Abstract
Objective: To investigate the efficacy of noninvasive ventilator usage for type II respiratory failure in patients with severe chronic obstructive pulmonary disease (COPD). Methods: A total of 124 patients with COPD complicated with type II respiratory failure were divided into an observation group (n = 63) and a control group (n = 61) according to their intervention protocols. The patients in the observation group received noninvasive ventilator intervention, and the patients in the control group received bronchodilators, cough suppressants, oxygen therapy, anti-infection medications, nutritional support, and correction of electrolyte imbalances. Lung function indexes, arterial blood gas index, and inflammatory indicators were collected to assess the efficacy of noninvasive ventilator treatment in patients with severe COPD and type II respiratory failure. Results: The levels of FEV1, FEV1/FVC and FEV1% of the two groups after treatment were significantly higher than those before treatment (P<0.05), with significantly higher levels in the observation group than the control group (all P<0.05). Post-treatment levels of PaCO2 decreased significantly while the post-treatment levels of PaO2 increased significantly (all P<0.05). Additionally, the levels of WBC, CRP and PCT of the control group was significantly higher than that of the observation group after treatment (all P<0.05). Conclusion: Noninvasive ventilator treatment can improve hypoxemia, improve lung function and reduce inflammatory responses in patients with COPD complicated with type II respiratory failure, suggesting its potential for wider clinical application.
Keywords: Noninvasive ventilator, severe chronic obstructive pulmonary disease, type II respiratory failure
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic, yet preventable and treatable illness characterized by irreversible airflow limitation [1]. Globally recognized as a major contributor to chronic morbidity, COPD is projected to rise from the fourth to the third leading cause of death by 2020 [2]. Annually, it is responsible for over three million deaths worldwide [3]. Acute exacerbations of COPD (AECOPD) often impair expiratory capacity, leading to CO2 retention and subsequent type II respiratory failure. This condition not only induces respiratory acidosis but also accelerates the decline in lung function. Type II respiratory failure associated with AECOPD greatly increases mortality rates and severely compromises patient outcomes [4].
Current clinical treatments for managing COPD include oxygen therapy, pharmacotherapy (such as bronchodilators and glucocorticoids), and respiratory rehabilitation. Progress in the field has led to the emergence of new drug formulations and innovative treatment strategies. Notably, advancements in bronchodilator technology have enhanced symptom relief effectiveness; similarly, respiratory rehabilitation methods have become more diverse and tailored to individual needs. However, limitations also exist: some drugs induce side effects, and their long-term effect warrants further investigation. Additionally, there is still room for improvement in the compliance and popularity of respiratory rehabilitation [5].
Noninvasive ventilation represents a significant advancement in treating COPD [6]. This treatment for COPD combined with type II respiratory failure is favored in the clinic with the advantages of simple operation, no intubation and high safety. Moreover, non-invasive ventilators aid in enhancing gas exchange, alleviating respiratory muscle fatigue, stabilizing patient condition, and providing critical time to pursue further treatments. Moreover, it can also shorten the hospital stay and reduce medical costs. However, potential adverse reactions still exist while using this kind of ventilator, such as bloating, facial discomfort, and, in rare cases, risks to patient safety, thereby limiting their application in treating COPD [7]. To address this issue, our study aims to deepen the understanding of noninvasive ventilator treatment in patients with severe COPD complicated with type II respiratory failure, focusing on patient experiences and treatment efficacy.
Data and methods
Study population
This retrospective study involved 124 patients with severe COPD and type II respiratory failure treated at the Fifth Affiliated Hospital of Xinjiang Medical University between January 2021 and January 2024. Among the included patients, 61 patients who received routine treatment were assigned to the control group, while the other 63 patients who received noninvasive ventilator based on routine treatment were assigned to the observation group. The study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Xinjiang Medical University.
Inclusion and exclusion standards
Inclusion criteria: ① Patients diagnosed with COPD at GOLD stage C or D; ② PCO2 levels between 55-80 mmHg in arterial blood gas analysis [8]; ③ Aged 18 years or older; ④ Post-bronchodilator forced expiratory volume in one second (FEV1) <45% of the predicted value; ⑤ Complete clinical data available.
Exclusion criteria: ① Patients with severe coagulation dysfunction or thrombocytopenia; ② Patients with a history of mental illness; ③ Patients with serious cardiac disorders, severe liver malfunction or renal failure; ④ Patients who are pregnant or lactating; ⑤ Patients with incomplete clinical data.
Methods
Control group: The patients received routine treatment, mainly including administration of bronchodilators, cough suppressants, oxygen therapy, anti-infection medications, nutritional support, and correction of electrolyte imbalances.
Observation group: In addition to the routine treatments received by the control group, patients in the observation group were treated with a noninvasive ventilator. Philips Weikang BiPAP S/T noninvasive ventilator (Weinmann, Germany) was used in this study. Treatment was administered through a mouth and nose mask. The respiratory rate was set at approximately 15 breaths/min; the initial inspiratory pressure was set at 7 cm H2O (1 cm H2O = 0.098 kPa) and adjusted to about 16 cm H2O based on the patient’s condition. Similarly, the initial expiratory pressure was set at 5 cm H2O and adjusted to about 10 cm H2O as required.
Routine nursing care for both groups included monitoring of body temperature, oxygen saturation and other vital signs. Nurses also regularly assisted patients with turning over and expectoration to ensure comfort and prevent complications.
Evaluation index
① Lung function indexes: Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), and the ratio of FEV1:FVC were compared between the two groups. ② Arterial blood gas index: Blood oxygen saturation (PaO2) and arterial partial pressure of carbon dioxide (PaCO2) were compared between the two groups. ③ Biomarker analysis: Blood samples (5 ml) were collected using EDTA-K2 anticoagulant vacuum tubes from the cubital vein at four time points: pre-anesthesia, 1-hour post-surgery, 6 hours post-surgery, and 24 hours post-surgery. The collected venous blood was immediately labeled and stored at 4°C. The samples were centrifuged at 4000 r/min for 10 minutes to obtain the serum, which were then stored at -70°C. The concentrations of White Blood Cells (WBC), C-Reactive Protein (CRP), and Procalcitonin (PCT) in the serum was determined using the Enzyme-Linked Immunosorbent Assay (ELISA) method. Assays were conducted according to manufacturer’s protocols using ELISA kits for WBC (Wuhan Yipu Biotechnology Co., Ltd., Cat. No. CK-E10083), CRP (Shanghai Guduo Biotechnology Co., Ltd., Cat. No. GD-S0135-B), and PCT (IL-6; Chuzhou Shinuoda Biological Technology Co., Ltd., Cat. No. SND-H1925). ④ Therapeutic effects: Marked effective: Symptoms such as shortness of breath of the patient disappear, and blood gas, vital signs, and pulmonary function indicators basically return to normal; Effective: Symptoms such as shortness of breath and indicators such as blood gas, vital signs and pulmonary function were significantly improved; Ineffective: Neither the patient’s symptoms nor the vital signs improved. The effective rate = (cases of marked effective + effective)/total cases * 100%.
Statistical analysis
All data were analyzed using SPSS 25.0. The measurement data were expressed as mean ± standard deviation (x̅ ± SD), and the comparison between the two groups were conducted using t-test. Count data were expressed as numbers and percentages, and the comparison was conducted using Chi-square test. P<0.05 was considered with statistical significance. Additionally, data visualizations were generated using GraphPad Prism software (Version 7, GraphPad Prism, San Diego, CA).
Results
Clinical data
As shown in Table 1, the mean age was (68±12.26) years in the observation group and (65±11.47) years in the control group, the mean Body Mass Index (BMI) was (20.4±1.66) kg/m2 in the observation group and (21.1±1.76) kg/m2 in the control group, showing no significant statistical difference (P = 0.55, P = 0.21). Besides, there were no statistical differences in terms of gender composition, smoking history or GOLD stage between the two groups, indicating the comparability between the two groups (all P>0.05).
Table 1.
Comparison of clinical data between the two groups
| Observation group (n = 63) | Control group (n = 61) | t/χ2 | P | |
|---|---|---|---|---|
| Age (years) | 68±12.26 | 65±11.47 | 2.15 | 0.55 |
| Sex | 1.156 | 0.64 | ||
| Male (n%) | 36 (57.14%) | 35 (57.38%) | ||
| Female (n%) | 27 (42.86%) | 25 (39.68%) | ||
| BMI | 20.4±1.66 | 21.1±1.76 | 5.74 | 0.21 |
| Smoking Status | 6.85 | 0.34 | ||
| Smoking | 55 (87.30%) | 54 (88.52%) | ||
| Never Smoke | 7 (11.11%) | 8 (13.11%) | ||
| Chronic emphysema | 29 (46.03%) | 33 (54.10%) | 8.76 | 0.11 |
| Asthma | 16 (25.40%) | 19 (31.15%) | 5.72 | 0.31 |
| Disease severity | 6.17 | 0.17 | ||
| GOLD Stage C | 46 (73.02%) | 29 (68.85%) | ||
| GOLD Stage D | 15 (23.81%) | 18 (29.51%) | ||
| APACHE II score | 16.71±1.36 | 17.02±1.26 | 3.22 | 0.21 |
Comparison of lung function indexes between the two groups
As shown in Figure 1, the pre-treatment FVC in the observation group was (1.38±0.22) L, and that in the control group was (1.44±0.25) L (P>0.05); Post-treatment, the level of FVC in the observation group was (2.97±0.54) L, and that in the control group was (1.81±0.21) L; the level of FVC in the observation group increased significantly after treatment, and was obviously higher than that in the control group (all P<0.05). The pre-treatment level of FEV1 in the observation group was (1.22±0.21) L, and that in the control group was (1.31±0.24) L (P>0.05); post-treatment, the FEV1 in the observation group was (1.91±0.22) L, and that in the control group was (1.42±0.21) L, with significantly pronounced improvement in the observation group (P<0.05). The level of FEV1/FVC in the observation group before treatment was (44.7±4.5)%, and that in the control group was (40.1±6.4)% (P>0.05); post-treatment, the level of FEV1/FVC in the observation group was (79.8±5.3)%, and that in the control group was (62.6±4.3)%, also with significantly pronounced improvement in the observation group (P<0.05).
Figure 1.
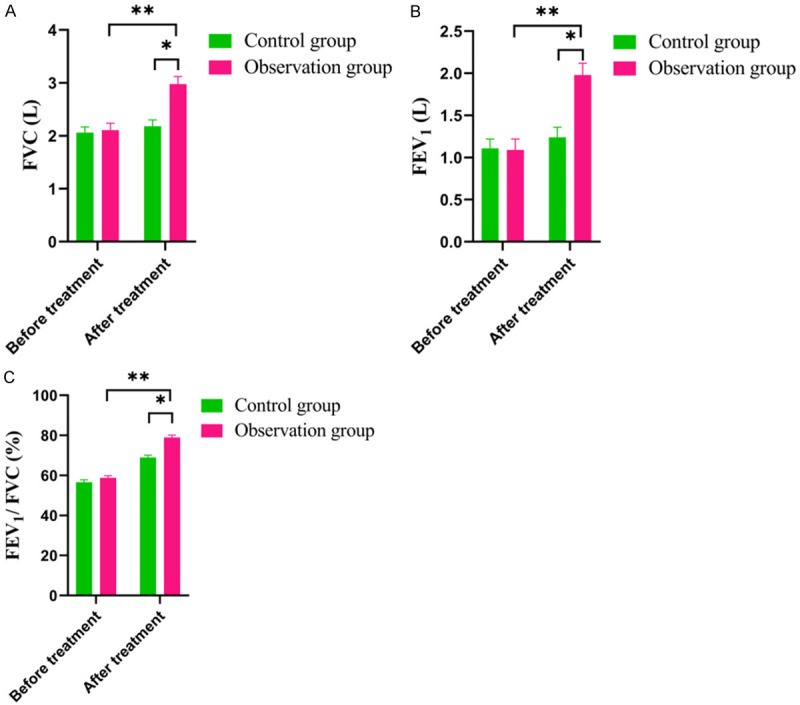
Comparison of Lung function indexes between the two groups. A. FVC. B. FEV1. C. FEV1/FVC. Note: FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in the first second. Compared with control group, *P<0.05, **P<0.01.
Analysis of blood gas index level
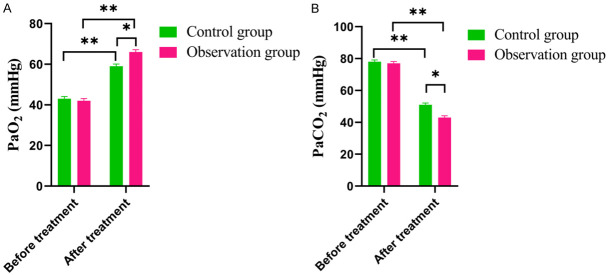
Post-treatment analysis revealed a significant improvement in blood gas indices. The PaCO2 levels were lower and the PaO2 levels were higher than before treatment. Specifically, in the observation group, the post-treatment PaCO2 level (56.78±7.37) was significantly lower compared to the control group (63.60±6.81) (P = 0.031), and the PaO2 level was significantly higher (70.63±6.53) vs. (59.14±4.92) (P = 0.003), as illustrated in Figure 2.
Figure 2.
Comparison of Blood gas index level between the two groups before and after treatment. A. PaO2. B. PaCO2. Note: PaO2: Partial Pressure of Arterial Oxygen; PaCO2: Arterial partial pressure of carbon dioxide. Compared with the control group, *P<0.05, **P<0.01.
Therapeutic effects
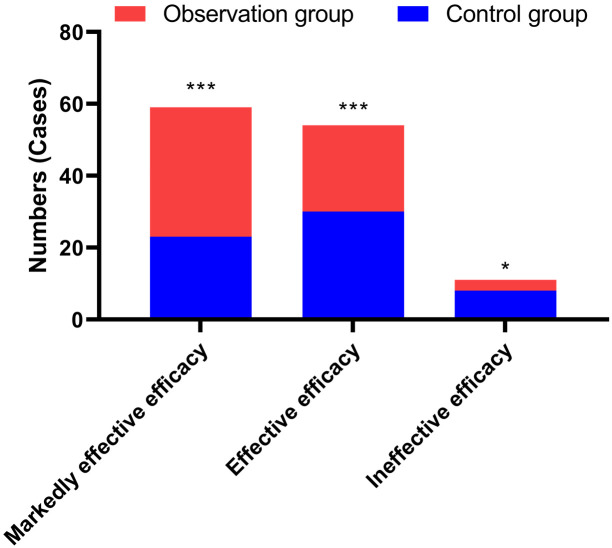
After intervention, we evaluated the efficacy on the patients and found the efficacy of the observation group was greater than that on the control groups (93.8% VS. 86.9%, P<0.05) (Figure 3).
Figure 3.
Comparison of therapeutic effects between the two groups. *P<0.05, ***P<0.001, compared to the control group.
Complications
As shown in the Table 2, there was no significance between two groups in terms of incidence of complications (P>0.05).
Table 2.
Comparison of incidence of complications between the two groups
| Group | Observation Group (n = 63) | Control group (n = 61) | χ2 | P |
|---|---|---|---|---|
| Pulmonary infection | 2 | 6 | 0.5 | 0.11 |
| Pulmonary heart and brain disease | 0/0.00 | 0/0.00 | - | - |
| Atelectasis | 0/0.00 | 0/0.00 | - | - |
| Pneumothorax | 0/0.00 | 0/0.00 | - | - |
| Total incidence rate | 2 | 6 | 0.5 | 0.11 |
Inflammatory indicators
The WBC, CRP and PCT level of the two groups of patients was significantly decreased after treatment. Notably, the WBC, CRP and PCT levels in the observation group were significantly lower than those in the control group after treatment (all P<0.05) (Table 3).
Table 3.
Comparison of inflammatory indicators between the two groups
| Time | Observation Group (n = 63) | Control group (n = 61) | t | P | |
|---|---|---|---|---|---|
| WBC (*109/L) | Before treatment | 13.37±2.10 | 13.19±2.01 | 0.523 | 0.096 |
| After treatment | 7.63±2.35 | 10.09±2.42 | 5.784 | 0.003 | |
| χ2 | 24.921 | 5.785 | - | - | |
| P | 0.001 | 0.022 | - | - | |
| CRP (mg/L) | Before treatment | 12.67±2.37 | 12.31±2.09 | 0.545 | 0.272 |
| After treatment | 6.31±2.89 | 9.39±2.32 | 6.325 | 0.002 | |
| χ2 | 16.915 | 7.154 | - | - | |
| P | 0.001 | 0.001 | - | - | |
| PCT (ug/L) | Before treatment | 0.87±0.02 | 0.82±0.09 | 0.645 | 0.172 |
| After treatment | 0.12±0.01 | 0.34±0.03 | 8.325 | 0.006 | |
| χ2 | 7.915 | 8.154 | - | - | |
| P | 0.011 | 0.009 | - | - |
Note: WBC: White Blood Cell; CRP: C-reactive protein; PCT: Procalcitonin.
Discussion
The prevalence of Chronic Obstructive Pulmonary Disease (COPD) is on the rise, fueled by an increase in smoking rates and the ongoing deterioration of environmental conditions. This trend is alarming as most COPD patients suffer from severe respiratory complications, particularly type II respiratory failure, which profoundly impacts their quality of life. Glucocorticoids, antibiotics and bronchodilators are commonly used in the treatment of COPD-associated type II respiratory failure, but their therapeutic effects are not satisfying [9]. Mechanical ventilation provides respiratory support and plays an important role in the treatment of respiratory diseases. However, noninvasive ventilator treatment introduces challenges, including misunderstandings about the precautions among patients and their families, skin damage from mask compression, and complications from excessive gas entering the gastrointestinal tract. These issues can compromise the treatment effectiveness. Moreover, the frequent use of ventilator can inhibit the immune system of patients, which is prone to bacterial invasion and ventilator-associated pneumonia [10-13].
Our study demonstrates significant improvements in FEV1, FEV1/FVC ratio, and FEV1% post-treatment in both groups, with even greater enhancements observed in the observation group. This demonstrate that the noninvasive ventilator can effectively improve lung function of the patients. Furthermore, we observed significant reductions in PaCO2 and increases in PaO2 levels post-treatment, underscoring the efficacy of noninvasive ventilators in enhancing therapeutic outcomes and optimizing blood gas levels.
In our study, we demonstrated that non-invasive ventilation (NIV) is an effective treatment for COPD patients with type II respiratory failure. NIV enhances oxygenation and reduces the respiratory effort by providing positive pressure to the airways. This intervention offers multiple benefits: Firstly, NIV enhances oxygenation and carbon dioxide removal, alleviating symptoms associated with respiratory failure [14,15]. Secondly, by providing positive pressure to the airways, NIV reduces the effort required for breathing. This lessens respiratory muscle fatigue, making it easier for COPD patients to breathe and reducing the risk of further respiratory complications [16,17]. Thirdly, NIV alleviates symptoms such as shortness of breath and respiratory distress, improving the overall quality of life for COPD patients [18,19].
The mechanism of non-invasive ventilators in treatment of COPD-associated type II respiratory failure involves delivering pressurized air to the patient’s airways through a mask or nasal prongs, which helps to improve oxygenation and remove carbon dioxide from the body [20-22], consequently reducing the work of breathing and allowing the respiratory muscles to rest [23]. Additionally, NIV improves lung function by enhancing gas exchange efficiency in the alveoli, which leads to better oxygenation of the blood and improved respiratory function. This intervention not only helps in managing current symptoms but also aids in preventing further deterioration of lung function and reducing the risk of respiratory failure [24,25].
We also found that non-invasive ventilators can reduce the inflammatory indicators in patients with COPD-associated type II respiratory failure. By delivering appropriate positive pressure ventilation, these devices improve alveolar ventilation and gas exchange. Improved oxygen supply and reduced carbon dioxide retention can alleviate the hypoxic and hypercapnic states that can trigger and exacerbate inflammation. Enhanced oxygenation and ventilation can reduce the stress response and the production of inflammatory mediators. In addition, non-invasive ventilation supports the stability of the chest wall and respiratory muscles, reducing the effort required for breathing and muscle fatigue, which in turn can positively affect inflammation levels. Reduced muscle fatigue means less release of inflammatory substances related to muscle damage. Moreover, proper ventilation support can regulate the immune system to a certain extent, potentially influencing the balance of cytokines and other immune factors. This promotes a more favorable immune environment and reduces the excessive activation of inflammatory responses.
However, our study has some limitation. Firstly, the limited number of participants may hinder the generalizability of the results to a larger population. Secondly, the retrospective nature of our research could introduce bias and confounding factors, potentially affecting the accuracy and reliability of the findings. Thirdly, conducting this research in a single center may limit the diversity of the study population and may not represent the broader population with the same condition. Additionally, the absence of long-term follow-up data restricts our ability to assess the sustained effectiveness of non-invasive ventilation over time.
In conclusion, the use of a noninvasive ventilator can significantly improve blood gas index and lung function of patients with COPD-associated type II respiratory failure.
Disclosure of conflict of interest
None.
References
- 1.Ramakrishnan S. Chronic obstructive pulmonary disease: 10 years of precision-guided success. Lancet Respir Med. 2023;11:227–228. doi: 10.1016/S2213-2600(23)00013-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Kuhn M, Prettner K, Yu F, Yang T, Bärnighausen T, Bloom DE, Wang C. The global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020-50: a health-augmented macroeconomic modelling study. Lancet Glob Health. 2023;11:e1183–e1193. doi: 10.1016/S2214-109X(23)00217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaron SD, Montes de Oca M, Celli B, Bhatt SP, Bourbeau J, Criner GJ, DeMeo DL, Halpin DMG, Han MK, Hurst JR, Krishnan JK, Mannino D, van Boven JFM, Vogelmeier CF, Wedzicha JA, Yawn BP, Martinez FJ. Early diagnosis and treatment of chronic obstructive pulmonary disease: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209:928–937. doi: 10.1164/rccm.202311-2120PP. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Yang B, Qiao J, Bai L, Li Z, Sun W, Liu Q, Yang S, Cui L. Serum exosomal microRNA-1258 may as a novel biomarker for the diagnosis of acute exacerbations of chronic obstructive pulmonary disease. Sci Rep. 2023;13:18332. doi: 10.1038/s41598-023-45592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pérez-Nieto OR, Gómez-Oropeza I, Quintero-Leyra A, Kammar-García A, Zamarrón-López ÉI, Soto-Estrada M, Morgado-Villaseñor LA, Meza-Comparán HD. Hemodynamic and respiratory support in pulmonary embolism: a narrative review. Front Med (Lausanne) 2023;10:1123793. doi: 10.3389/fmed.2023.1123793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess DR. Respiratory care management of COPD exacerbations. Respir Care. 2023;68:821–837. doi: 10.4187/respcare.11069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J, Luo W, Mei Y, Xu Y, Ding S. Nalmefene combined noninvasive positive-pressure ventilation in Chinese patients with chronic obstructive pulmonary disease coupled with type II respiratory failure: a meta-analysis. Medicine (Baltimore) 2023;102:e34624. doi: 10.1097/MD.0000000000034624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng Y, Wu H. Analysis of the prognostic impact of staged nursing interventions on the treatment of patients with COPD combined with type II respiratory failure. Appl Bionics Biomech. 2022;2022:4498161. doi: 10.1155/2022/4498161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang P, Lin X, Liu Y, Hou Z. The efficacy and safety of combined traditional Chinese and western medicine in the treatment of chronic obstructive pulmonary disease complicated with respiratory failure: a systematic review and meta-analysis study. Ann Palliat Med. 2022;11:1102–1111. doi: 10.21037/apm-22-272. [DOI] [PubMed] [Google Scholar]
- 10.Özden G, Parlar Kılıç S. Breathing better: a tech-monitored study of positive expiratory pressure and reading aloud for chronic obstructive pulmonary disease. Int J Nurs Pract. 2023;29:e13198. doi: 10.1111/ijn.13198. [DOI] [PubMed] [Google Scholar]
- 11.Norweg A, Hofferber B, Oh C, Spinner M, Stavrolakes K, Pavol M, DiMango A, Raveis VH, Murphy CG, Allegrante JP, Buchholz D, Zarate A, Simon N. Capnography-assisted learned, monitored (CALM) breathing therapy for dysfunctional breathing in COPD: a bridge to pulmonary rehabilitation. Contemp Clin Trials. 2023;134:107340. doi: 10.1016/j.cct.2023.107340. [DOI] [PubMed] [Google Scholar]
- 12.Laratta CR, Moore LE, Jen R, Campbell SM, MacLean JE, Pendharkar SR, Rowe BH. Acceptance of and adherence with long-term positive airway pressure treatment in adults with chronic obstructive pulmonary disease: a systematic review protocol. PLoS One. 2023;18:e0287887. doi: 10.1371/journal.pone.0287887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jubran A. Setting positive end-expiratory pressure in the severely obstructive patient. Curr Opin Crit Care. 2024;30:89–96. doi: 10.1097/MCC.0000000000001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourke SC, Piraino T, Pisani L, Brochard L, Elliott MW. Beyond the guidelines for non-invasive ventilation in acute respiratory failure: implications for practice. Lancet Respir Med. 2018;6:935–947. doi: 10.1016/S2213-2600(18)30388-6. [DOI] [PubMed] [Google Scholar]
- 15.Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, Barbas CSV, de Acilu MG, Cutuli SL, Bongiovanni F, Amato M, Frat JP, Mauri T, Kress JP, Mancebo J, Antonelli M. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021;47:851–866. doi: 10.1007/s00134-021-06459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Meng W, Zeng H, Ma Y, Chen Y. Relationship between nighttime symptoms and clinical features in COPD patients: a cross-sectional multicenter study in China. Heart Lung. 2023;62:168–174. doi: 10.1016/j.hrtlng.2023.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Pérez-Fernández XL, Sabater-Riera J, Fuset-Cabanes M. COVID-19 ARDS: getting ventilation right. Lancet. 2022;399:22. doi: 10.1016/S0140-6736(21)02439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J, Bone L, Gurses AP, Knowlton A, Pronovost P, Putcha N, Rand C, Roter D, Sylvester C, Thompson C, Wolff JL, Hibbard J, Wise RA. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322:1371–1380. doi: 10.1001/jama.2019.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeung J, Couper K, Ryan EG, Gates S, Hart N, Perkins GD. Non-invasive ventilation as a strategy for weaning from invasive mechanical ventilation: a systematic review and Bayesian meta-analysis. Intensive Care Med. 2018;44:2192–2204. doi: 10.1007/s00134-018-5434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ul Mannan MA, Shahzad MI, Afzal MW, Murtaza HG, Waseem M, Khan T. Outcome of non-invasive ventilation (NIV) among patients with type II respiratory failure due to acute exacerbation of chronic obstructive pulmonary disease (COPD) The Professional Medical Journal. 2020;27:1027–1031. [Google Scholar]
- 21.Cheng Y. Application of continuous nursing intervention for chronic obstructive pulmonary disease patients with type II respiratory failure receiving non-invasive ventilation treatment. CJIN. 2017;3:42–50. [Google Scholar]
- 22.Zhu F. Clinical research of bilevel non-invasive mechanical ventilation in the treatment of chronic obstructive pulmonary disease with respiratory failure. Chin J Prim Med Pharm. 2017;24:1536–1539. [Google Scholar]
- 23.Shi ZX, Gao X, Di KX, Hu YJ. The application of whole-course care under the guidance of the collaborative theory for patients with chronic obstructive pulmonary disease and type II respiratory failure treated with non-invasive ventilation. Int J Nurs. 2017;36:145–148. [Google Scholar]
- 24.Dwyer TJ, Robbins L, Kelly P, Piper AJ, Bell SC, Bye PT. Non-invasive ventilation used as an adjunct to airway clearance treatments improves lung function during an acute exacerbation of cystic fibrosis: a randomised trial. J Physiother. 2015;61:142–147. doi: 10.1016/j.jphys.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Prigent H, Fauroux B, Attarian S, Annane D, Lofaso F. Tracheostomy in ventilator-dependent patients with slowly progressive neuromuscular disease. Lancet Respir Med. 2023;11:410–411. doi: 10.1016/S2213-2600(23)00040-1. [DOI] [PubMed] [Google Scholar]