Abstract
Objectives: To analyze the factors influencing chronic pain in patients with knee osteoarthritis after total knee replacement surgery (TKRS) and to construct a nomogram risk prediction model, providing an economically effective screening method for clinical use. Methods: This retrospective study included 100 consecutive patients at the Jinan Central Hospital, with knee osteoarthritis who underwent TKRS from January 2023 to December 2023. Patients were divided into the observation group (n=55) and the control group (n=45) based on the presence of chronic pain. Logistic regression was performed to explore factors associated with chronic pain, including medical records, laboratory data, previous history, and independent clinical risk factors. The identified independent factors were then incorporated to construct a nomogram for chronic pain prediction. Results: Six variables were identified as independent predictors of chronic pain after TKRS: age, BMI, diabetes, severity of preoperative pain, severity of postoperative acute pain, and postoperative wound infection (P<0.05). The area under the curve (AUC) of this nomogram was 0.836 [95% confidence interval (CI): 0.615-0.884], demonstrating good calibration and clinical practicability. Conclusion: Age, BMI, diabetes, severity of preoperative pain, severity of postoperative acute pain, and postoperative wound infection are risk factors for chronic pain after TKRS. The predictive nomogram developed in this study shows good prediction ability and accuracy for chronic pain in patients with knee osteoarthritis after surgery.
Keywords: Chronic pain, knee osteoarthritis, total knee replacement surgery, nomogram
Introduction
Knee osteoarthritis is the most common chronic disease seen in orthopedics, predominantly affecting middle-aged and elderly women over 50 years old, with a prevalence rate of 25% to 40% [1,2]. The pathological characteristics of knee osteoarthritis include destruction of knee joint cartilage, leading to pain, stiffness, and reduced range of motion. This can result in the formation of bone spurs, inflammation, and osteophytes [3]. As the disease progresses, the joint space narrows, and surrounding tissues may become inflamed. Clinically, knee osteoarthritis presents symptoms such as pain, swelling, and knee joint stiffness [4]. When the condition worsens, total knee replacement surgery (TKRS) is the primary treatment option [5]. Currently, over 600,000 knee joint replacement surgeries are performed annually in the United States, and over 200,000 patients undergo TKRS each year in China [6].
A major complication after TKRS is postoperative chronic pain [7]. Studies indicate that approximately 10% to 34% of patients undergoing TKRS will experience chronic pain postoperatively, which can persist for months to years [8]. The International Association for the Study of Pain (IASP) defines chronic pain as pain persisting beyond the expected healing time, not attributable to other causes such as infection, surgical failure, or malignant tumor recurrence [9]. Multiple factors can influence the occurrence of chronic pain after knee replacement surgery. Research has investigated whether social factors such as gender, age, education level, economic status, and working conditions are related to chronic pain post-surgery [10]. For example, Duong et al. found that patients with lower education levels were three times more likely to experience chronic postsurgical pain (CPSP) [11]. Lower education levels may be associated with poorer pain awareness and coping abilities for the negative emotions brought about by pain [12].
However, some factors remain controversial. Some studies suggest that perioperative factors, such as the type of surgery, implant type, and surgery duration, have little impact on long-term pain outcomes [13]. In contrast, other studies indicate that surgery duration does affect the occurrence of chronic pain after knee replacement surgery [14]. Therefore, there is a need for more risk prediction tools to identify high-risk patients for chronic pain after TKRS in advance and to provide targeted interventions. The application of multidisciplinary and individualized intervention measures is essential to ensure optimal treatment for patients.
This study adopts a retrospective analysis to investigate the independent risk factors for chronic pain in patients with knee osteoarthritis after TKRS and to construct a clinical nomogram model. The aim is to provide a scientific basis for assessing the risk of chronic pain in patients with knee osteoarthritis after TKRS and to implement appropriate preventive measures, thereby reducing complications and improving postoperative quality of life for patients.
Materials and methods
Research design and participants
This retrospective study was reviewed and approved by the Ethics and Research Committee of Jinan Central Hospital.
Inclusion criteria: 1) Patients aged 18 and above. 2) Patients with knee osteoarthritis who underwent TKRS from January 2023 to December 2023.
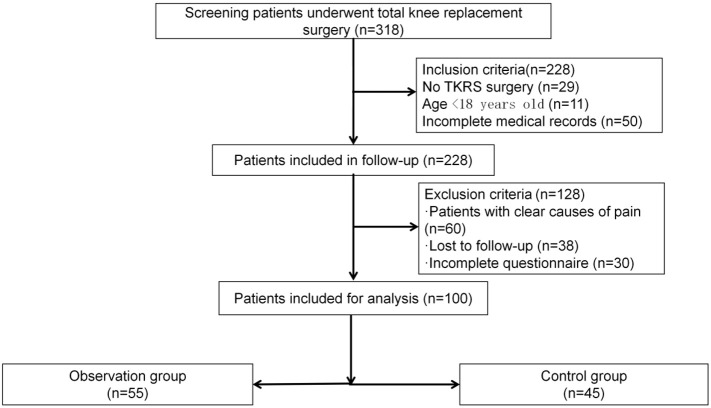
Exclusion criteria: 1) Patients with clear causes of pain such as a history of trauma, infection, previous knee surgery, postoperative deep vein thrombosis, diabetic peripheral neuropathy, or a history of chronic low back and leg pain. 2) Patients unable to cooperate due to communication barriers. 3) Patients with rheumatoid arthritis, a history of long-term drug abuse, muscle diseases or neurological disorders restricting lower limb movement, or mental disorders hindering questionnaire comprehension. Based on these criteria, 100 TKRS patients were included. The experimental design flowchart is shown in Figure 1.
Figure 1.
The flowchart of the experimental design. TKRS, total knee replacement surgery.
Definitions of chronic pain
The International Association for the Study of Pain (IASP) defines chronic pain as pain persisting for more than 3 months postoperatively, excluding other causes such as infection, surgical failure, or malignant tumor recurrence [15].
Data collection
Demographic and clinical characteristics were obtained from the hospital’s electronic case system, including: age, sex, education, body mass index (BMI), activities of daily living, length of disease, preoperative visual analog scale (VAS) score, distance of preoperative walking, hip-knee-ankle angle (HKA), medical complications (e.g., hypertension, diabetes, coronary heart disease, stroke), anxiety and depression, American Society of Anesthesiologists (ASA) score, New York Heart Association (NYHA) functional classification, operation time, amount of bleeding during surgery, transfusion, tourniquet time during surgery, single or double joint replacement, anesthesia and surgical data, presence of drainage tube after surgery, early postoperative pain intensity and duration, use of diazepam and dezocine during hospitalization, patient-controlled analgesia, postoperative medication and quadriceps muscle training, deep heat therapy during hospitalization, and postoperative VAS score. Hospitalization time was precisely recorded from admission to discharge to ensure accuracy and reliability.
Statistical analysis
SPSS 26.0 and R (R4.0.2) software were used for statistical analysis. The sample size was calculated by power analysis and estimated as: corrected sample size = sample size/(1 - [% attrition/100]), resulting in a sample size of around 100. All count and measurement variables were converted into categorical variables (percentage representation), and then single-factor and multi-factor binary logistic regression was used to analyze risk factors, determining independent risk factors for chronic pain after surgery. The least absolute shrinkage and selection operator (LASSO) regression method was used to select data dimensions and prognosis factors. Independent risk factors were introduced into R software to construct a nomogram model for predicting chronic pain after knee arthroplasty. The model was evaluated by calculating the C-index, drawing ROC curves, and calculating the area under the curve (AUC) to assess accuracy and discrimination, as well as drawing calibration curves to evaluate the model’s calibration for predicting chronic pain. The model was internally validated through bootstrapping. Decision curve analysis was used to evaluate clinical utility. A difference was considered statistically significant at P<0.05.
Results
Comparison of clinical characteristics between the observation and control group
The characteristics in terms of gender, educational level, disease duration, preoperative walking distance, hospitalization time, surgical time, unilateral/bilateral knee replacement, surgical bleeding volume, anesthesia ASA score, NYHA score, and physical therapy were similar in both groups (all P>0.05). However, significant differences were observed between the two groups in terms of age, body mass index (BMI), hip-knee-ankle angle, preoperative VAS score, postoperative functional exercise, diabetes, postoperative VAS score, and postoperative wound infection (all P<0.05) (Table 1).
Table 1.
Comparison of demographic and clinical characteristics between the observation and control group
| Influence factor | Observation group (n=55) | Control group (n=45) | t | P |
|---|---|---|---|---|
| Gender | 0.562 | 0.757 | ||
| Male | 12 (21.82%) | 10 (22.22%) | ||
| Female | 43 (78.18%) | 35 (77.78%) | ||
| Age | 10.285 | 0.003 | ||
| ≤60 | 5 (9.09%) | 4 (8.89%) | ||
| 60-70 | 17 (30.91%) | 15 (33.33%) | ||
| ≥70 | 33 (60.00%) | 26 (57.78%) | ||
| BMI | 8.474 | 0.010 | ||
| 18.5-23.9 | 10 (18.18%) | 9 (20.00%) | ||
| 24-27.9 | 23 (41.82%) | 22 (48.89%) | ||
| ≥28 | 22 (40.00%) | 14 (31.11%) | ||
| Educational level | 0.381 | 0.537 | ||
| Junior high school or below | 30 (54.55%) | 25 (25.56%) | ||
| Middle school above high school below | 18 (32.73%) | 12 (26.67%) | ||
| High school or above | 7 (12.72%) | 8 (17.77%) | ||
| Sleep | ||||
| Good | 20 (36.36%) | 32 (71.11%) | ||
| Commonly | 18 (32.73%) | 10 (22.22%) | ||
| Bad | 17 (30.91%) | 3 (6.67%) | ||
| Disease duration | 0.628 | 0.428 | ||
| <5 years | 18 (32.73%) | 16 (35.56%) | ||
| 5-10 years | 21 (38.18%) | 14 (31.11%) | ||
| >10 years | 16 (29.09%) | 15 (33.33%) | ||
| Preoperative walking distance | 0.186 | 0.666 | ||
| >100 kilometre | 40 (72.73%) | 34 (75.56%) | ||
| ≤100 kilometre | 15 (17.27%) | 11 (24.44%) | ||
| Hospitalization time | 0.247 | 0.619 | ||
| <11.3 | 27 (49.09%) | 24 (53.33%) | ||
| ≥11.3 | 28 (50.91%) | 21 (46.67%) | ||
| Hip knee ankle angle | 8.756 | 0.009 | ||
| -3(-3)-3(3) | 4 (7.27%) | 12 (26.67%) | ||
| 3-10/-10~-3 | 18 (32.73%) | 23 (51.11%) | ||
| ≥10 | 33 (60.00%) | 10 (22.22%) | ||
| Preoperative VAS score | 8.756 | 0.001 | ||
| <6 | 18 (32.73%) | 30 (66.67%) | ||
| ≥6 | 37 (62.27%) | 15 (33.33%) | ||
| Postoperative functional exercise | 0.342 | 0.036 | ||
| Good | 9 (16.36%) | 14 (31.11%) | ||
| Commonly | 22 (40.00%) | 16 (35.56%) | ||
| Bad | 24 (43.64%) | 15 (33.33%) | ||
| Diabetes | 11.286 | 0.007 | ||
| No | 15 (27.27%) | 21 (46.67%) | ||
| Had | 40 (72.73%) | 24 (53.33%) | ||
| Surgical time | 0.247 | 0.119 | ||
| <150 | 19 (34.55%) | 17 (37.78%) | ||
| 150-165 | 13 (23.64%) | 14 (31.11%) | ||
| ≥165 | 23 (41.81%) | 14 (31.11%) | ||
| Unilateral/Bilateral Knee Replacement | 2.206 | 0.137 | ||
| Unilateral | 45 (81.82%) | 32 (71.11%) | ||
| Bilateral | 10 (18.18%) | 23 (28.89%) | ||
| Surgical bleeding volume | 0.628 | 0.428 | ||
| ≤50 | 14 (25.45%) | 9 (20.00%) | ||
| 50-100 | 17 (30.91%) | 16 (35.56%) | ||
| 100-200 | 19 (34.55%) | 10 (22.22%) | ||
| >200 | 5 (9.09%) | 10 (22.22%) | ||
| Postoperative VAS score | 8.474 | 0.010 | ||
| <3 | 27 (49.09%) | 32 (71.11%) | ||
| ≥3 | 28 (50.91%) | 13 (28.89%) | ||
| Postoperative wound infection | 10.285 | 0.003 | ||
| No | 17 (30.91%) | 36 (57.78%) | ||
| Had | 38 (69.09%) | 19 (42.22%) | ||
| Anesthesia ASA score | 0.628 | 0.428 | ||
| ≤2 | 34 (61.82%) | 28 (62.22%) | ||
| ≥3 | 21 (38.18%) | 17 (37.78%) | ||
| Heart NYHA score | 0.381 | 0.537 | ||
| ≤2 | 37 (67.27%) | 28 (62.22%) | ||
| ≥3 | 18 (32.73%) | 17 (37.78%) | ||
| Physical therapy | 0.628 | 0.428 | ||
| No | 28 (50.91%) | 21 (46.67%) | ||
| Yes | 27 (49.09%) | 24 (53.33%) |
BMI, body mass index; VAS, visual analog scale; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association.
Univariate and multivariate analysis
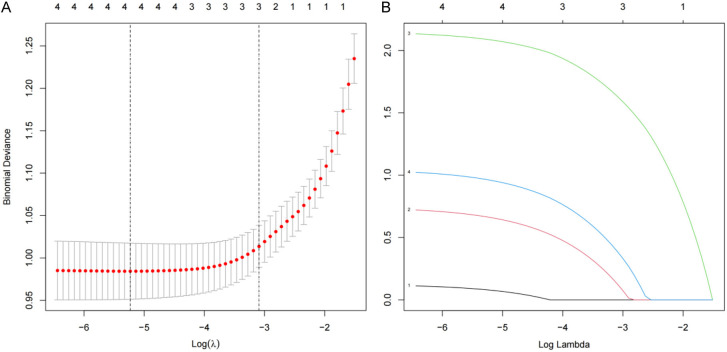
Univariate and multivariate analysis confirmed risk factors associated with chronic pain after TKRS surgery (Tables 2 and 3). Independent risk factors included: age (95% CI 0.0008-0.0321; P=0.0085), BMI (95% CI 1.6987-14.6525; P=0.0048), preoperative VAS score (95% CI 1.8974-38.2054; P=0.0074), postoperative VAS score (95% CI 1.2354-6.5974; P=0.0165), postoperative wound infection (95% CI 2.3247-12.2506; P=0.001), and diabetes (95% CI 1.5874-8.9874; P=0.0045). Additionally, a predictive model for chronic pain after TKRS was established using the LASSO regression technique for multivariate logistic regression analysis of the six variables (Figure 2).
Table 2.
Logistic univariate analysis
| Intercept and variables | Prediction model | ||||
|---|---|---|---|---|---|
|
| |||||
| Regression coefficient | SE | Wald | Odds ratio (95% CI) | P | |
| Age | -0.536 | 0.254 | 3.874 | 0.587 (0.354-0.998) | 0.048 |
| BMI | 0.874 | 0.223 | 14.587 | 2.356 (1.523-3.674) | 0.000 |
| Hip knee ankle angle | 1.087 | 0.278 | 15.981 | 2.987 (1.747-5.087) | 0.001 |
| Preoperative VAS score | 1.114 | 0.338 | 10.897 | 3.057 (1.587-5.980) | 0.001 |
| Postoperative VAS pain score | 1.487 | 0.354 | 17.985 | 4.652 (2.178-8.369) | 0.003 |
| Postoperative wound infection | 1.087 | 0.398 | 8.697 | 2.987 (1.421-6.038) | 0.001 |
| Postoperative functional exercise | 1.487 | 0.368 | 4.528 | 1.558 (1.023-3.754) | 0.037 |
| Diabetes | 0.987 | 0.368 | 4.129 | 1.987 (1.024-3.726) | 0.045 |
BMI, body mass index; VAS, visual analog scale.
Table 3.
Multivariate regression analysis
| Intercept and variables | Prediction model | ||
|---|---|---|---|
|
| |||
| Regression coefficient | Odds ratio (95% CI) | P | |
| Age | -5.1465 | 0.0059 (0.0008-0.0321) | 0.0085 |
| BMI | 1.5748 | 4.9874 (1.6987-14.6525) | 0.0048 |
| Preoperative VAS score | 1.9874 | 7.3354 (1.8974-38.2054) | 0.0074 |
| Postoperative VAS score | 1.0245 | 2.8974 (1.2354-6.5974) | 0.0165 |
| Postoperative wound infection | 1.5598 | 4.7852 (2.3247-12.2506) | 0.0001 |
| Diabetes | 1.2587 | 3.5478 (1.5874-8.9874) | 0.0045 |
BMI, body mass index; VAS, visual analog scale.
Figure 2.
Predictor selection using LASSO regression analysis with 10-fold cross-validation. A. Tuning parameter (lambda) selection of deviance in the LASSO regression based on the minimum criteria (left dotted line) and the 1-SE criteria (right dotted line); B. A coefficient profile plot was created against the log (lambda) sequence. LASSO, least absolute shrinkage and selection operator; SE, standard error.
Comparison of clinical baseline characteristics between training and validation sets
Patients were divided into a training set (n=71) and a validation set (n=29) in a 7:3 ratio. Characteristics such as age, BMI, gender, educational level, disease duration, preoperative walking distance, hospitalization time, surgical time, unilateral/bilateral knee replacement, surgical bleeding volume, anesthesia ASA score, NYHA score, and physical therapy were similar in both groups (all P>0.05). However, significant differences were found in hip-knee-ankle angle, preoperative VAS score, postoperative functional exercise, diabetes, postoperative VAS score, and postoperative wound infection (all P<0.05) (Table 4).
Table 4.
Comparison of clinical baseline characteristics between the training and validation sets
| Training set (n=71) | Validation set (n=29) | t/χ2 | P | |
|---|---|---|---|---|
| Gender | 0.197 | 0.874 | ||
| Male | 19 (26.76%) | 3 (10.34%) | ||
| Female | 52 (73.24%) | 26 (89.66%) | ||
| Age | 0.489 | 0.628 | ||
| ≤60 | 6 (8.45%) | 3 (10.34%) | ||
| 60-70 | 21 (29.58%) | 11 (37.93%) | ||
| ≥70 | 44 (6.97%) | 15 (51.72%) | ||
| BMI | 0.412 | 0.687 | ||
| 18.5-23.9 | 14 (19.72%) | 5 (17.24%) | ||
| 24-27.9 | 31 (43.66%) | 14 (48.28%) | ||
| ≥28 | 26 (36.62%) | 10 (34.48%) | ||
| Educational level | 0.347 | 0.741 | ||
| Junior high school or below | 38 (53.52%) | 17 (58.62%) | ||
| Middle school above high school below | 21 (29.58%) | 9 (31.04%) | ||
| High school or above | 12 (16.90%) | 3 (10.34%) | ||
| Sleep | 0.321 | 0.746 | ||
| Good | 36 (50.70%) | 16 (55.17%) | ||
| Commonly | 20 (28.17%) | 8 (27.59%) | ||
| Bad | 15 (21.13%) | 5 (17.24%) | ||
| Disease duration | 1.089 | 0.274 | ||
| <5 years | 23 (32.39%) | 11 (37.93%) | ||
| 5-10 years | 23 (32.39%) | 12 (41.38%) | ||
| >10 years | 25 (35.22%) | 6 (20.69%) | ||
| Preoperative walking distance | 1.234 | 0.066 | ||
| >100 kilometre | 50 (70.24%) | 24 (82.76%) | ||
| ≤100 kilometre | 21 (29.58%) | 5 (17.24%) | ||
| Hospitalization time | 1.089 | 0.274 | ||
| <11.3 | 37 (52.11%) | 17 (58.62%) | ||
| ≥11.3 | 34 (47.89%) | 12 (41.38%) | ||
| Hip knee ankle angle | 11.578 | 0.007 | ||
| -3(-3)-3(3) | 11 (15.49%) | 5 (17.24%) | ||
| 3-10/-10~-3 | 28 (39.44%) | 13 (44.83%) | ||
| ≥10 | 32 (45.07%) | 11 (37.93%) | ||
| Preoperative VAS pain score | 9.562 | 0.037 | ||
| <6 | 25 (35.21%) | 13 (44.83%) | ||
| ≥6 | 46 (64.79%) | 16 (55.17%) | ||
| Postoperative functional exercise | 8.074 | 0.032 | ||
| Good | 15 (21.13%) | 8 (27.59%) | ||
| Commonly | 26 (36.62%) | 12 (41.38%) | ||
| Bad | 30 (42.25%) | 9 (31.03%) | ||
| Diabetes | 7.406 | 0.028 | ||
| No | 21 (29.58%) | 15 (51.72%) | ||
| Had | 50 (70.42%) | 14 (48.28%) | ||
| Surgical time | 0.597 | 0.487 | ||
| <150 | 27 (38.03%) | 9 (31.03%) | ||
| 150-165 | 18 (25.35%) | 9 (31.03%) | ||
| ≥165 | 26 (36.62%) | 11 (37.94%) | ||
| Unilateral/Bilateral Knee Replacement | 2.874 | 0.074 | ||
| Unilateral | 50 (70.42%) | 7 (24.14%) | ||
| Bilateral | 21 (29.58%) | 22 (75.86%) | ||
| Surgical bleeding volume | 0.978 | 0.354 | ||
| ≤50 | 14 (19.72%) | 9 (31.03%) | ||
| 50-100 | 25 (35.21%) | 8 (27.59%) | ||
| 100-200 | 24 (33.80%) | 5 (17.24%) | ||
| >200 | 8 (11.27%) | 7 (24.14%) | ||
| Postoperative VAS score at | 10.687 | 0.005 | ||
| <3 | 42 (59.15%) | 11 (37.93%) | ||
| ≥3 | 29 (40.85%) | 18 (62.07%) | ||
| Postoperative wound infection | 6.142 | 0.045 | ||
| No | 36 (50.70%) | 17 (58.62%) | ||
| Had | 35 (49.30%) | 12 (41.38%) | ||
| Anesthesia ASA score | 0.468 | 0.524 | ||
| ≤2 | 42 (59.15%) | 20 (68.97%) | ||
| ≥3 | 29 (40.85%) | 9 (31.03%) | ||
| Heart NYHA score | 0.084 | 0.781 | ||
| ≤2 | 45 (63.38%) | 20 (68.97%) | ||
| ≥3 | 26 (36.62%) | 9 (31.03%) | ||
| Physical therapy | 0.247 | 0.619 | ||
| No | 36 (50.70%) | 13 (44.83%) | ||
| Yes | 35 (49.30%) | 16 (55.17%) |
TKRS, total knee replacement surgery; BMI, body mass index; VAS, visual analog scale; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association.
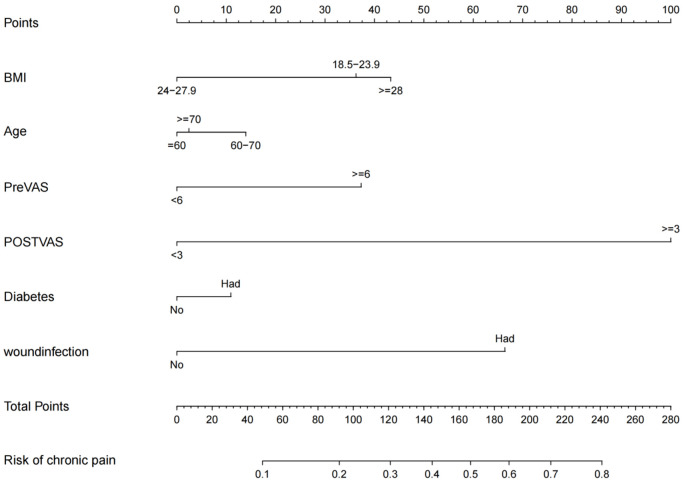
Development of the nomogram model
Risk predictors for chronic pain were included in a prediction model established using R software (R 3.6.3). The prediction probability corresponding to the sum of the integral of each factor was the risk value of chronic pain after TKRS (Figure 3). The regression equation model based on these factors was: logit (P) = -3.820 + 0.220 * age + BMI * 0.212 + preoperative VAS score * 0.219 + postoperative VAS score * 0.416 + postoperative wound infection * 0.328 + diabetes * 0.218.
Figure 3.
The nomogram for predicting the risk of chronic pain after TKRS. TKRS, total knee replacement surgery; BMI, body mass index; VAS, visual analog scale.
Discrimination and calibration of the prediction model
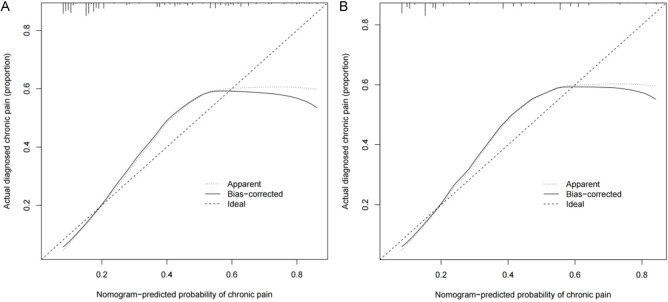
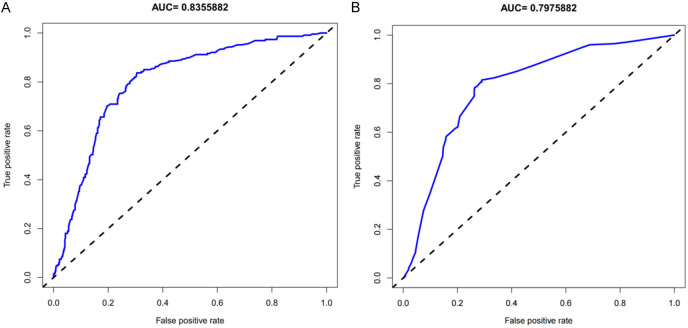
The unadjusted concordance index (C-index) for the training set was 0.792 [95% CI, 0.615-0.934], and for the validation set was 0.782 [95% CI, 0.703-0.924]. The calibration plot for the training and validation sets is shown in Figure 4. The AUC for the training set was 0.8355882 (Figure 5A), and the validation set was 0.7975882 (Figure 5B), indicating that the nomogram model had good discrimination and consistency in predicting the risk of chronic pain after TKRS.
Figure 4.
The calibration curves for predicting the risk of chronic pain after TKRS. A. The Training set; B. The Validation set. TKRS, total knee replacement surgery.
Figure 5.
ROC curves for the predicting the risk of chronic pain after TKRS. A. The Training set; B. The Validation set. TKRS, total knee replacement surgery.
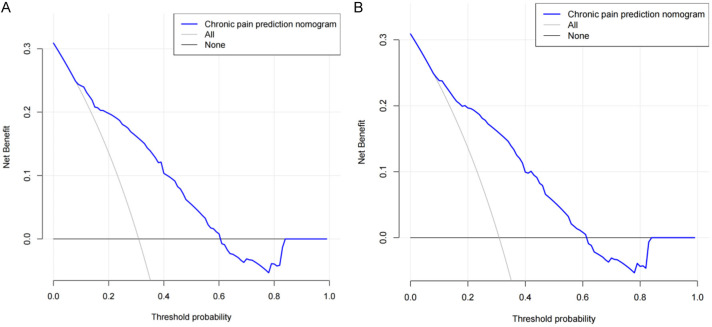
Decision curve analysis (DCA)
DCA demonstrated that if the threshold probability of chronic pain was 15% to 60%, the validity of the model was increased (Figure 6). DCA findings suggest that with a predicted occurrence probability of chronic pain after TKRS in the range of 15%-60%, application of the nomogram to predict the risk of chronic pain is more beneficial.
Figure 6.
Decision curve analysis for the predicting the risk of chronic pain after TKRS. A. The Training set; B. The Validation set. TKRS, total knee replacement surgery.
Discussion
This study found that age is an independent risk factor for chronic pain, with younger patients being more likely to experience it. Some scholars have observed that younger age can predict the severity of postoperative acute pain [16]. Several large-scale surveys have shown that the prevalence of chronic neuropathic pain in women is significantly higher than in men [17,18]. However, studies focusing on children have found that the incidence of chronic pain in children is lower compared to adults, which may be related to the greater plasticity of the central nervous system in children [19]. The higher incidence of chronic pain in young adults is estimated to be related to their higher levels of daily physical activity [20], and the pain perception system gradually decreases in function with age, leading to a decrease in sensitivity to pain in older individuals [21]. Therefore, younger adults should pay more attention to pain assessment and prevention before surgery.
Postoperative wound infection is also a significant risk factor for chronic pain. The presence of an infection at the surgical site can cause inflammation and tissue damage, resulting in persistent pain even after the infection has been treated [22]. Infections can also delay the healing process, leading to prolonged pain and discomfort [23]. Additionally, postoperative wound infections can increase the risk of complications such as implant loosening or failure, which can contribute to chronic pain in the affected joint. In some cases, the infection may spread to the surrounding tissues or bones, causing further damage and pain [24]. To prevent postoperative wound infections and subsequent chronic pain after TKRS, it is essential to follow proper infection control protocols before, during, and after the surgery. This includes ensuring a sterile surgical environment, using prophylactic antibiotics, and closely monitoring the wound for signs of infection postoperatively. Patients should also be educated on proper wound care and hygiene practices to reduce the risk of infection. Early detection and prompt treatment of any signs of infection are crucial in preventing complications and minimizing the risk of chronic pain following surgery.
Our research also found that the severity of postoperative acute pain is a risk factor for chronic pain. Studies have shown that acute pain during thoracotomy surgery is associated with the development of CPSP [25]. Other research has indicated that both the severity of acute postoperative pain and the dosage of analgesic drugs used postoperatively are the best predictors of chronic pain [26-29]. Intense early postoperative pain is a manifestation of a large number of noxious stimuli entering the central nervous system, leading to central sensitization. Therefore, even after peripheral noxious stimuli weaken or disappear, pain can persist for a long time [30]. It is evident that adequately controlling acute postoperative pain is an effective measure to reduce the occurrence of CPSP. However, despite the availability of various drugs and techniques for controlling early postoperative pain, managing acute postoperative pain remains a challenging issue in clinical practice [31]. Currently, there is no unified evidence to suggest that preemptive analgesia, local anesthetic techniques, or multimodal analgesia have significant advantages in reducing the severity of acute postoperative pain and the incidence of CPSP [32].
The severity of preoperative pain is also a risk factor for CPSP. Severe preoperative pain can result in central sensitization, where the nervous system becomes hypersensitive to pain signals, leading to an increased perception of pain postoperatively [33]. Additionally, preexisting inflammation and damage in the knee joint can contribute to persistent pain after surgery, as the surgical procedure may not completely resolve these underlying issues [34]. To prevent chronic pain after TKRS in patients with severe preoperative pain, several strategies can be implemented. Firstly, effective preoperative pain management, such as the use of medications, physical therapy, and other non-pharmacological interventions, can help reduce pain levels before surgery, potentially minimizing the risk of developing chronic pain postoperatively. Secondly, optimizing the surgical technique and postoperative care to minimize tissue damage and inflammation can also help reduce the likelihood of persistent pain after surgery. Lastly, providing comprehensive postoperative rehabilitation and pain management programs can aid in the recovery process and improve outcomes, potentially reducing the risk of chronic pain development.
A higher BMI increases the likelihood of experiencing chronic pain after TKRS. Excess body weight puts additional stress on the knee joint, leading to increased wear and tear on the joint components. This can result in a higher risk of complications during and after surgery, such as poor wound healing, infection, and implant failure, all of which can contribute to chronic pain [35]. Furthermore, individuals with a higher BMI often have poorer overall physical health, including reduced muscle strength and flexibility, which can affect their ability to recover effectively from surgery. This can lead to prolonged rehabilitation periods and a higher likelihood of developing chronic pain due to muscle imbalances and poor joint mechanics [36]. To prevent chronic pain after TKRS in individuals with a higher BMI, it is important to address these factors both before and after surgery. Preoperative interventions such as weight loss programs, physical therapy to improve muscle strength and flexibility, and optimizing overall health can help reduce the risk of complications and improve surgical outcomes. Postoperative rehabilitation should focus on gradual and progressive strengthening exercises, range of motion exercises, and proper biomechanics to promote optimal healing and reduce the likelihood of chronic pain development.
Diabetes is another risk factor for chronic pain after TKRS. Diabetes can lead to nerve damage, known as diabetic neuropathy, which can result in heightened sensitivity to pain and difficulty in pain management post-surgery [37]. Additionally, diabetes can impair the body’s ability to heal and recover, prolonging the post-operative pain experience. Furthermore, diabetic patients may have pre-existing vascular issues that can affect blood flow to the surgical site, leading to delayed healing and increased pain [38]. To prevent chronic pain in diabetic patients following TKRS, several strategies can be implemented. Firstly, optimizing blood sugar control before and after surgery is crucial in reducing the risk of complications and promoting proper healing. Close monitoring of blood glucose levels and adherence to a diabetic diet and medication regimen can help in this regard. Secondly, early mobilization and physical therapy post-surgery can aid in improving circulation, reducing inflammation, and promoting faster recovery, thereby minimizing the risk of chronic pain development. Lastly, proper pain management strategies, including the use of medications, physical therapy, and alternative therapies such as acupuncture or nerve blocks, should be tailored to the individual needs of diabetic patients to effectively manage post-operative pain and prevent the development of chronic pain.
This study also has certain limitations. Firstly, it is a retrospective study, which may result in lower validation strength. Additionally, since perioperative data of patients were collected in a retrospective manner, the completeness and consistency of the data cannot be fully guaranteed, making it difficult to comprehensively evaluate the factors contributing to the occurrence of chronic pain. Therefore, in future studies, we will adopt a prospective research design to further investigate this topic. We will comprehensively, objectively, and accurately collect clinical data, strengthen postoperative follow-up, increase the number and duration of follow-up visits, in order to obtain more convincing evidence to guide clinical practice.
In summary, we developed a clinical risk prediction model to determine the probability of developing chronic pain in patients with knee osteoarthritis after TKRS. Our prediction model showed good accuracy for both the development and validation datasets. Moreover, we determined that age, BMI, diabetes, the severity of preoperative pain and postoperative acute pain, and postoperative wound infection were the risk factors for chronic pain after TKRS.
Disclosure of conflict of interest
None.
References
- 1.Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384:51–59. doi: 10.1056/NEJMcp1903768. [DOI] [PubMed] [Google Scholar]
- 2.Reichenbach S, Felson DT, Hincapié CA, Heldner S, Bütikofer L, Lenz A, da Costa BR, Bonel HM, Jones RK, Hawker GA, Jüni P. Effect of biomechanical footwear on knee pain in people with knee osteoarthritis: the BIOTOK randomized clinical trial. JAMA. 2020;323:1802–1812. doi: 10.1001/jama.2020.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu KT, Wang YW, Wu RW, Huang CC, Chen YC. Association of a femoral neck T score with knee joint osteophyte formation but not with skeletal muscle mass. Clin Rheumatol. 2023;42:917–922. doi: 10.1007/s10067-022-06410-w. [DOI] [PubMed] [Google Scholar]
- 4.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325:568–578. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AT, Bronsther CI, Stanley EE, Paltiel AD, Sullivan JK, Collins JE, Neogi T, Katz JN, Losina E. The value of total knee replacement in patients with knee osteoarthritis and a body mass index of 40 kg/m2 or greater: a cost-effectiveness analysis. Ann Intern Med. 2021;174:747–757. doi: 10.7326/M20-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez PM, McKeon JF, Spitzer AI, Krueger CA, Pigott M, Li M, Vajapey SP. Race, Utilization, and outcomes in total hip and knee arthroplasty: a systematic review on health-care disparities. JBJS Rev. 2022;10:e21. doi: 10.2106/JBJS.RVW.21.00161. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-de-Las-Peñas C, Florencio LL, de-la-Llave-Rincón AI, Ortega-Santiago R, Cigarán-Méndez M, Fuensalida-Novo S, Plaza-Manzano G, Arendt-Nielsen L, Valera-Calero JA, Navarro-Santana MJ. Prognostic factors for postoperative chronic pain after knee or hip replacement in patients with knee or hip osteoarthritis: an umbrella review. J Clin Med. 2023;12:6624–6640. doi: 10.3390/jcm12206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jurewicz A, Gasiorowska A, Leźnicka K, Pawlak M, Sochacka M, Machoy-Mokrzyńska A, Bohatyrewicz A, Maciejewska-Skrendo A, Pawlus G. Individual factors modifying postoperative pain management in elective total hip and total knee replacement surgery. Life (Basel) 2024;14:211. doi: 10.3390/life14020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, Keefe FJ, Mogil JS, Ringkamp M, Sluka KA, Song XJ, Stevens B, Sullivan MD, Tutelman PR, Ushida T, Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalid S, Mohammad HR, Gooberman-Hill R, Garriga C, Pinedo-Villanueva R, Arden N, Price A, Wylde V, Peters TJ, Blom A, Judge A. Post-operative determinants of chronic pain after primary knee replacement surgery: analysis of data on 258,386 patients from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man (NJR) Osteoarthr Cartil Open. 2021;3:100139. doi: 10.1016/j.ocarto.2021.100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duong V, Oo WM, Ding C, Culvenor AG, Hunter DJ. Evaluation and treatment of knee pain: a review. JAMA. 2023;330:1568–1580. doi: 10.1001/jama.2023.19675. [DOI] [PubMed] [Google Scholar]
- 12.Pinedo-Villanueva R, Khalid S, Wylde V, Gooberman-Hill R, Soni A, Judge A. Identifying individuals with chronic pain after knee replacement: a population-cohort, cluster-analysis of Oxford knee scores in 128,145 patients from the English National Health Service. BMC Musculoskelet Disord. 2018;19:354. doi: 10.1186/s12891-018-2270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sellevold VB, Olsen U, Lindberg MF, Steindal SA, Aamodt A, Lerdal A, Dihle A. “I am accustomed to something in my body causing pain”: a qualitative study of knee replacement non-improvers’ stories of previous painful and stressful experiences. BMC Musculoskelet Disord. 2023;24:305. doi: 10.1186/s12891-023-06423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podmore B, Hutchings A, Konan S, Robson J, van der Meulen J. Access to hip and knee replacement surgery in patients with chronic diseases according to patient-reported pain and functional status. BMC Health Serv Res. 2020;20:602. doi: 10.1186/s12913-020-05464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11) Pain. 2019;160:19–27. doi: 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 16.Ashoorion V, Sadeghirad B, Wang L, Noori A, Abdar M, Kim Y, Chang Y, Rehman N, Lopes LC, Couban RJ, Aminilari M, Malektojari A, Ghazizadeh S, Rehman Y, Ghasemi M, Adili A, Guyatt GH, Busse JW. Predictors of persistent post-surgical pain following total knee arthroplasty: a systematic review and meta-analysis of observational studies. Pain Med. 2023;24:369–381. doi: 10.1093/pm/pnac154. [DOI] [PubMed] [Google Scholar]
- 17.Şahin F, Beyaz SG, Karakuş N, İnanmaz ME. Total knee arthroplasty postsurgical chronic pain, neuropathic pain, and the prevalence of neuropathic symptoms: a prospective observational study in Turkey. J Pain Res. 2021;14:1315–1321. doi: 10.2147/JPR.S293856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groenewald CB, Murray CB, Battaglia M, Scaini S, Quinn PD. Prevalence of pain management techniques among adults with chronic pain in the United States, 2019. JAMA Netw Open. 2022;5:e2146697. doi: 10.1001/jamanetworkopen.2021.46697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hechler T, Wager J, Zernikow B. Chronic pain treatment in children and adolescents: less is good, more is sometimes better. BMC Pediatr. 2014;14:262. doi: 10.1186/1471-2431-14-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson EMJ, Omura JD, Boring MA, Odom EL, Foster AL, Olivari BS, McGuire LC, Croft JB. Prevalence and characteristics of arthritis among caregivers - 17 states, 2017 and 2019. MMWR Morb Mortal Wkly Rep. 2022;71:1389–1395. doi: 10.15585/mmwr.mm7144a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson JA, Crow JA, Johnson AJ, Meng L, Rani A, Huo Z, Foster TC, Fillingim RB, Cruz-Almeida Y. Pain interference mediates the association between epigenetic aging and grip strength in middle to older aged males and females with chronic pain. Front Aging Neurosci. 2023;15:1122364. doi: 10.3389/fnagi.2023.1122364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazzaniga S, Real G, Finazzi S, Lorini LF, Forget P, Bugada D. How to modulate peripheral and central nervous system to treat acute postoperative pain and prevent pain persistence. Curr Neuropharmacol. 2024;22:23–37. doi: 10.2174/1570159X21666230810103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alven S, Peter S, Aderibigbe BA. Polymer-based hydrogels enriched with essential oils: a promising approach for the treatment of infected wounds. Polymers (Basel) 2022;14:3772. doi: 10.3390/polym14183772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiraga SI, Itokazu T, Nishibe M, Yamashita T. Neuroplasticity related to chronic pain and its modulation by microglia. Inflamm Regen. 2022;42:15. doi: 10.1186/s41232-022-00199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian MP, Dong MR, Li J, Kang F. The duration of chronic low back pain is associated with acute postoperative pain intensity in lumbar fusion surgery: a prospective observational study. BMC Anesthesiol. 2022;22:129. doi: 10.1186/s12871-022-01674-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Drongelen S, Wesseling M, Holder J, Meurer A, Stief F. Knee load distribution in hip osteoarthritis patients after total hip replacement. Front Bioeng Biotechnol. 2020;8:578030. doi: 10.3389/fbioe.2020.578030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hah JM, Cramer E, Hilmoe H, Schmidt P, McCue R, Trafton J, Clay D, Sharifzadeh Y, Ruchelli G, Goodman S, Huddleston J, Maloney WJ, Dirbas FM, Shrager J, Costouros JG, Curtin C, Mackey SC, Carroll I. Factors associated with acute pain estimation, postoperative pain resolution, opioid cessation, and recovery: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2019;2:e190168. doi: 10.1001/jamanetworkopen.2019.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skrejborg P, Petersen KK, Beck J, Ulrich M, Simonsen O, Nielsen PT, Arendt-Nielsen L, Laursen M. Investigating the effect of perioperative chlorzoxazone on acute postoperative pain after total hip and knee replacement surgery. Clin J Pain. 2020;36:352–358. doi: 10.1097/AJP.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 29.Cao L, Yang H, Sun K, Wang H, Fan H, Cheng W. The role of knee position in blood loss and enhancement of recovery after total knee arthroplasty. J Knee Surg. 2021;34:1304–1309. doi: 10.1055/s-0040-1708042. [DOI] [PubMed] [Google Scholar]
- 30.Price AJ, Alvand A, Troelsen A, Katz JN, Hooper G, Gray A, Carr A, Beard D. Knee replacement. Lancet. 2018;392:1672–1682. doi: 10.1016/S0140-6736(18)32344-4. [DOI] [PubMed] [Google Scholar]
- 31.Ursavaş FE, Yaradılmış YU. Relationship between pain beliefs and postoperative pain outcomes after total knee and hip replacement surgery. J Perianesth Nurs. 2021;36:187–193. doi: 10.1016/j.jopan.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Yajnik M, Hill JN, Hunter OO, Howard SK, Kim TE, Harrison TK, Mariano ER. Patient education and engagement in postoperative pain management decreases opioid use following knee replacement surgery. Patient Educ Couns. 2019;102:383–387. doi: 10.1016/j.pec.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Hanley AW, Gililland J, Erickson J, Pelt C, Peters C, Rojas J, Garland EL. Brief preoperative mind-body therapies for total joint arthroplasty patients: a randomized controlled trial. Pain. 2021;162:1749–1757. doi: 10.1097/j.pain.0000000000002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Bruggen W, Hirschmann MT, Strobel K, Kampen WU, Kuwert T, Gnanasegaran G, Van den Wyngaert T, Paycha F. SPECT/CT in the postoperative painful knee. Semin Nucl Med. 2018;48:439–453. doi: 10.1053/j.semnuclmed.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Puijk R, Sierevelt IN, Pijls BGCW, Spekenbrink-Spooren A, Nolte PA. Increased risk of aseptic loosening for posterior stabilized compared with posterior cruciate-retaining uncemented total knee replacements: a cohort study of 13,667 knees from the Dutch Arthroplasty Registry. Acta Orthop. 2023;94:600–606. doi: 10.2340/17453674.2023.33283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulford JS, Ackerman I, Holder C, Cashman KS, Graves SE, Harris IA. The association between body mass index and patient-reported outcome measures before and after primary total hip or knee arthroplasty: a registry. ANZ J Surg. 2023;93:1665–1673. doi: 10.1111/ans.18449. [DOI] [PubMed] [Google Scholar]
- 37.Lai FTT, Yip BHK, Hunter DJ, Rabago DP, Mallen CD, Yeoh EK, Wong SY, Sit RW. Metformin use and the risk of total knee replacement among diabetic patients: a propensity-score-matched retrospective cohort study. Sci Rep. 2022;12:11571. doi: 10.1038/s41598-022-15871-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbass Reslan H, Moustafa SM, Saghieh S, Sharara ES, Badr LK. Does intervention improve the outcomes of patients after total knee replacement surgery? Int J Orthop Trauma Nurs. 2018;31:26–31. doi: 10.1016/j.ijotn.2018.08.001. [DOI] [PubMed] [Google Scholar]