Abstract
Objective: Esketamine (ESK), an intravenous anesthetic, exerts antidepressant effects; however, the antidepression mechanism is not clear. The aim of this study was to explore whether microglial cannabinoid type 2 (CB2) receptor and protein kinase C (PKC) are involved in the antidepressant effects of ESK. Methods: In this investigation, lipopolysaccharide (LPS) was used to stimulate BV-2 microglia to mimic neuroinflammation. An enzyme-linked immunosorbent assay (ELISA) and Griess reagent kits were used to determine cytokine and nitrite concentrations in the medium. CB2, inducible nitric oxide synthase (iNOS) and nuclear factor (NF)-κB (p65) protein expression were evaluated by immunocytochemistry and western blot analysis. Results: Compared with the control, LPS enhanced proinflammatory factor and nitrite concentration in the medium, upregulated iNOS and NF-κB (p65) expressions, and coadministration of ESK decreased proinflammatory cytokine and nitrite levels, and downregulated iNOS and NF-κB (p65) expression. Moreover, ESK exposure enhanced CB2 receptor expression; coadministration of the CB2 receptor antagonist AM630 or the PKC inhibitor chelerythrine (Che), however, markedly blocked the anti-inflammatory effect of ESK in reducing cytokine and nitrite concentration, and downregulating iNOS and NF-κB (p65) expression. Conclusions: These observations demonstrated that the microglial CB2-PKC pathway mediates ESK-induced anti-inflammation in LPS-stimulated microglial cells.
Keywords: Esketamine, microglia, neuroinflammation, lipopolysaccharide, cannabinoid CB2 receptor, protein kinase C
Introduction
Psychiatric disorders account for 22.8% of the global burden of diseases [1]. Depression is one of the leading psychiatric diseases. Globally, there are approximately 350 million patients with depression. In the US alone, the economic burden of depression is 210 billion USD, causing great economic distress to individuals and the whole country [2]. Unfortunately, the incidence of depression is increasing annually. Therefore, exploring effective and safe therapies for depression is an urgent and important task.
Traditionally, monoamine neurotransmission system modulation has been used to effectively treat depression [3]. However, traditional antidepressant therapy requires several weeks to induce obvious clinical effects [4,5]. Suicidal tendency is a severe clinical feature of depression [6,7]. Therefore, exploring a fast-acting therapy to block the suicidal tendency is of great importance. Esketamine (S-ketamine, ESK) is an intravenous anesthetic agent that is the enantiomer of ketamine. An increasing number of investigations have shown that ESK can exert antidepressant effects [8,9]. Moreover, many investigations have indicated that microglial activation-induced neuroinflammation is a key mechanism in the development of depression, and inhibiting the secretion of proinflammatory cytokines from microglial cells is regarded to be useful in controlling depression [10,11], especially for blocking suicidal tendencies. The N-methyl-D-aspartate (NMDA) receptor, expressed in the cell membrane, is usually considered to be the therapeutic target of ESK/ketamine in treating depression; nevertheless, several recent studies have shown that the endocannabinoid system is also involved in the antidepressant effects of ketamine [12,13]. At present, two main types of cannabinoid receptors, CB1 and CB2, have been discovered in brain tissue. CB1 is found in neuronal cells and astrocytes; in contrast, the CB2 receptor is located in glial cells, including microglia and astrocytes [14]. Upregulation of microglial CB2 receptors can reduce the generation of inflammatory cytokines and alleviate neuroinflammation [15]. Protein kinase C is commonly involved in the inhibition of microglial inflammation induced by various types of medicine [16,17].
In the current study, BV-2 microglial cells (a microglial cell line) were stimulated with lipopolysaccharide (LPS) to imitate the neuroinflammation associated with depression, and we hypothesized that the microglial CB2-PKC pathway mediates ESK-induced anti-inflammation in microglial cells exposed to LPS.
Materials and methods
Cells and reagents
BV-2 microglial cells were obtained as a gift from Air Force Medical University (Xi’an, China). Dulbecco’s Modified Eagle Medium (DMEM, Product No. D5796), fetal bovine serum (FBS, Product No. 12103C), lipopolysaccharide (O55:B5, Product No. L2880) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Product No. 2003) were purchased from Sigma-Aldrich (St. Louis, MO, USA). ESK was obtained from Hengrui Pharmaceuticals Co., Ltd. (Jiangsu, China). The penicillin-streptomycin mixed solution was obtained from Solarbio (Beijing, China). Enzyme-linked immunosorbent assay (ELISA) reagent kits and Griess Reagent Kit, were purchased from Abcam (Cambridge, UK). The anti-CB2 (ab3561), anti-inducible nitric oxide synthase (iNOS) (ab178945) and anti-NF-κB p65 (ab32536) rabbit anti-mouse primary antibodies were purchased from Abcam (Cambridge, UK), and the Cy3-labeled fluorescence antibody (red) was purchased from Beyotime Biotechnology (China). The horseradish peroxidase (HRP)-conjugated secondary antibody was purchased from Thermo Fisher Scientific, Inc. (cat. No. PA1-86329, USA).
Cell culture and treatments
The BV-2 microglial cells used in this study were cultured in DMEM medium containing 10% FBS and 1% penicillin-streptomycin solution (v:v). The air in the incubator consisted of 5% CO2 and 95% O2 with a humidity of 100%, and the temperature of the incubator was 37°C. The cell culture medium was replaced once every 2-3 days, and the BV-2 cells were passaged 2-3 times per week.
To determine an ideal ESK concentration, BV-2 cells were divided into five groups: control (cultured in drug-free medium), LPS (exposed to medium with 1 μg/mL LPS) and three ESK treatment groups (cultured in medium with 1, 5 or 10 μg/mL ESK plus 1 μg/mL LPS). After incubation for 24 h, ELISA was performed to evaluate cytokine release, and immunocytochemistry staining and western blot analysis were performed to measure iNOS and cannabinoid CB2 receptor expression. Then, to exclude the potential neurotoxic effects of ESK, the cells were divided into four groups: one control and three ESK exposure groups (medium with 1, 5 or 10 μg/mL ESK). After 24 h of treatment, the cell viability was measured.
To explore the role of the CB2 cannabinoid receptor in this study, the cells were divided into five groups: control (cultured in drug-free DMEM), LPS (exposed to medium with 1 μg/mL LPS), ESK+LPS (exposed to medium with 10 μg/mL ESK plus 1 μg/mL LPS), AM630+ESK+LPS (exposed to medium with 10 μM CB2 receptor antagonist AM630, 10 μg/mL ESK and 1 μg/mL LPS) and AM630+LPS (exposed to medium with 10 μM AM630 and 1 μg/mL LPS). After incubation for 24 h, cytokine release, iNOS, CB2, NF-κB (p65) protein expression, nitrite level and cell viability were evaluated.
We also investigated the function of protein kinase C in this study. There were five cell groups: control, LPS, ESK+LPS (same as the experiments above), Che+ESK+LPS (exposed to medium with 10 μM PKC inhibitor Che, 10 μg/mL ESK and 1 μg/mL LPS) and Che+LPS (exposed to medium with 10 μM Che and 1 μg/mL LPS). Pro-inflammatory cytokine and nitrite concentrations, iNOS expression and cell viability were measured after the treatments.
Cytokine release measurement
BV-2 microglial cells were plated and cultured in a 96-well cell culture plate at a density of 1×105 cells per well. As the treatment was completed, 20 μL of supernatant from each well was sampled. The cytokine levels in the supernatants were evaluated according to the instructions of the ELISA reagent kits respectively [17], including TNF-α (ab285327, sensitivity: 1 pg/mL, range: 15.6 pg/mL-1000 pg/mL), IL-1β (ab197742, sensitivity: 1 pg/mL, range: 1.56 pg/mL-100 pg/mL) and IL-6 (ab222503, sensitivity: 11.3 pg/mL, range: 15.6 pg/mL-1000 pg/mL). After 90-min incubation, the absorbance of each well was evaluated at a wavelength of 405 nm by using an enzyme-labeled instrument (TECAN, CH). The cytokine concentration (pg/mL) was calculated from a standard curve provided by the kits.
Nitrite concentration measurement
The BV-2 microglial cells were seeded into a 96-well plate at a density of 2×104 cells per well. The level of nitrite, a stable NO oxidation product, in the medium was measured by using a Griess Reagent Kit (ab234044, Abcam, Cambridge, UK), After the treatments, 50 μL supernatant from each well was collected and mixed with 50 μL Griess Reagent. Then, the absorbance of the mixture (50 μL supernatant plus 50 μL Griess Reagent) was measured by using an enzyme-labeled instrument (TECAN, CH), and the wavelength was 570 nm. The data of the samples were determined by the external calibration curve.
Western blot analysis
A 6-well cell culture plate was used to culture the BV-2 cells, and the cell seeding density was 1×106 cells per well. As the treatment was completed, the supernatants of the medium were removed and discarded, and then the culture plate was washed three times with phosphate-buffered saline (PBS) for 5 min/wash. Then, 200 μL lysis buffer was added into every well, and the plate was placed on ice. The lysis buffer consisted of NaCl (0.15 M), sucrose (0.3 M), PMSF (0.3 mM), Tris-HCl (20 mM), EDTA (2 mM) and leupeptin (10 μg/ml). For detecting the microglial NF-κB p65 expression, the nuclei protein was extracted by using a Cytoplasmic and Nuclear Protein Extraction Kit (cat. No. KGB5302-50, Keygen Biotech, Jiangsu, China). Next, the protein level of the treated cells was assessed by using the Bradford method [18]. Next, equal amounts of protein (25 μL, 500 ng/mL) were separated on 10% SDS-PAGE mini gels, and then transferred to nitrocellulose membranes. Then 5% skimmed milk was used to block the membranes for 45 min, then the primary antibodies, including anti-iNOS, anti-CB2, anti-NF-κB (p65) and anti-GAPDH (1:1000 in dilution), were used to incubate the membranes overnight at 4°C. Next, the membranes were washed three times with Tris-buffered saline with Tween-20 (0.05%; TBST), 10 min, one time. Subsequently, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2000 in dilution) for 2 h at room temperature. Then, the blots were washed three times again with TBST, 10 min for once, and the target proteins in the cells were determined by using chemiluminescence kit (SLF1021, Thermo Fisher Scientiffc, Inc.). The images of this study were assessed by using computerized analysis software (Bio-Rad, USA).
Immunocytochemistry staining
BV-2 microglial cells were plated into a confocal microscope-specific dish (Corning, USA), and the cell density was 1×105 cells per dish. As the treatments were completed, the medium in the dish was discarded, and 1 mL of PBS at room temperature was used to wash each dish, three times in total, 5 min per time. Then, 4% paraformaldehyde (500 μL) was added to one dish to fix the microglia, and 30 min later, the paraformaldehyde was removed. Then, PBS was used to wash the dishes three times. Then, to block the heterogenetic antigen of the cells, 100 μL of 5% bovine serum albumin (BSA) solution was added to every dish. Thirty minutes later, the BSA was removed, and 100 μL anti-CB2 primary antibody (1:100 dilution) was added to every dish. Then, the dishes were placed in a refrigerator at 4°C overnight. Then, the dishes were washed three times again by using PBS at room temperature. After washing, 100 μL of Cy-3-labeled secondary antibody (red) was added to each dish. After incubation in the dark at room temperature for 1 h, to mark the cell nuclei (blue), 50 μL DAPI staining solution was added to each dish. After incubation in the dark for 10 min, the solution was removed. After washing with PBS, the dish was observed under a confocal fluorescence microscope (Olympus, Japan), and pictures were randomly taken.
Cell viability
A 96-well cell culture plate was used to culture the cells, and the cell seeding density was 1×105 cells/well. As the treatments were completed, a total volume of 20 μL MTT solution was added into each well, and the MTT concentration was 5 mg/mL. After incubation at 37°C in the cell incubator for 3 h, the supernatants of the cells were discarded. Then, 150 μL dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan. After 15 min of shaking with an agitator, as DMSO dissolved all the formazan, the absorbance was evaluated by using an enzyme-labeled instrument (TECAN, CH).
Statistical analysis
SPSS 13.0 (SPSS, USA) was used to analyze the data of the present investigation. Each experiment was repeated at least three times, and all the data of this work are expressed as the mean ± standard deviation (SD). One-way ANOVA followed by Tukey’s multiple comparison was used to analyze the differences between different groups. P < 0.05 indicated significance.
Results
ESK decreased LPS-induced inflammatory responses in microglial cells
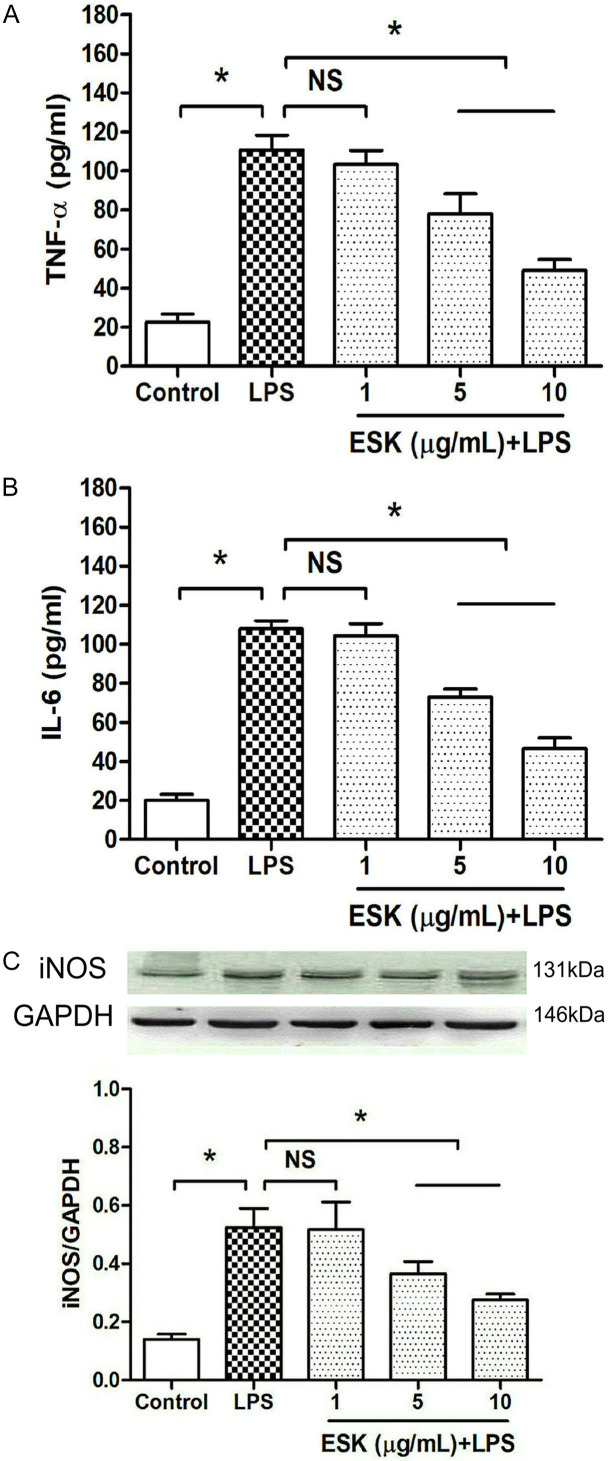
To search for a suitable ESK concentration in LPS-stimulated BV-2 microglia, proinflammatory factor release and the expression of inducible nitric oxide synthase (iNOS), a microglial inflammatory biomarker, were measured (Figure 1). Compared with the control group, the LPS stimulation significantly increased TNF-α and IL-6 release and upregulated iNOS expression (P < 0.05), and the presence of 5 μg/mL or 10 μg/mL ESK obviously reduced the release of TNF-α and IL-6 into the medium and downregulated iNOS expression (P < 0.05), indicating that ESK can alleviate LPS-induced inflammation in microglial cells.
Figure 1.
Esketamine inhibited LPS-induced proinflammatory cytokine release and iNOS protein upregulation. The number of cell groups was five in total, including the control group, 1 μg/mL LPS-stimulated group, and three esketamine (ESK) treatment groups (cultured in medium with 1, 5 or 10 μg/mL ESK plus 1 μg/mL LPS). After 24 h of incubation, TNF-α and IL-6 concentrations and iNOS expression were measured. A. The TNF-α concentration in the medium (n = 10). B. The IL-6 concentration in the medium (n = 10). C. iNOS expression (n = 4). *: P < 0.05; NS: no significance.
ESK upregulated cannabinoid CB2 receptor expression in microglial cells
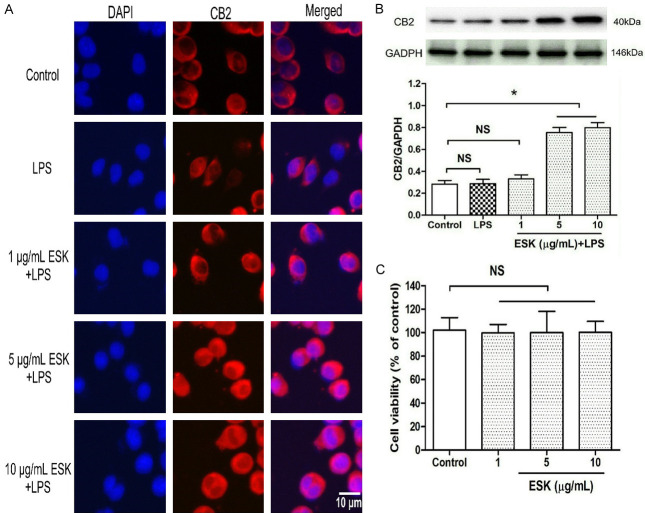
To investigate the effects of ESK on cannabinoid CB2 expression, immunocytochemistry staining and western blot analysis were performed to evaluate CB2 protein levels (Figure 2A, 2B). Compared with the control, LPS exposure for 24 h did not cause marked effects on CB2 expression (P > 0.05); however, the coadministration of 5 μg/mL or 10 μg/mL ESK, but not 1 μg/mL (P > 0.05), obviously upregulated CB2 expression (P < 0.05).
Figure 2.
Esketamine upregulated CB2 protein expression in microglial cells. The grouping and treatments were the same as in Figure 1, and immunocytochemistry staining and western blot analysis were performed to assess CB2 expression. Then, the cells were assigned into four groups, including the normal cultured control and three concentrations of esketamine (ESK) groups. After 24 h of treatment, the cell injury was assessed by using the MTT assay. A. CB2 receptor staining (magnification: 40×10). B. CB2 expression level (n = 4). C. Microglial viability (n = 10). *: P < 0.05; NS: no significance.
Moreover, to rule out the possible toxic effects induced by ESK, the cells were divided into four groups (Figure 2C): control and three ESK treatment groups (1, 5 and 10 μg/mL ESK). Compared with the control group, the ESK treatment groups did not exhibit significant cell injury (P > 0.05). The above results showed that the ESK-induced anti-inflammation and CB2 upregulation occurred via its pharmacological effects rather than toxic effects. Thus, 10 μg/mL ESK was chosen for subsequent experiments.
CB2 receptor antagonist blocked ESK-induced anti-inflammation in BV-2 microglial cells
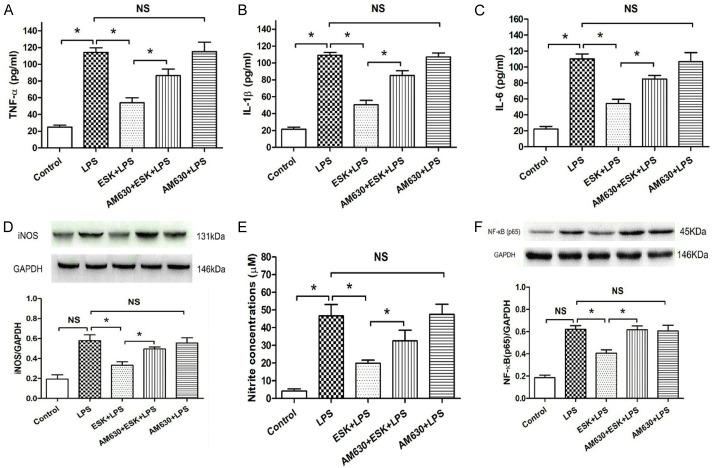
To explore the action of the CB2 receptor in cells treated with ESK, a CB2 receptor antagonist, AM630, was used in this study. The number of cell groups was five in total. Compared with the control, LPS stimulation enhanced TNF-α, IL-1β and IL-6 release from BV-2 microglia (Figure 3A-C) and upregulated iNOS (Figure 3D) and NF-κB (p65) expression (Figure 3F) (P < 0.05), meanwhile, increased the nitrite (a stable nitric oxide oxidation product) concentration in the medium (Figure 3E); however, coadministration of ESK inhibited the secretion of the three cytokines, downregulated iNOS and NF-κB (p65) expression (P < 0.05), and decreased the nitrite level. The addition of AM630 markedly blocked the ESK-induced inhibition of cytokine release and iNOS and NF-κB (p65) expression (P < 0.05), and also the nitrite concentration. The AM630 group did not show obvious changes in the LPS-induced inflammatory factor release, upregulation of iNOS and NF-κB (p65) or the nitrite increase (P > 0.05), compared with those in the LPS group. These results showed that the cannabinoid CB2 receptor mediates the anti-inflammatory effect of ESK in LPS-stimulated microglial cells.
Figure 3.
CB2 antagonist AM630 blocked esketamine-induced inhibition of pro-inflammatory cytokine release, nitrite accumulation and downregulation of iNOS and NF-κB (p65) expression. ELISA was used to measure cytokine release, and western blot analysis was performed to evaluate iNOS and NF-κB (p65) expression. A. The TNF-α concentration (n = 10). B. The IL-1β concentration (n = 10). C. The IL-6 concentration (n = 10). D. iNOS expression (n = 4). E. Nitrite concentration (n = 8). F. NF-κB (p65) expression (n = 4). *: P < 0.05; NS: no significance.
CB2 receptor antagonist inhibited ESK-induced CB2 receptor upregulation in microglia
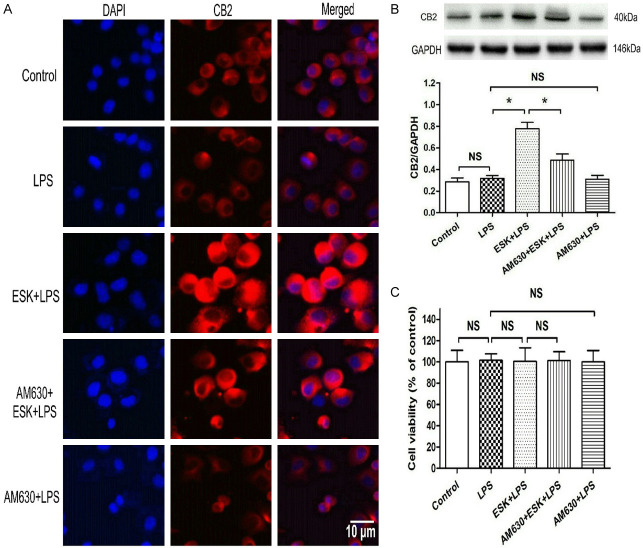
The microglial CB2 protein expression level was determined by immunocytochemistry and western blot analysis (Figure 4A, 4B). Compared with the control, LPS did not change CB2 expression (P > 0.05), and ESK obviously upregulated CB2 expression; however, the CB2 antagonist AM630 partially blocked the ESK-induced CB2 protein elevation (P < 0.05), and no obvious changes in CB2 expression were observed in the AM630 group (P > 0.05) compared with the LPS group.
Figure 4.
The CB2 antagonist AM630 blocked esketamine-induced CB2 protein upregulation in LPS-stimulated microglia. Immunocytochemistry staining and western blotting were used to determine microglial CB2 receptor levels, and the cell injury was assessed by using the MTT assay. A. Immunocytochemistry results of CB2 expression (magnification: 40×10). B. Western blot results of microglial CB2 expression (n = 4). C. Microglial viability (n = 10). *: P < 0.05; NS: no significance.
To exclude the potential neurotoxicity of the drugs used in this experiment, an MTT assay was performed (Figure 4C). No significant cell injury was observed among the five groups (P > 0.05). These results support that the CB2 receptor antagonist AM630 can reverse ESK-induced CB2 upregulation in BV-2 microglial cells.
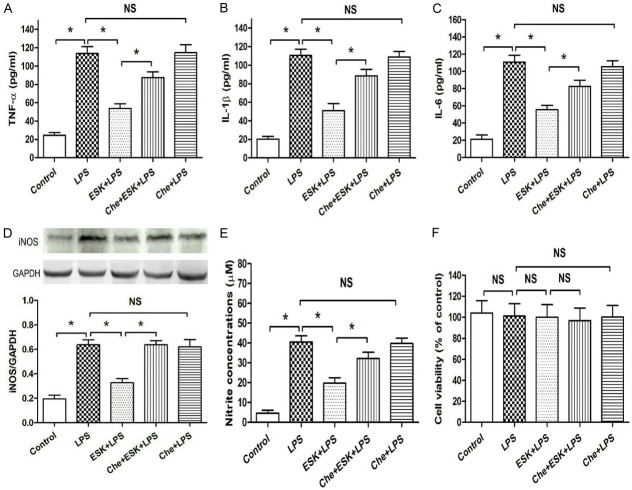
Protein kinase C inhibitor blocked ESK-induced anti-inflammation in microglia
Protein kinase C (PKC) is an intracellular protein that is involved in many drug-induced anti-inflammatory effects in microglial cells and other immune cells [16,17]. In the present investigation, we explored whether PKC is related to the anti-inflammatory effect of ESK by using chelerythrine (Che), a PKC inhibitor. Compared with the control (Figure 5A-E), LPS significantly increased the TNF-α, IL-1β and IL-6 releases, iNOS expression and nitrite level in the medium (P < 0.05), and ESK decreased the three inflammatory factor releases, iNOS expression and nitrite level (P < 0.05). However, coadministration of the PKC inhibitor Che partially blocked the ESK-induced actions on the three cytokine releases, iNOS expression and the nitrite level; and compared with the LPS group, the Che group did not show obvious changes in the LPS-induced pro-inflammation metrics (P > 0.05). In addition, no marked difference in cellular injury (Figure 5F) was observed in the five groups (P > 0.05), showing that the changes in inflammatory level among the five groups were induced by pharmacological effects, not by cytotoxic effects, of the drugs used in this study. These findings demonstrated that PKC mediates the anti-inflammatory effect of ESK in LPS-stimulated BV-2 cells.
Figure 5.
Protein kinase C inhibitor reversed esketamine-induced inhibition of pro-inflammatory cytokine release, nitrite accumulation and iNOS expression. ELISA was used to measure cytokine release, and western blot and MTT assays were used to evaluate iNOS expression and cell injury, respectively. A. The TNF-α concentration (n = 10). B. The IL-1β concentration (n = 10). C. The IL-6 concentration (n = 10). D. The iNOS expression level (n = 4). E. Nitrite concentration (n = 8). F. Microglial viability (n = 10). *: P < 0.05; NS: no significance.
Discussion
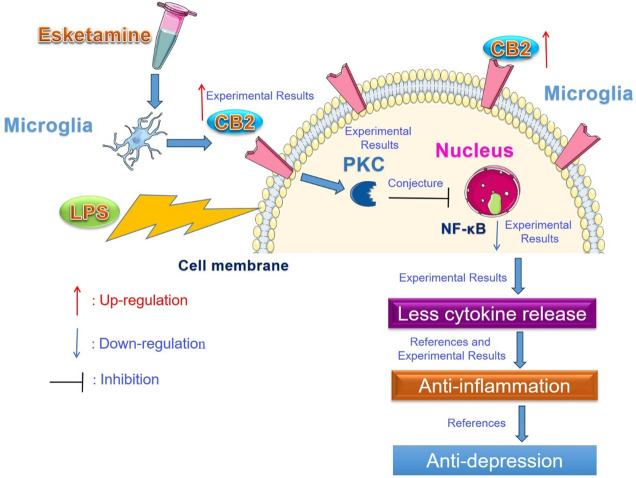
In the present investigation, we explored the CB2-PKC pathway in ESK-induced anti-inflammation in LPS-stimulated BV-2 microglia. We noticed that LPS exposure enhanced the secretion of proinflammatory cytokines, including TNF-α, IL-1β and IL-6, from microglial cells, upregulated the expression of iNOS and NF-κB, and increased the nitrite level in the medium; coadministration of the intravenous anesthetic agent ESK reduced cytokine and the nitrite concentration, and downregulated iNOS and NF-κB levels in BV-2 cells while increasing microglial cannabinoid CB2 protein expression; and the CB2 receptor antagonist AM630 or PKC inhibitor Che markedly blocked the ESK-induced changes in cytokine release, nitrite production and the expression of iNOS and NF-κB proteins. These observations showed that ESK can decrease LPS-induced inflammation in BV-2 cells, and the CB2-PKC pathway might mediate the anti-inflammatory effects of ESK, causing anti-depression efficacy ultimately (Figure 6).
Figure 6.
Esketamine exerts anti-inflammatory effects on LPS-stimulated microglia via the CB2-PKC pathway. Esketamine upregulated microglial cannabinoid CB2 receptors and then downregulated microglial NF-κB protein in the nucleus via protein kinase C (PKC), and fewer cytokines were released from the microglia, ultimately leading to anti-inflammatory and antidepressant effects.
Depression is an extremely common and severe psychiatric disease, and the incidence of depression is approximately 15% in the general population, leading to cardiovascular diseases and even suicide [19,20]. At present, two main pathophysiological mechanisms of depression have been reported: regulatory dysfunction of the hypothalamus-pituitary-adrenal (HPA) axis and production of adult-generated neurons in hippocampal dentate gyrus tissue [20]. Unfortunately, only approximately 33% of patients with depression can obtain complete remission after a single antidepressant administration, and an approximately 60% remission rate can be obtained after the use of two different antidepressants [21]. This evidence showed that the occurrence and development of depression could not be completely explained by the above two pathological mechanisms, and there may be other mechanisms associated with the development of depression. Several recent studies have indicated that alleviating neuroinflammation can control the symptoms of depression, and nonsteroidal anti-inflammatory drugs (NSAIDs) and other medicines with anti-inflammatory abilities are effective in treating depression [22]. In addition, microglial cells are vital immune cells in the brain and spinal cord, and reducing microglial activation can obviously decrease neuroinflammation [23]. Therefore, inhibiting the activation status of microglia is considered to be a useful method in alleviating many neurological and psychological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD) and depression. ESK (the S enantiomer of ketamine) is a general intravenous anesthetic drug and NMDA receptor antagonist that is used widely in pediatric and short-term anesthesia [8]. In the pathophysiological process of severe depression, microglial cells can be activated, and secrete proinflammatory cytokines, in addition, the NF-κB protein and iNOS protein of microglial cells can be up-regulated [24,25], which are very similar with the observed indicators of this study. Moreover, the secretion of inflammation cytokines and up-regulation of iNOS protein are very classic features of activated microglial cells, for these reasons aforementioned, we tested these indictors to evaluate the anti-depressive ability of ESK. BV-2 microglial cell exposed to LPS is used very widely to mimic depression in the in vitro investigation, and this cell model was used in many recent studies to explore the occurrence and treatment of depression [26-28]. Recently, several investigations showed that ESK was effective in alleviating neuroinflammation and symptoms of depression [9,29], so in the present investigation, we used LPS-treated BV-2 microglial cells to imitate the neuroinflammation associated with depression and explored the anti-inflammation and anti-depression mechanism of ESK. The LPS exposure can enhance iNOS expression and nitric oxide (NO) production in microglial cells, and the produced NO exists as the nitrite, a stable NO oxidation product in the medium. We found that ESK administration obviously reduced LPS-induced proinflammatory cytokine release from BV-2 microglial cells, downregulated iNOS and NF-κB (p65) expression, attenuated the nitrite accumulation in the medium and upregulated cannabinoid CB2 expression; however, coadministration of the CB2 antagonist AM630 obviously blocked the ESK-induced changes in cytokine release, nitrite production and the expression of iNOS, NF-κB (p65) and CB2 proteins, indicating that the cannabinoid CB2 receptor might mediate the anti-inflammatory effect of ESK on LPS-treated microglia. The PKC inhibitor Che induced similar effects as AM630 in reversing the ESK-induced anti-inflammation described above. In fact, ESK and cannabinoids are similar in many ways; for instance, they are addictive, analgesic, anti-inflammatory and neuroprotective. Moreover, ESK/ketamine and cannabinoids can also be used in treating psychiatric disorders, including schizophrenia, anxiety and depression [30,31]. However, although it has been widely accepted that ESK/ketamine induces pharmacological effects by inhibiting NMDA receptors, several recent studies have revealed that ESK or ketamine can also activate cannabinoid CB1 and CB2 receptors [12]. As the CB2 receptor is located in immune cells and is involved in modulating inflammation in the central nervous system (CNS) [32], in this investigation, we studied the CB2 receptor’s action in ESK-induced anti-inflammation. In addition, in basic research, administration of a CB2 agonist alleviated depression-like behaviors in rats [33]. In another in vivo study, Khakpai et al. reported that ketamine exerted antidepressant effects by modulating cannabinoid CB1 and CB2 receptors [12]. In addition, several clinical trials showed that ESK induced obvious and rapid antidepressant effects with fewer and mild or moderate side effects [34]. Traditional anti-depressants, including monoamine oxidase inhibitors (MAOIs), tricyclic agents (TCAs) and selective serotonin reuptake inhibitors (SSRIs) [35], require approximately three to six weeks to exert obvious antidepressant effects, and the remission rate with single medicine is approximately 30%. In addition, traditional antidepressants usually bring about obvious side effects, such as marked elevated blood pressure, anxiety, tachycardia and orthostatic hypotension. Moreover, patients with depression usually commit suicide, so it is crucial to control suicidal tendency. ESK takes only several days to control depressive behaviors, especially suicidal tendency [7]. As a result of its active therapeutic effects and fewer side effects, ESK has been authorized by the Food and Drug Administration (FDA) of the US to treat depression [36].
PKC is localized in the cytoplasm and is a kinase family of phospholipid-dependent serine/threonine kinases consisting of three subfamilies, including classic (PKCα, PKCβI, PKCβII and PKCγ), non-classic (PKCε, PKCδ, PKCη, PKCθ and PKCμ) and atypical PKC (PKCξ) [37]. Many investigations have shown that PKC is associated with cell proliferation, differentiation and death, and PKC is also a regulator of host defense and inflammation. As several studies have shown that PKC is related to anesthetic-induced anti-inflammation in immune cells, including microglia and macrophages [16,17,38], in the present investigation, we studied PKC in ESK-induced anti-inflammatory effects in microglia. We found that the PKC inhibitor Che reversed the ESK-induced anti-inflammatory effects in LPS-stimulated microglia, showing that PKC might mediate ESK-induced anti-inflammation in BV-2 cells. As a key inflammatory regulator of microglial cells, NF-κB upregulation increases proinflammatory cytokine release. In this study, we noticed that ESK decreased NF-κB upregulation in microglia exposed to LPS, and the CB2 cannabinoid receptor antagonist AM630 blocked the ESK-induced upregulation of NF-κB, indicating CB2 receptor mediated ESK-induced anti-inflammatory effects. However, there are also some limitations in this study. First, what we found in the present research was completely in vitro, and whether the futural findings from in vivo or clinical studies are in accordance with this study is unknown. Second, although we found that PKC was related to ESK-induced anti-inflammation in microglial cells, which subtype of PKC played a more vital role is still obscure. Third, there are still some controversies regarding the modulatory role of CB2 in inflammation, Olabiyi et al. reported that depletion of CB2 receptor or CB2 receptor antagonist SR144528 can make microglial cells less responsive to inflammation stimulation [39], and the genetic deletion of CB2 in mice reduced the severity of dementia [40]. In future work, we will verify what we found in the present study in vivo and explore which subtype of PKC mediates the anti-inflammatory and antidepressant effects of ESK.
Briefly, in this investigation, we found that ESK reduces inflammation in BV-2 microglial cells treated with LPS, and the CB2-PKC pathway mediates anti-inflammation. These observations indicated that the antidepressant effects exerted by ESK may be mediated by the microglial CB2-PKC pathway.
Acknowledgements
This investigation was funded by the National Natural Science Foundation of China (Program No. 81801304).
Disclosure of conflict of interest
None.
References
- 1.Liu Y, Wang W, Huang X, Zhang X, Lin L, Qin JJ, Lei F, Cai J, Cheng B. Global disease burden of stroke attributable to high fasting plasma glucose in 204 countries and territories from 1990 to 2019: an analysis of the global burden of disease study. J Diabetes. 2022;14:495–513. doi: 10.1111/1753-0407.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Caballero L, Torres-Sanchez S, Romero-López-Alberca C, González-Saiz F, Mico JA, Berrocoso E. Monoaminergic system and depression. Cell Tissue Res. 2019;377:107–13. doi: 10.1007/s00441-018-2978-8. [DOI] [PubMed] [Google Scholar]
- 4.Molero P, Ramos-Quiroga JA, Martin-Santos R, Calvo-Sánchez E, Gutiérrez-Rojas L, Meana JJ. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018;32:411–420. doi: 10.1007/s40263-018-0519-3. [DOI] [PubMed] [Google Scholar]
- 5.Swainson J, Thomas RK, Archer S, Chrenek C, MacKay MA, Baker G, Dursun S, Klassen LJ, Chokka P, Demas ML. Esketamine for treatment resistant depression. Expert Rev Neurother. 2019;19:899–911. doi: 10.1080/14737175.2019.1640604. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Hong S, Cao J, Zhou Y, Xu X, Ai M, Kuang L. Hippocampal subfield volumes in major depressive disorder adolescents with a history of suicide attempt. Biomed Res Int. 2021;2021:5524846. doi: 10.1155/2021/5524846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175:327–335. doi: 10.1176/appi.ajp.2017.17060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozymski KM, Crouse EL, Titus-Lay EN, Ott CA, Nofziger JL, Kirkwood CK. Esketamine: a novel option for treatment-resistant depression. Ann Pharmacother. 2020;54:567–576. doi: 10.1177/1060028019892644. [DOI] [PubMed] [Google Scholar]
- 9.Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, Lane R, Lim P, Duca AR, Hough D, Thase ME, Zajecka J, Winokur A, Divacka I, Fagiolini A, Cubala WJ, Bitter I, Blier P, Shelton RC, Molero P, Manji H, Drevets WC, Singh JB. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893–903. doi: 10.1001/jamapsychiatry.2019.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepanichev M, Dygalo NN, Grigoryan G, Shishkina GT, Gulyaeva N. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res Int. 2014;2014:932757. doi: 10.1155/2014/932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villanueva R. Neurobiology of major depressive disorder. Neural Plast. 2013;2013:873278. doi: 10.1155/2013/873278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khakpai F, Ebrahimi-Ghiri M, Alijanpour S, Zarrindast MR. Ketamine-induced antidepressant like effects in mice: a possible involvement of cannabinoid system. Biomed Pharmacother. 2019;112:108717. doi: 10.1016/j.biopha.2019.108717. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira RCM, Castor MGM, Piscitelli F, Di Marzo V, Duarte IDG, Romero TRL. The involvement of the endocannabinoid system in the peripheral antinociceptive action of ketamine. J Pain. 2018;19:487–495. doi: 10.1016/j.jpain.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 15.Bie B, Wu J, Foss JF, Naguib M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol. 2018;31:407–414. doi: 10.1097/ACO.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Zuo Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell-surface protein level and activity of glutamate transporter type 3. Mol Pharmacol. 2005;67:1522–1533. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- 17.Jia J, Peng J, Li Z, Wu Y, Wu Q, Tu W, Wu M. Cannabinoid CB2 receptor mediates nicotine-induced anti-inflammation in N9 microglial cells exposed to β amyloid via protein kinase C. Mediators Inflamm. 2016;2016:4854378. doi: 10.1155/2016/4854378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Bea A, Walker MA, Hyde TM, Kleinman JE, Harrison PJ, Lane TA. Metabotropic glutamate receptor 3 (mGlu3; mGluR3; GRM3) in schizophrenia: antibody characterisation and a semi-quantitative western blot study. Schizophr Res. 2016;177:18–27. doi: 10.1016/j.schres.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szałach ŁP, Lisowska KA, Cubała WJ. The influence of antidepressants on the immune system. Arch Immunol Ther Exp (Warsz) 2019;67:143–151. doi: 10.1007/s00005-019-00543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kiliç C, Offord D, Ustun TB, Wittchen HU. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) surveys. Int J Methods Psychiatr Res. 2003;12:3–21. doi: 10.1002/mpr.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, Okazaki S, Yamashita S, Miura S, Miki H, Matsuda H, Koike M, Izuhara M, Araki T, Tsuchie K, Azis IA, Arauchi R, Abdullah RA, Oh-Nishi A, Horiguchi J. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin Neuropharmacol. 2018;41:151–155. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 22.Woelfer M, Kasties V, Kahlfuss S, Walter M. The role of depressive subtypes within the neuroinflammation hypothesis of major depressive disorder. Neuroscience. 2019;403:93–110. doi: 10.1016/j.neuroscience.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Mohanraj M, Sekar P, Liou HH, Chang SF, Lin WW. The mycobacterial adjuvant analogue TDB attenuates neuroinflammation via mincle-independent PLC-γ1/PKC/ERK signaling and microglial polarization. Mol Neurobiol. 2019;56:1167–1187. doi: 10.1007/s12035-018-1135-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, Yang J. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflammation. 2022;19:132. doi: 10.1186/s12974-022-02492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahimian R, Belliveau C, Chen R, Mechawar N. Microglial inflammatory-metabolic pathways and their potential therapeutic implication in major depressive disorder. Front Psychiatry. 2022;13:871997. doi: 10.3389/fpsyt.2022.871997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Huang Y, Ye W, Chen Z, Yuan Z. Cynaroside improved depressive-like behavior in CUMS mice by suppressing microglial inflammation and ferroptosis. Biomed Pharmacother. 2024;173:116425. doi: 10.1016/j.biopha.2024.116425. [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Wang J, Quan Q, Zhang H, Tian Y, Wang L, Liu L. Sestrin2 attenuates depressive-like behaviors and neuroinflammation in CUMS mice through inhibiting ferroptosis. Neuroreport. 2024;35:143–151. doi: 10.1097/WNR.0000000000001988. [DOI] [PubMed] [Google Scholar]
- 28.Wei X, Wang D, Liu J, Zhu Q, Xu Z, Niu J, Xu W. Interpreting the mechanism of active ingredients in polygonati rhizoma in treating depression by combining systemic pharmacology and in vitro experiments. Nutrients. 2024;16:1167. doi: 10.3390/nu16081167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisłowska-Stanek A, Kołosowska K, Maciejak P. Neurobiological basis of increased risk for suicidal behaviour. Cells. 2021;10:2519. doi: 10.3390/cells10102519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garakani A, Murrough JW, Freire RC, Thom RP, Larkin K, Buono FD, Iosifescu DV. Pharmacotherapy of anxiety disorders: current and emerging treatment options. Front Psychiatry. 2020;11:595584. doi: 10.3389/fpsyt.2020.595584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonaccorso S, Ricciardi A, Zangani C, Chiappini S, Schifano F. Cannabidiol (CBD) use in psychiatric disorders: a systematic review. Neurotoxicology. 2019;74:282–298. doi: 10.1016/j.neuro.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 32.García-Baos A, Alegre-Zurano L, Cantacorps L, Martín-Sánchez A, Valverde O. Role of cannabinoids in alcohol-induced neuroinflammation. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110054. doi: 10.1016/j.pnpbp.2020.110054. [DOI] [PubMed] [Google Scholar]
- 33.Wang S, Sun H, Liu S, Wang T, Guan J, Jia J. Role of hypothalamic cannabinoid receptors in post-stroke depression in rats. Brain Res Bull. 2016;121:91–97. doi: 10.1016/j.brainresbull.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176:428–438. doi: 10.1176/appi.ajp.2019.19020172. [DOI] [PubMed] [Google Scholar]
- 35.Doboszewska U, Wlaź P, Nowak G, Radziwoń-Zaleska M, Cui R, Młyniec K. Zinc in the monoaminergic theory of depression: its relationship to neural plasticity. Neural Plast. 2017;2017:3682752. doi: 10.1155/2017/3682752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pochwat B, Nowak G, Szewczyk B. An update on NMDA antagonists in depression. Expert Rev Neurother. 2019;19:1055–1067. doi: 10.1080/14737175.2019.1643237. [DOI] [PubMed] [Google Scholar]
- 37.Fu SY, Xiong RP, Peng Y, Zhang ZH, Chen X, Zhao Y, Ning YL, Yang N, Zhou YG, Li P. PKC mediates LPS-induced IL-1β expression and participates in the pro-inflammatory effect of A2AR under high glutamate concentrations in mouse microglia. Neurochem Res. 2019;44:2755–2764. doi: 10.1007/s11064-019-02895-1. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–650. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- 39.Olabiyi BF, Schmoele AC, Beins EC, Zimmer A. Pharmacological blockade of cannabinoid receptor 2 signaling does not affect LPS/IFN-γ-induced microglial activation. Sci Rep. 2023;13:11105. doi: 10.1038/s41598-023-37702-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmöle AC, Lundt R, Toporowski G, Hansen JN, Beins E, Halle A, Zimmer A. Cannabinoid receptor 2-deficiency ameliorates disease symptoms in a mouse model with Alzheimer’s disease-like pathology. J Alzheimers Dis. 2018;64:379–392. doi: 10.3233/JAD-180230. [DOI] [PubMed] [Google Scholar]