Trop Dis. 2021;2:719369. doi: 10.3389/fitd.2021.719369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eyayu T, Zeleke AJ, Worku L. Current status and future prospects of protein vaccine candidates against Schistosoma mansoni infection. Parasite Epidemiol Control. 2020;11:e00176. doi: 10.1016/j.parepi.2020.e00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al-Naseri A, Al-Absi S, El Ridi R, Mahana N. A comprehensive and critical overview of schistosomiasis vaccine candidates. J Parasit Dis. 2021;45:557–580. doi: 10.1007/s12639-021-01387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 122.Huberts DH, van der Klei IJ. Moonlighting proteins: an intriguing mode of multitasking. Biochim Biophys Acta. 2010;1803:520–525. doi: 10.1016/j.bbamcr.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 123.Jeffery CJ. Protein moonlighting: what is it, and why is it important? Philos Trans R Soc Lond B Biol Sci. 2018;373:20160523. doi: 10.1098/rstb.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karkowska-Kuleta J, Kozik A. Moonlighting proteins as virulence factors of pathogenic fungi, parasitic protozoa and multicellular parasites. Mol Oral Microbiol. 2014;29:270–283. doi: 10.1111/omi.12078. [DOI] [PubMed] [Google Scholar]
- 125.Copley SD. An evolutionary perspective on protein moonlighting. Biochem Soc Trans. 2014;42:1684–1691. doi: 10.1042/BST20140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jeffery CJ. Why study moonlighting proteins? Front Genet. 2015;6:211. doi: 10.3389/fgene.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gupta MN, Pandey S, Ehtesham NZ, Hasnain SE. Medical implications of protein moonlighting. Indian J Med Res. 2019;149:322–325. doi: 10.4103/ijmr.IJMR_2192_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yadav P, Singh R, Sur S, Bansal S, Chaudhry U, Tandon V. Moonlighting proteins: beacon of hope in era of drug resistance in bacteria. Crit Rev Microbiol. 2023;49:57–81. doi: 10.1080/1040841X.2022.2036695. [DOI] [PubMed] [Google Scholar]
- 129.Huerta M, Franco-Serrano L, Amela I, Perez-Pons JA, Piñol J, Mozo-Villarías A, Querol E, Cedano J. Role of moonlighting proteins in disease: analyzing the contribution of canonical and moonlighting functions in disease progression. Cells. 2023;12:235. doi: 10.3390/cells12020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Henderson B. Moonlighting proteins: novel virulence factors in bacterial infections. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2016. [Google Scholar]
- 131.Sengupta S, Ghosh S, Nagaraja V. Moonlighting function of glutamate racemase from Mycobacterium tuberculosis: racemization and DNA gyrase inhibition are two independent activities of the enzyme. Microbiology (Reading) 2008;154:2796–2803. doi: 10.1099/mic.0.2008/020933-0. [DOI] [PubMed] [Google Scholar]
- 132.Franco-Serrano L, Huerta M, Hernández S, Cedano J, Perez-Pons J, Piñol J, Mozo-Villarias A, Amela I, Querol E. Multifunctional proteins: involvement in human diseases and targets of current drugs. Protein J. 2018;37:444–453. doi: 10.1007/s10930-018-9790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wierenga RK, Kapetaniou EG, Venkatesan R. Triosephosphate isomerase: a highly evolved biocatalyst. Cell Mol Life Sci. 2010;67:3961–3982. doi: 10.1007/s00018-010-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferraro F, Corvo I, Bergalli L, Ilarraz A, Cabrera M, Gil J, Susuki BM, Caffrey CR, Timson DJ, Robert X, Guillon C, Freire T, Álvarez G. Novel and selective inactivators of Triosephosphate isomerase with anti-trematode activity. Sci Rep. 2020;10:2587. doi: 10.1038/s41598-020-59460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mathur D, Malik G, Garg LC. Biochemical and functional characterization of triosephosphate isomerase from Mycobacterium tuberculosis H37Rv. FEMS Microbiol Lett. 2006;263:229–235. doi: 10.1111/j.1574-6968.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 136.Espinoza-Fonseca LM, Trujillo-Ferrara JG. Exploring the possible binding sites at the interface of triosephosphate isomerase dimer as a potential target for anti-trypanosomal drug design. Bioorg Med Chem Lett. 2004;14:3151–3154. doi: 10.1016/j.bmcl.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 137.Roland BP, Stuchul KA, Larsen SB, Amrich CG, VanDemark AP, Celotto AM, Palladino MJ. Evidence of a triosephosphate isomerase non-catalytic function critical to behavior and longevity. J Cell Sci. 2013;126:3151–3158. doi: 10.1242/jcs.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmed N, Battah S, Karachalias N, Babaei-Jadidi R, Horányi M, Baróti K, Hollan S, Thornalley PJ. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim Biophys Acta. 2003;1639:121–132. doi: 10.1016/j.bbadis.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 139.Rodríguez-Bolaños M, Perez-Montfort R. Medical and veterinary importance of the moonlighting functions of Triosephosphate isomerase. Curr Protein Pept Sci. 2019;20:304–315. doi: 10.2174/1389203719666181026170751. [DOI] [PubMed] [Google Scholar]
- 140.Pirovich D, Da’dara AA, Skelly PJ. Why do intravascular schistosomes coat themselves in glycolytic enzymes? Bioessays. 2019;41:e1900103. doi: 10.1002/bies.201900103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Da’Dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, Feng Z, Yu XL, Gray DJ, Harn DA. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen B, Wen JF. The adaptive evolution divergence of triosephosphate isomerases between parasitic and free-living flatworms and the discovery of a potential universal target against flatworm parasites. Parasitol Res. 2011;109:283–289. doi: 10.1007/s00436-010-2249-4. [DOI] [PubMed] [Google Scholar]
- 143.Sun W, Liu S, Brindley PJ, McManus DP. Bacterial expression and characterization of functional recombinant Triosephosphate isomerase from Schistosoma japonicum. Protein Expr Purif. 1999;17:410–413. doi: 10.1006/prep.1999.1140. [DOI] [PubMed] [Google Scholar]
- 144.Zinsser VL, Farnell E, Dunne DW, Timson DJ. Triose phosphate isomerase from the blood fluke Schistosoma mansoni: biochemical characterisation of a potential drug and vaccine target. FEBS Lett. 2013;587:3422–3427. doi: 10.1016/j.febslet.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 145.Jimenez-Sandoval P, Castro-Torres E, González-González R, Díaz-Quezada C, Gurrola M, Camacho-Manriquez LD, Leyva-Navarro L, Brieba LG. Crystal structures of Triosephosphate Isomerases from Taenia solium and Schistosoma mansoni provide insights for vaccine rationale and drug design against helminth parasites. PLoS Negl Trop Dis. 2020;14:e0007815. doi: 10.1371/journal.pntd.0007815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Olivares-Illana V, Rodríguez-Romero A, Becker I, Berzunza M, García J, Pérez-Montfort R, Cabrera N, López-Calahorra F, de Gómez-Puyou MT, Gómez-Puyou A. Perturbation of the dimer interface of triosephosphate isomerase and its effect on Trypanosoma cruzi. PLoS Negl Trop Dis. 2007;1:e1. doi: 10.1371/journal.pntd.0000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Téllez-Valencia A, Ávila-Ríos S, Pérez-Montfort R, Rodríguez-Romero A, Tuena de Gómez-Puyou M, López-Calahorra F, Gómez-Puyou A. Highly specific inactivation of triosephosphate isomerase from Trypanosoma cruzi. Biochem Biophys Res Commun. 2002;295:958–963. doi: 10.1016/s0006-291x(02)00796-9. [DOI] [PubMed] [Google Scholar]
- 148.Enríquez-Flores S, Rodríguez-Romero A, Hernández-Alcántara G, Oria-Hernández J, Gutiérrez-Castrellón P, Pérez-Hernández G, de la Mora-de la Mora I, Castillo-Villanueva A, García-Torres I, Méndez ST, Gómez-Manzo S, Torres-Arroyo A, López-Velázquez G, Reyes-Vivas H. Determining the molecular mechanism of inactivation by chemical modification of triosephosphate isomerase from the human parasite Giardia lamblia: a study for antiparasitic drug design. Proteins. 2011;79:2711–2724. doi: 10.1002/prot.23100. [DOI] [PubMed] [Google Scholar]
- 149.Hernández-Alcántara G, Torres-Larios A, Enríquez-Flores S, García-Torres I, Castillo-Villanueva A, Méndez ST, de la Mora-de la Mora I, Gómez-Manzo S, Torres-Arroyo A, López-Velázquez G, Reyes-Vivas H, Oria-Hernández J. Structural and functional perturbation of giardia lamblia triosephosphate isomerase by modification of a non-catalytic, non-conserved region. PLoS One. 2013;8:e69031. doi: 10.1371/journal.pone.0069031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Son J, Kim S, Kim SE, Lee H, Lee MR, Hwang KY. Structural analysis of an epitope candidate of triosephosphate isomerase in opisthorchis viverrini. Sci Rep. 2018;8:15075. doi: 10.1038/s41598-018-33479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zinsser VL, Hoey EM, Trudgett A, Timson DJ. Biochemical characterisation of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from the liver fluke, Fasciola hepatica. Biochim Biophys Acta. 2014;1844:744–749. doi: 10.1016/j.bbapap.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 152.Sotillo J, Pearson M, Becker L, Mulvenna J, Loukas A. A quantitative proteomic analysis of the tegumental proteins from Schistosoma mansoni schistosomula reveals novel potential therapeutic targets. Int J Parasitol. 2015;45:505–516. doi: 10.1016/j.ijpara.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 153.Gómez-Arreaza A, Acosta H, Quiñones W, Concepción JL, Michels PA, Avilán L. Extracellular functions of glycolytic enzymes of parasites: unpredicted use of ancient proteins. Mol Biochem Parasitol. 2014;193:75–81. doi: 10.1016/j.molbiopara.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 154.Sahoo S, Murugavel S, Devi IK, Vedamurthy GV, Gupta SC, Singh BP, Joshi P. Glyceraldehyde-3-phosphate dehydrogenase of the parasitic nematode Haemonchus contortus binds to complement C3 and inhibits its activity. Parasite Immunol. 2013;35:457–467. doi: 10.1111/pim.12058. [DOI] [PubMed] [Google Scholar]
- 155.Perez-Casal J, Potter AA. Glyceradehyde-3-phosphate dehydrogenase as a suitable vaccine candidate for protection against bacterial and parasitic diseases. Vaccine. 2016;34:1012–1017. doi: 10.1016/j.vaccine.2015.11.072. [DOI] [PubMed] [Google Scholar]
- 156.Pérez-Sánchez R, Valero ML, Ramajo-Hernández A, Siles-Lucas M, Ramajo-Martín V, Oleaga A. A proteomic approach to the identification of tegumental proteins of male and female Schistosoma bovis worms. Mol Biochem Parasitol. 2008;161:112–123. doi: 10.1016/j.molbiopara.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 157.González-Miguel J, Morchón R, Siles-Lucas M, Oleaga A, Simón F. Surface-displayed glyceraldehyde 3-phosphate dehydrogenase and galectin from Dirofilaria immitis enhance the activation of the fibrinolytic system of the host. Acta Trop. 2015;145:8–16. doi: 10.1016/j.actatropica.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 158.Steisslinger V, Korten S, Brattig NW, Erttmann KD. DNA vaccine encoding the moonlighting protein Onchocerca volvulus glyceraldehyde-3-phosphate dehydrogenase (Ov-GAPDH) leads to partial protection in a mouse model of human filariasis. Vaccine. 2015;33:5861–5867. doi: 10.1016/j.vaccine.2015.07.110. [DOI] [PubMed] [Google Scholar]
- 159.Bourguignon SC, Alves CR, Giovanni-De-Simone S. Detrimental effect of nitric oxide on Trypanosoma cruzi and Leishmania major like cells. Acta Trop. 1997;66:109–118. doi: 10.1016/s0001-706x(97)00033-8. [DOI] [PubMed] [Google Scholar]
- 160.Argiro LL, Kohlstädt SS, Henri SS, Dessein HH, Matabiau VV, Paris PP, Bourgois AA, Dessein AJ. Identification of a candidate vaccine peptide on the 37 kDa Schistosoma mansoni GAPDH. Vaccine. 2000;18:2039–2048. doi: 10.1016/s0264-410x(99)00521-6. [DOI] [PubMed] [Google Scholar]
- 161.Yang J, Qiu C, Xia Y, Yao L, Fu Z, Yuan C, Feng X, Lin J. Molecular cloning and functional characterization of Schistosoma japonicum enolase which is highly expressed at the schistosomulum stage. Parasitol Res. 2010;107:667–677. doi: 10.1007/s00436-010-1913-z. [DOI] [PubMed] [Google Scholar]
- 162.Avilán L, Gualdrón-López M, Quiñones W, González-González L, Hannaert V, Michels PA, Concepción JL. Enolase: a key player in the metabolism and a probable virulence factor of trypanosomatid parasites-perspectives for its use as a therapeutic target. Enzyme Res. 2011;2011:932549. doi: 10.4061/2011/932549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pancholi V. Multifunctional α-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lorenzatto KR, Monteiro KM, Paredes R, Paludo GP, da Fonsêca MM, Galanti N, Zaha A, Ferreira HB. Fructose-bisphosphate aldolase and enolase from Echinococcus granulosus: genes, expression patterns and protein interactions of two potential moonlighting proteins. Gene. 2012;506:76–84. doi: 10.1016/j.gene.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 165.Pal-Bhowmick I, Mehta M, Coppens I, Sharma S, Jarori GK. Protective properties and surface localization of plasmodium falciparum enolase. Infect Immun. 2007;75:5500–5508. doi: 10.1128/IAI.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mundodi V, Kucknoor AS, Alderete JF. Immunogenic and plasminogen-binding surface-associated α-enolase of trichomonas vaginalis. Infect Immun. 2008;76:523–531. doi: 10.1128/IAI.01352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Castillo-Romero A, Davids BJ, Lauwaet T, Gillin FD. Importance of enolase in Giardia lamblia differentiation. Mol Biochem Parasitol. 2012;184:122–125. doi: 10.1016/j.molbiopara.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.de la Torre-Escudero E, Manzano-Román R, Pérez-Sánchez R, Siles-Lucas M, Oleaga A. Cloning and characterization of a plasminogen-binding surface-associated enolase from Schistosoma bovis. Vet Parasitol. 2010;173:76–84. doi: 10.1016/j.vetpar.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 169.Figueiredo BC, Da’dara AA, Oliveira SC, Skelly PJ. Schistosomes enhance plasminogen activation: the role of tegumental enolase. PLoS Pathog. 2015;11:e1005335. doi: 10.1371/journal.ppat.1005335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ramajo-Hernández A, Pérez-Sánchez R, Ramajo-Martín V, Oleaga A. Schistosoma bovis: plasminogen binding in adults and the identification of plasminogen-binding proteins from the worm tegument. Exp Parasitol. 2007;115:83–91. doi: 10.1016/j.exppara.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 171.Manneck T, Keiser J, Müller J. Mefloquine interferes with glycolysis in schistosomula of Schistosoma mansoni via inhibition of enolase. Parasitology. 2012;139:497–505. doi: 10.1017/S0031182011002204. [DOI] [PubMed] [Google Scholar]
- 172.Warburg O, Christian W. Chemischer mechanismus der fluorid-hemmung der Gärung. Naturwissenschaften. 1941;29:590. [Google Scholar]
- 173.Qin J, Chai G, Brewer JM, Lovelace LL, Lebioda L. Fluoride inhibition of enolase: crystal structure and thermodynamics. Biochemistry. 2006;45:793–800. doi: 10.1021/bi051558s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 175.Abou-El-Naga IF. Heat shock protein 70 (Hsp70) in Schistosoma mansoni and its role in decreased adult worm sensitivity to praziquantel. Parasitology. 2020;147:634–642. doi: 10.1017/S0031182020000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics. 2007;8:125. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.LoVerde PT. Do antioxidants play a role in schistosome host-parasite interactions? Parasitol Today. 1998;14:284–289. doi: 10.1016/s0169-4758(98)01261-7. [DOI] [PubMed] [Google Scholar]
- 178.Qokoyi NK, Masamba P, Kappo AP. Proteins as targets in anti-schistosomal drug discovery and vaccine development. Vaccines (Basel) 2021;9:762. doi: 10.3390/vaccines9070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hedstrom R, Culpepper J, Harrison RA, Agabian N, Newport G. A major immunogen in Schistosoma mansoni infections is homologous to the heat-shock protein Hsp70. J Exp Med. 1987;165:1430–1435. doi: 10.1084/jem.165.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jeremias WJ, Araújo FMG, Queiroz FR, Pais FSM, Mattos ACA, Salim ACM, Coelho PMZ, Oliveira GC, Kusel JR, Guerra-Sá R, Coimbra RS, Babá ÉH. Comparative sequence analysis reveals regulation of genes in developing schistosomula of Schistosoma mansoni exposed to host portal serum. PLoS One. 2017;12:e0178829. doi: 10.1371/journal.pone.0178829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Day J, Passecker A, Beck H, Vakonakis I. The plasmodium falciparum Hsp70-x chaperone assists the heat stress response of the malaria parasite. FASEB J. 2019;33:14611–14624. doi: 10.1096/fj.201901741R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumar S, Stokes J 3rd, Singh UP, Scissum Gunn K, Acharya A, Manne U, Mishra M. Targeting Hsp70: a possible therapy for cancer. Cancer Lett. 2016;374:156–166. doi: 10.1016/j.canlet.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ishida Y, Nagata K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy. 2009;5:1217–1219. doi: 10.4161/auto.5.8.10168. [DOI] [PubMed] [Google Scholar]
- 184.Assimon VA, Gillies AT, Rauch JN, Gestwicki JE. Hsp70 protein complexes as drug targets. Curr Pharm Des. 2013;19:404–417. doi: 10.2174/138161213804143699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Qiu XB, Shao YM, Miao S, Wang L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell Mol Life Sci. 2006;63:2560–2570. doi: 10.1007/s00018-006-6192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Harrison C. GrpE, a nucleotide exchange factor for DnaK. Cell Stress Chaperones. 2003;8:218–24. doi: 10.1379/1466-1268(2003)008<0218:ganeff>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Allan RK, Ratajczak T. Versatile TPR domains accommodate different modes of target protein recognition and function. Cell Stress Chaperones. 2011;16:353–367. doi: 10.1007/s12192-010-0248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zininga T, Shonhai A. Small molecule inhibitors targeting the heat shock protein system of human obligate protozoan parasites. Int J Mol Sci. 2019;20:5930. doi: 10.3390/ijms20235930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Armstrong HK, Koay YC, Irani S, Das R, Nassar ZD Australian Prostate Cancer BioResource. Selth LA, Centenera MM, McAlpine SR, Butler LM. A novel class of Hsp90 C-terminal modulators have pre-clinical efficacy in prostate tumor cells without induction of a heat shock response. Prostate. 2016;76:1546–1559. doi: 10.1002/pros.23239. [DOI] [PubMed] [Google Scholar]