Abstract
Lung cancer is the most prevalent and lethal disease globally, with approximately 80% of cases being non-small cell lung cancer (NSCLC). NSCLC is primarily composed of lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD). Despite chemotherapy currently being the primary treatment for NSCLC, chemotherapy resistance remains a significant challenge for patients. Recent studies have proposed immunotherapy as a promising new avenue for treating NSCLC. The association between the lysyl oxidase-like 2 (LOXL2) gene and NSCLC was explored using multiple online tools and bioinformatics analysis software based on the available datasets from TCGA. The immune microenvironment of the tumor was explored by calculating ImmuneScore, StromalScore, and TumorPurity of LUAD and LUSC and analyzing the infiltration of 22 immune cells in lung cancer tissues. LOXL2-related loads were obtained from the Xena database for LUSC and LUAD patients, and relevant prognostic genes were identified by analyzing survival curves. Functional and pathway enrichment analyses of prognostic, predictive genes were performed using Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG). The expression of LOXL2 in NSCLC was detected by RT-qPCR. LOXL2 may be involved in the progression of LUAD and LUSC and is closely related to the T-lymphocyte subpopulation, T-reg cells. SEMA7A and VEGFC are identified as the genes that interact with LOXL2 and could be used as prognostic signature genes in NSCLC patients. LOXL2 may become a prognostic marker and a new target for immunotherapy.
Keywords: NSCLC, bioinformatics, immune infiltration, LOXL2, doxorubicin
Introduction
Lung cancer stands as a leading cause of cancer-related mortality worldwide, contributing to an estimated 26% of global cancer-related fatalities [1-3]. Amongst all lung cancer cases, non-small cell lung cancer (NSCLC) accounts for roughly 80%, further classify into lung squamous cell carcinoma (LUSC) and lung adenocarcinoma (LUAD) [4,5]. Decades of intensive genomic and signaling pathway research have revealed that NSCLC constitutes a diverse group of diseases characterized by genetic and cellular heterogeneity [6], primarily encompassing LUSC and LUAD [7]. Notably, early-stage NSCLC often exhibits mild symptoms and clinical signs, posing challenges for early detection. The aggressive nature and rapid progression of NSCLC frequently lead to patients presenting with locally advanced or metastatic disease at diagnosis [8].
Surgical resection is the preferred therapeutic modality for patients diagnosed with early-stage NSCLC, whereas a multimodal approach combining radiotherapy and chemotherapy is typically employed for those presenting with advanced, inoperable disease stages [9]. However, resistance to chemotherapeutic agents such as cisplatin has been a significant challenge, necessitating the development of new approaches to treat NSCLC [10]. In recent years, there has been growing recognition of the benefits of harnessing the host’s immune system to combat malignancy [11,12]. The emergence of immunotherapy has revolutionized the landscape of NSCLC treatment by capitalizing on this concept [13]. The underlying principle of immunotherapy involves the stimulation of the patient’s endogenous T-cells and the subsequent release of cytokines, which facilitate the targeted destruction of tumor cells [14]. At present, the principal immune checkpoints are PD-1 and PD-2 [15]. Prior investigations have demonstrated the promise of neoadjuvant immunotherapy in patients with substantial tumor burden [16]. Furthermore, an intricate interplay between regulatory T-cells (Tregs) and the tumor microenvironment has been well-documented in numerous studies, underscoring the importance of further exploration into novel targets for immunotherapy [17]. It is therefore of interest to explore new targets for the immunotherapy of NSCLC. The expression of LOXL2 is markedly elevated in tumors, contributing to tumor invasion and migration [18]. However, the utility of LOXL2 as a target for immunotherapy in NSCLC remains uncharted territory and merits further investigation.
In this study, we conducted a bioinformatics analysis of the risk factors influencing the prognosis of non-small cell lung cancer (NSCLC) using differential gene expression, survival curves, the tumor immune microenvironment, associations with other molecules, and KEGG and GO analysis. Furthermore, RT-qPCR analysis demonstrated that LOXL2 was highly expressed in NSCLC. The aforementioned results indicate that LOXL2 may serve as a promising target for immunotherapy in NSCLC patients.
Material and methods
Gene expression data analysis
Inclusion criteria for this study were as follows: (a) pathologic diagnosis of NSCLC (biopsy or surgically resected tissue), (b) no experience of immunotherapy and other radiotherapy, and (c) having complete gene sequencing results. The exclusion criteria were as follows: (a) incomplete information; (b) combination with other tumors. In the TCGA database, GEPIA identifies the top 500 differentially expressed genes (DEGs) in LUAD and LUSC patients, and subsequently identified 11 commonly related genes by intersecting the results. Subsequently, Kaplan-Meier plotter 2 was employed to assess the overall survival (OS) of LUAD and LUSC cancer patients. The samples were divided into two groups according to gene expression levels in order to determine the significance of each gene in predicting the patient’s prognosis. Subsequently, a Kaplan-Meier survival curve was constructed to compare the two groups and contrast the log10HR value (HRs) and P-value. The two risk factors, LOXL2 and TLDC1, with the most unfavorable prognosis in LUAD and LUSC, were identified. A total of 483 cases of lung adenocarcinoma (LUAD) and 486 cases of lung squamous cell carcinoma (LUSC) were included in the analysis from the GEPIA database. The cases were designated as the “tumor group”, and the expression of LOXL2 in this group was compared with that of the “control group”.
Tumor purity and immune cell infiltration analysis
The ESTIMATE algorithm was employed to calculate ImmuneScore, StromalScore, and Tumor Purity for LUAD and LUSC patients, respectively. The proportion of 22 TILs in LUSC and LUAD was determined using the R package CIBERSORT. Furthermore, GEPIA2021 was employed to visualize gene expression profiles in order to explore the differences in immune cell subtypes. The immune cells included in this study were neutrophils, eosinophils, mast cells, and mast cells in different states of activation. The following cell types were identified: dendritic cells activated, dendritic cells quiescent, macrophages M2, T cell follicular helper cells, T cell regulatory (Tregs), T cell γδ, NK cells quiescent, NK cells activated, monocytes, macrophages M0, macrophages M1, T cell CD4 memory activated, T cell CD4 memory quiescent, T cell CD4 naive, T cell CD8, plasma cells, B cell memory, and B cell naive.
Immune prognosis analysis of LOXL2
ssGSEA was used to analyze the immune profile of LOXL2 in LUAD and LUSC, and the tumor tissues of LUAD and LUSC in the GEPIA database were compared to the adjacent tissues in normal lungs. The results demonstrated a significant positive correlation between LUAD and T-reg (rho=0.311, P=5.71e-13) and between LUSC and T-reg (rho=0.494, P<2.2e-16).
Correlated gene analysis
LOXL2-associated genes from LUAD and LUSC patients were obtained using the Xena database, P-values and R-values were calculated, and differentially expressed genes (DEGs) were analyzed. Cross-tabulation analysis was performed on genes that were upregulated in LOXL2-associated genes in LUAD and LUSC patients. The eight cross-over genes associated with OS were extracted by univariate Cox regression analysis of the results and then the median of their characteristic scores. Patients were finally categorized as high and low risk. Patient survival was analyzed by the Kaplan-Meier method. Time-dependent receiver operating characteristic (ROC) curves were used to predict the accuracy and characteristics of clinical characteristics. The R package rms was employed to generate the nomograms.
Genetic interaction analysis
The search tool for interacting genes/proteins (STRING) database (http://cn.string-bd.org) was used to establish a PPI network of LOXL2.
Gene enrichment analysis
GO and KEGG analysis was carried out by using the R package “clusterProfiler”. Gene Ontology (GO) was used for functional enrichment analysis, while the Kyoto Encyclopedia of Genes and Genomes (KEGG) was used for pathway enrichment analysis. The effects of functional enrichment included molecular functions (MF), cellular components (CC), and biological processes (BP). The top 10 pathway enrichment was analyzed.
Drug sensitivity analysis
We used the “oncoPredict” R package to analyze the batch-corrected prioritization of different drugs in the DREAMT database.
Cell culture
Human bronchial epithelial cell line, human non-small cell lung cancer cell line PC9, A549, H1299 were provided by Jiangsu Province Key Laboratory of Geriatrics, and cultured in DMEM (Sigma-Aldrich) supplemented with 100 μg/ml streptomycin, 100 μg/ml penicillin (Gibco), and 10% fetal bovine serum (FBS) (Invitrogen). All cells were maintained in a 5% CO2 incubator (Thermo Fisher Scientific) at 37°C. Cells in the logarithmic growth phase were used for experiments.
RT-PCR
Total RNA was extracted from the cells using Trizol reagent (Invitrogen). RNA was reverse transcribed to cDNA using Primescript™ RT Master Mix (Vazyme). Quantitative RT-PCR was performed using ChamQTM Universal SYBR QPCR Master Mix (Vazyme) and Steppe One PlusTM Real-Time PCR System (Application Biosystems, Foster City, CA, USA). The primers for LOXL2 and GAPDH (used as an internal reference) were purchased from Genscript. RT-PCR was performed under the following conditions: Fluorescence signal was acquired at 94° for 60 seconds, 95° for 10 seconds and 60° for 30 seconds, and after 40 cycles, 60° were acquired. Target gene expression levels were normalized to GAPDH expression and then calculated using the 2-ΔΔCt method. The primer sequences are listed in Table 1.
Table 1.
List of primers
| Gene | Primer (5’-3’) |
|---|---|
| LOXL2 | F: AACGAGGCGACCCTTGCAGC |
| R: GGGTGCGCTTGCGGTAGGTT | |
| GAPDH | F: CTCCTCCACCTTTGACGC |
| R: CCACCACCCTGTTGCTGT |
Cell viability
The Cell Counting Kit-8 (Vazyme) was performed to detect cell proliferation. We purchased doxorubicin from Beijing Huamei Company and used it to treat 1×10^4 cells in a 96-well plate. The cells were exposed to different concentrations of doxorubicin for 24 hours. 10 μl CCK8 reagent was added into each well according to the instruction of CCK8. Then, absorbance of samples was detected at 450 nm wavelength.
Statistical analysis
Kaplan-Meier plots used HR and P-value or Cox P-value for the log-rank test. Comparisons were made using unpaired and paired t-tests for normally distributed variables or Mann-Whitney U-test and Wilcoxon signed-rank test for non-normally distributed variables. R software was used to perform all statistical analyses. P-value <0.05 was considered significant.
Results
Expression feature of LOXL2
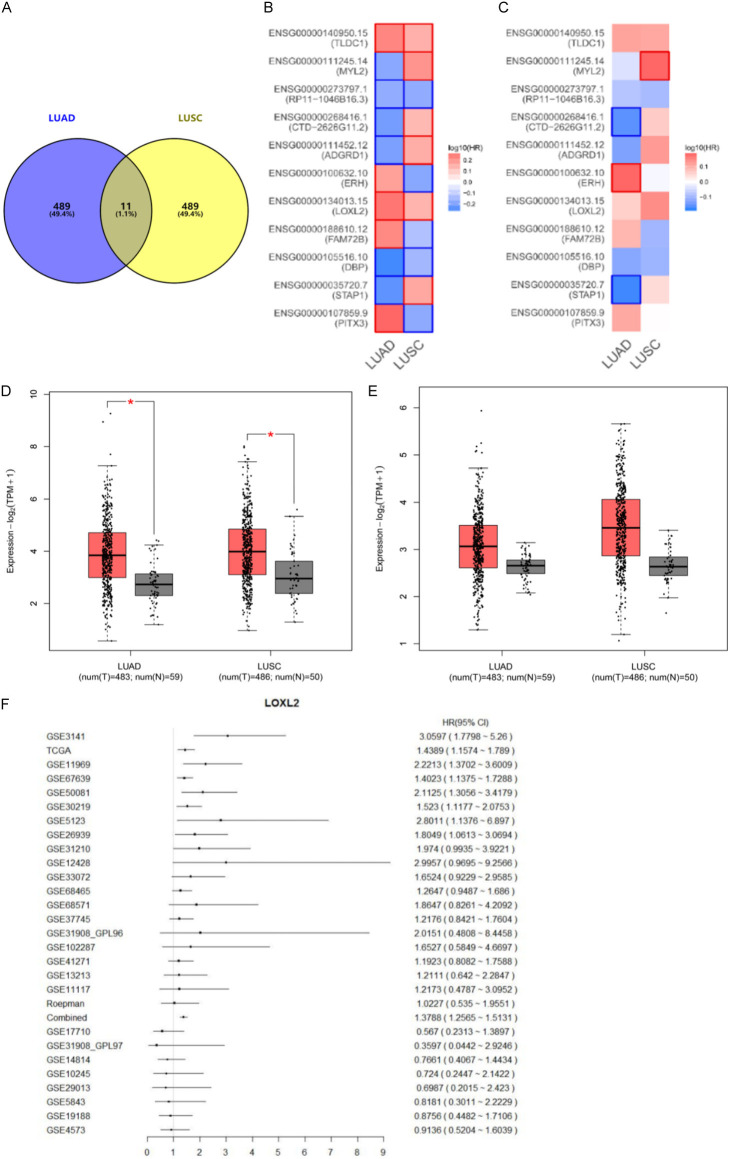
We identified the top 500 DEGs in LUAD and LUSC from the TCGA databases. We crossed them to obtain eleven common DEGs, including PITX3, STAP1, DBP, FAM72B, LOXL2, ERH, ADGRD1, CTD-2626G11.2, RP11-1046B16.3, MYL2, TLDC1 (Figure 1A). Next, we used Kaplan-Meier Plotter and GEPIA database to explore the relationship between this set of genetic changes and the survival rate of LUAD and LUDSC patients (Figures S1, S2). We found six protective factors: STAP1, DBP, ADGRD1, CTD-2626G11.2, 1046B16.3, MYL2 and five risk factors: PITX3, FAM72B, LOXL2, ERH, TLDC1. Among these, we focused on two risk factors LOXL2 and TLDC1, which had high-risk values in LUAD and LUSC (Figure 1B, 1C). Based on the GEPIA dataset, we analyzed LOXL2 and TLDC1 in lung cancer tissues and adjacent tissues. We included lung cancer cases (n=483 for LUAD; n=486 for LUSC) as the “tumor group”. Compared to the “control group”, we found that the expression level of LOXL2 in the “tumor group” was higher (Figure 1D, 1E).
Figure 1.
LOXL2 is highly expressed in non-small cell lung cancer. A. Venn diagrams of the top 500 abnormally expressed genes related to LUAD and LUSC (R package analysis). B. Survival analysis of 11 intersection gene expression levels in LUAD and LUSC. C. LOXL2 and TLDC1 are associated with shorter overall survival. D, E. The level of LOXL2 in LUAD and LUAC cancer tissues is higher than in para cancer. F. Univariate analysis was used to analyze the correlation between LOXL2 expression and clinical prognosis in NSCLC patients.
Furthermore, we verified that LOXL2 was highly expressed in LUAD and LUSC by calculating the p-value, HR value, and 95% cl of the LOXL2 gene in the Oncomine database and TCGA database (P<0.05) (Figure 1F; Table 2).
Table 2.
Univariate analysis of the correlation between LOXL2 expression and clinical features and OS in LUAD and LUSC patients
| Dataset | P value | HR | 95% CI | Prognostic | |
|---|---|---|---|---|---|
| GSE3141 | <0.0001 | 3.0597 | 1.7798-5.2600 | Poor | Go |
| TCGA | 0.0011 | 1.4389 | 1.1574-1.7890 | Poor | Go |
| GSE11969 | 0.0012 | 2.2213 | 1.3702-3.6009 | Poor | Go |
| Stage l | 0.1672 | 1.7682 | 0.7876-3.9693 | ||
| Stage ll | 0.516 | 1.5434 | 0.4166-5.7188 | ||
| Stage lll | 0.0014 | 3.4612 | 1.6128-7.4280 | Poor | Go |
| GSE67639 | 0.0015 | 1.4023 | 1.1375-1.7288 | Poor | Go |
| GSE50081 | 0.0023 | 2.1125 | 1.3056-3.4179 | Poor | Go |
| Stage l | 0.0428 | 1.8640 | 1.0203-3.4056 | Poor | Go |
| Stage ll | 0.1498 | 1.7766 | 0.8127-3.8839 | ||
| GSE30219 | 0.0077 | 1.5230 | 1.1177-2.0753 | Poor | Go |
| GSE5123 | 0.0251 | 2.8011 | 1.1376-6.8970 | Poor | Go |
| Stage l | 0.4481 | 1.7116 | 0.4269-6.8620 | ||
| Stage ll | 0.2313 | 2.4212 | 0.5692-10.3000 | ||
| Stage lll | 0.2918 | 3.6458 | 0.3290-40.3971 | ||
| GSE26939 | 0.0293 | 1.8049 | 1.0613-3.0694 | Poor | Go |
| Stage l | 0.1121 | 1.8641 | 0.8646-4.0194 | ||
| Stage lll | 0.1187 | 2.6001 | 0.7828-8.6362 | ||
| GSE31210 | 0.0522 | 1.9740 | 0.9935-3.9221 | ||
| Stage l | 0.0015 | 4.7791 | 1.8171-12.5698 | Poor | Go |
| Stage ll | 0.3536 | 0.5551 | 0.1601-1.9252 | ||
| GSE12428 | 0.0566 | 2.9957 | 0.9695-9.2566 | Go | |
| GSE33072 | 0.0910 | 1.6524 | 0.9229-2.9585 | Go | |
| GSE68465 | 0.1093 | 1.2647 | 0.9487-1.6860 | Go | |
| GSE68571 | 0.1336 | 1.8647 | 0.8261-4.2092 | Go | |
| Stage l | 0.2186 | 1.9920 | 0.6646-5.9708 | ||
| Stage ll | 0.4599 | 0.5556 | 0.1169-2.6405 | ||
| GSE17710 | 0.2148 | 0.5670 | 0.2313-1.3897 | Go | |
| Stage l | 0.6453 | 1.2765 | 0.4514-3.6101 | ||
| Stage ll | 0.0807 | 0.1560 | 0.0194-1.2551 | ||
| GSE37745 | 0.2954 | 1.2176 | 0.8421-1.7604 | Go | |
| Stage l | 0.3817 | 1.2318 | 0.7721-1.9651 | ||
| GSE37745 | 0.2954 | 1.2175 | 0.8421-1.7604 | Go | |
| Stage l | 0.3817 | 1.2176 | 0.7721-1.9651 | ||
| Stage ll | 0.2517 | 1.2318 | 0.7032-3.8342 | ||
| Stage lll | 0.0789 | 1.6420 | 0.1594-1.1054 | ||
| GSE31908_GPL96 | 0.3379 | 0.4198 | 0.4808-8.4458 | Go | |
| Stage l | 0.4208 | 2.0151 | 0.1952-49.9487 | ||
| GSE31908_GPL97 | 0.3389 | 3.1225 | 0.0442-2.9246 | Go | |
| GSE102287 | 0.3431 | 0.3697 | 0.5849-4.6697 | Go | |
| GSE41271 | 0.3752 | 1.6527 | 0.8082-1.7588 | Go | |
| Stage l | 0.0460 | 1.1923 | 1.0119-3.6844 | Poor | |
| Stage ll | 0.2885 | 1.9308 | 0.1872-1.6460 | ||
| Stage lll | 0.5018 | 0.5550 | 0.4432-1.4893 | ||
| GSE14814 | 0.4097 | 0.8125 | 0.4067-1.4434 | Go | |
| Stage l | 0.5010 | 0.7661 | 0.2710-1.8932 | ||
| Stage ll | 0.0585 | 0.7163 | 0.4185-2.0663 | ||
| GSE13213 | 0.5543 | 0.9299 | 0.6420-2.2847 | Go | |
| Stage lll | 0.1295 | 1.2111 | 0.7902-6.3195 | ||
| GSE10245 | 0.5596 | 2.2347 | 0.24472.1422 | Go | |
| GSE29013 | 0.5720 | 0.7240 | 0.2015-2.4230 | Go | |
| Stage l | 0.8679 | 0.6987 | 0.0916-7.5108 | ||
| Stage lll | 0.7513 | 0.8295 | 0.1581-3.7854 | ||
| GSE11117 | 0.6797 | 0.7736 | 0.4787-3.0952 | Go | |
| Stage lV | 0.7480 | 1.2173 | 0.3764-3.8970 | ||
| GSE5843 | 0.6938 | 1.2112 | 0.3011-2.2229 | Go | |
| Stage l | 0.6080 | 0.8181 | 0.2301-2.0696 | ||
| GSE19188 | 0.6975 | 0.6901 | 0.4482-1.7106 | Go | |
| GSE4573 | 0.7531 | 0.8756 | 0.5204-1.6039 | Go | |
| Stage l | 0.9703 | 1.0146 | 0.4719-2.1815 | ||
| Stage ll | 0.2390 | 1.8968 | 0.6522-5.5165 | ||
| Stage lll | 0.2249 | 0.4603 | 0.1315-1.6114 | ||
| Poepman | 0.9458 | 1.0227 | 0.5350-1.9551 | Go | |
| combined | <0.0001 | 1.3787 | 1.2564-1.5130 | Poor |
Note: cutoff: upper 25% VS other 75%.
LOXL2-related immune cell infiltration
We performed the immune score, stromal score, and tumor purity determinations of LOXL2 (Figure 2A-F), and the results showed that LOXL2 is negatively correlated with an immune score, positively correlated with a stromal score, and negatively associated with tumor purity. Considering that the tumor immune microenvironment plays a significant role in the development of tumorigenesis, we then used the R package CIBERSORT to determine the ratio of 22 TILs in LUAD and LUSC patients (Figures 2G, 2H, S3A, S3B).
Figure 2.
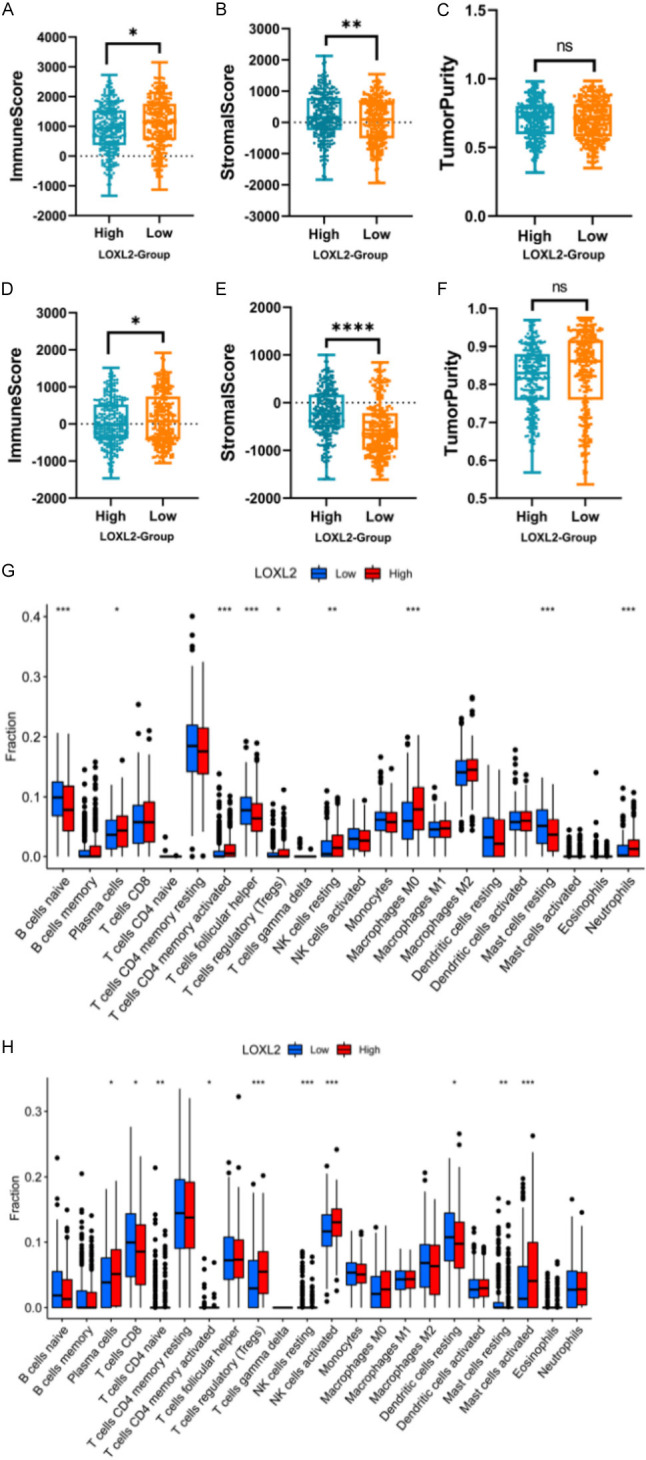
LOXL2 is associated with tumor microenvironment and tumor mutation burden. A-F. Use box plots to show the immune score, stromal score, and tumor purity of LOXL2 on LUAD and LUSC. G, H. Taking the median TMB value as a cutoff, the relative expressions of 22 tumor-infiltrating immune cells in the low- and high-TMB samples were determined (P<0.05, P<0.01, P<0.001, ns, not significant).
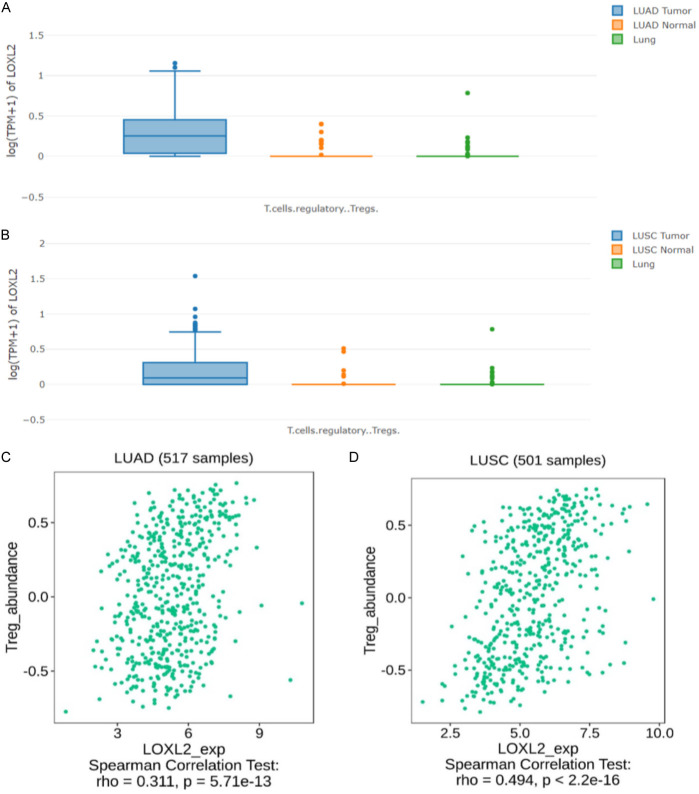
LOXL2 was positively correlated with T-reg
We characterized the immunology profile of LUAD and LUSC samples with low LOXL2 and high LOXL2 expression by ssGSEA. Found that LOXL2 in LUAD and LUSC patients is associated with T-reg. GEPIA, to compare LUAD and LUSC tumor tissues, adjacent tissues, and normal lungs (Figure 3A, 3B), found highly expressed T-reg in tumor tissues. In addition, Spearman Correlation Text confirmed the positive correlation between LUAD (Figure 3C) and LUSC (Figure 3D), and T-reg (LUAD, T-reg rho=0.311, P=5.71e-13; LUSC, T-reg, rho=0.494, P<2.2e-16). All these results indicated that LOXL2 might potentially regulate immune infiltration and the response to immunotherapy.
Figure 3.
LOXL2 was positively correlated with T-reg. A, B. Correlation between LOXL2 expression and T-reg in LUAD and LUSC patients. C, D. Associations between LOXL2 expression and immune subtypes in LUAD and LUSC.
Correlated gene expression
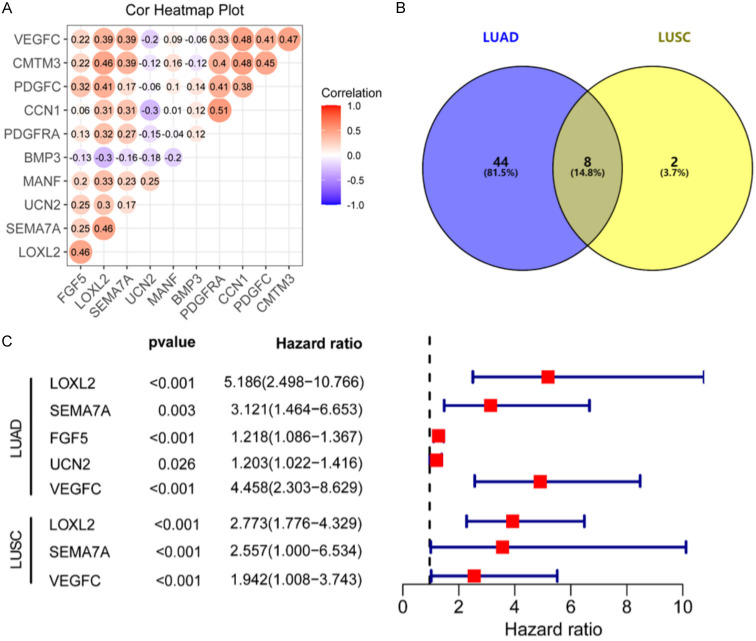
Furthermore, we evaluated the association of fifty-two corresponding bases of LUAD (Figure 4A; Table 3) and ten related genes of LUSC (Figure S4; Table 4) with LOXL2. Based on the intersection of the related genes of LUAD and LUSC and LOXL2, we identified eight common genes (Figure 4B): PDGFRA, SEMA7A, FGF5, CMTM3, UCN2, MANF, PDGFC, VEGFC. Among them, LOXL2, SEWA7A, FGF5, UCN2, and VEGFC were positively correlated with LUAD; while LOXL2, SEMA7A, and VEGFC were positively correlated with LUSC (all P<0.05) (Figure 4C).
Figure 4.
SEMA7A and VEGFC positively correlate with LOXL2 in non-small cell lung cancer. A. Correlation analysis of the differential up-regulation and down-regulation of related genes on LUAD and LUSC. B. Venn diagrams of genes related to LOXL2 in LUAD and LUSC. C. The 2 intersection genes that related to OS were extracted by univariate Cox regression analysis.
Table 3.
Association of fifty-two corresponding bases of LUAD with LOXL2
| GENE1 | GENE2 | P | R |
|---|---|---|---|
| LOXL2 | PDGFRA | 2.97444E-20 | 0.36848778 |
| LOXL2 | GDF11 | 2.49057E-14 | 0.308106677 |
| LOXL2 | SEMA4F | 2.31871E-18 | 0.350596022 |
| LOXL2 | IL1RN | 4.10483E-17 | 0.385049349 |
| LOXL2 | CCL21 | 9.49702E-19 | 0.35435584 |
| LOXL2 | SEMA7A | 2.8837E-58 | 0.599078545 |
| LOXL2 | FGF5 | 3.26866E-26 | 0.418530278 |
| LOXL2 | CMTM3 | 8.7196E-62 | 0.613532508 |
| LOXL2 | SECTM1 | 2.27652E-15 | 0.319714484 |
| LOXL2 | HDGF | 3.45809E-45 | 0.537939759 |
| LOXL2 | GDF7 | 1.51068E-17 | -0.342533156 |
| LOXL2 | UCN2 | 1.5078E-16 | 0.371179367 |
| LOXL2 | MANF | 2.4182E-42 | 0.522782038 |
| LOXL2 | PDGFC | 1.18991E-18 | 0.398061079 |
| LOXL2 | ADM | 3.65267E-40 | 0.510629978 |
| LOXL2 | VEGFC | 3.21155E-45 | 0.538106606 |
| LOXL2 | STC1 | 2.41538E-69 | 0.659673055 |
| LOXL2 | GIP | 6.12859E-12 | 0.336351246 |
| LOXL2 | TOR2A | 3.65868E-16 | 0.32825584 |
| LOXL2 | TGFA | 5.19121E-15 | 0.366262391 |
| LOXL2 | AIMP1 | 1.3148E-23 | 0.397702146 |
| LOXL2 | ESM1 | 1.50638E-46 | 0.569273722 |
| LOXL2 | GREM1 | 4.3189E-51 | 0.567128278 |
| LOXL2 | SEMA6B | 1.78133E-43 | 0.554551206 |
| LOXL2 | CD320 | 8.90038E-25 | 0.407236163 |
| LOXL2 | LTBP3 | 1.33309E-16 | 0.332859725 |
| LOXL2 | BMP1 | 2.52567E-83 | 0.688311411 |
| LOXL2 | SEMA4C | 5.06991E-51 | 0.566800334 |
| LOXL2 | CXCL8 | 7.83508E-26 | 0.452239551 |
| LOXL2 | APLN | 4.08522E-20 | 0.367221915 |
| LOXL2 | ANGPTL7 | 3.20052E-15 | -0.318092326 |
| LOXL2 | CCL11 | 3.29126E-16 | 0.328742125 |
| LOXL2 | SAA1 | 6.0212E-17 | 0.336430152 |
| LOXL2 | VEGFB | 2.92379E-25 | 0.411089378 |
| LOXL2 | CLCF1 | 5.6556E-24 | 0.400722429 |
| LOXL2 | DEFB103B | 1.09719E-15 | 0.323157274 |
| LOXL2 | RABEP2 | 9.27753E-14 | 0.301500619 |
| LOXL2 | BMP8A | 4.29787E-12 | 0.337930853 |
| LOXL2 | CSF1 | 4.10798E-12 | 0.338131359 |
| LOXL2 | JAG2 | 5.90689E-31 | 0.453085117 |
| LOXL2 | SEMA4B | 3.73141E-58 | 0.618957825 |
| LOXL2 | ANGPTL5 | 7.44501E-11 | -0.324968647 |
| LOXL2 | SEMA4D | 1.59881E-11 | 0.332036618 |
| LOXL2 | FAM3C | 1.75315E-35 | 0.512438649 |
| LOXL2 | GMFB | 2.65536E-18 | 0.350020547 |
| LOXL2 | NMB | 8.22844E-11 | 0.324502476 |
| LOXL2 | S100A6 | 8.25088E-21 | 0.415408511 |
| LOXL2 | DEFA1 | 3.48669E-14 | 0.306432507 |
| LOXL2 | CKLF | 1.41157E-25 | 0.413583014 |
| LOXL2 | MIF | 2.19098E-20 | 0.412079957 |
| LOXL2 | CCL16 | 1.58955E-15 | -0.321414289 |
| LOXL2 | CCL3 | 1.29679E-10 | 0.322372806 |
Table 4.
Association of ten related genes of LUSC with LOXL2
| GENE1 | GENE2 | P | R |
|---|---|---|---|
| LOXL2 | PDGFRA | 5.46926E-10 | 0.32491428 |
| LOXL2 | SEMA7A | 7.77056E-30 | 0.457736861 |
| LOXL2 | FGF5 | 1.305E-30 | 463270539 |
| LOXL2 | CMTM3 | 4.11249E-30 | 45972208 |
| LOXL2 | CCN1 | 1.83492E-13 | 306935752 |
| LOXL2 | UCN2 | 4.35921E-13 | 0.302312857 |
| LOXL2 | MANF | 1.4595E-11 | 0.331374895 |
| LOXL2 | PDGFC | 1.75505E-23 | 0.408057926 |
| LOXL2 | VEGFC | 7.30389E-17 | 0.393951854 |
| LOXL2 | BMP3 | 3.60237E-13 | -0.303338747 |
Predictive significance of SEMA7A and VEGFC in LUAD and LUSC
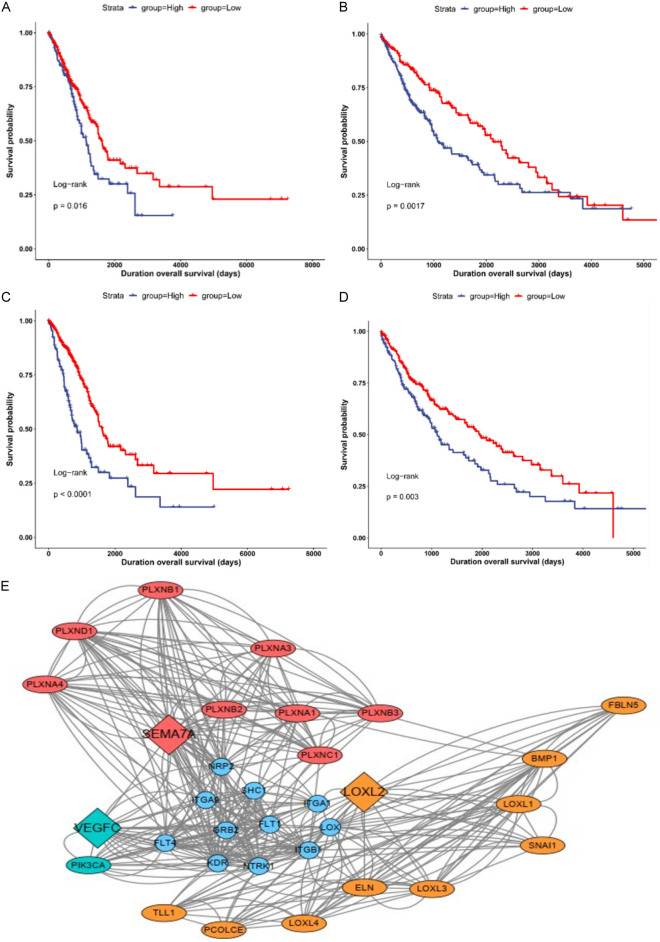
The common genes SEMA7A and VEGFC related to LUAD, LUSC, and LOXL2 were used as independent factors for Cox survival analysis (Figure 5A-D). SEMA7A (Log-rank P=0.016) and VEGFC (Log-rank P<0.01) were prognostic factors in LUAD. SEMA7A (Log-rank P=0.0017) and VEGFC (Log-rank P=0.003) were predictive factors in LUSC.
Figure 5.
SEMA7A and VEGFC are prognostic factors in LUAD and LUSC. A, B. The Kaplan-Meier plot of SEMA7A in LUAD and LUSC. C, D. The Kaplan-Meier plot of VEGFC in LUAD and LUSC. E. Protein-protein interaction (PPI) network. Molecules with the highest correlation with LOXL2.
Next, we built a PPI network to understand the mode of interaction of LOXL2 (Figure 5E). The PPI network comprised 31 nodes, showing the relationship between LOXL2, SEMA7A, and VEGFC. The results prove that SEMA7A and VEGFC are prognostic factors of LUAD and LUSC.
Enrichment of LOXL2-correlated gene
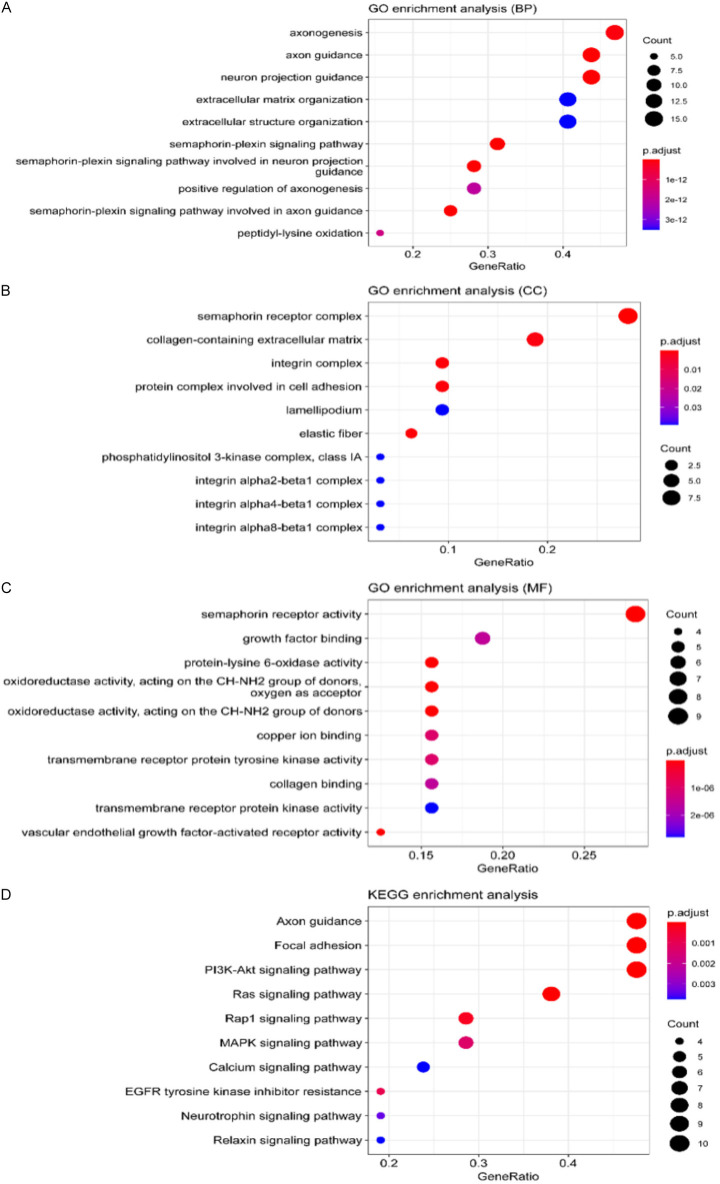
GO enrichment analysis in terms of biological processes (BP), cellular components (CC) and molecular functions (MF) revealed that the significant regulatory processes of LOXL2 on BP were axonogenesis, axon guidance, and neuronal projection guidance (Figure 6A). For CC, alterations in LOXL2 most clearly controlled processes in the semaphorin receptor complex and the collagen-containing extracellular matrix (Figure 6B). In the results shown for MF, semaphorin receptor activity was the most responsive to regulation by LOXL2, which was most clearly associated with different sites of regulation (Figure 6C).
Figure 6.
The tasks of LOXL2 and the correlations among their functions. A-D. Bar plot of Go and KEGG functional enrichment analyses. BP indicated biological process; CC indicated cellular component; MF indicated molecular function.
KEGG pathway enrichment of the LOXL2 interactive gene showed that axon guidance, focal adhesion, and PI3K-Akt signaling pathway were enriched pathways (Figure 6D).
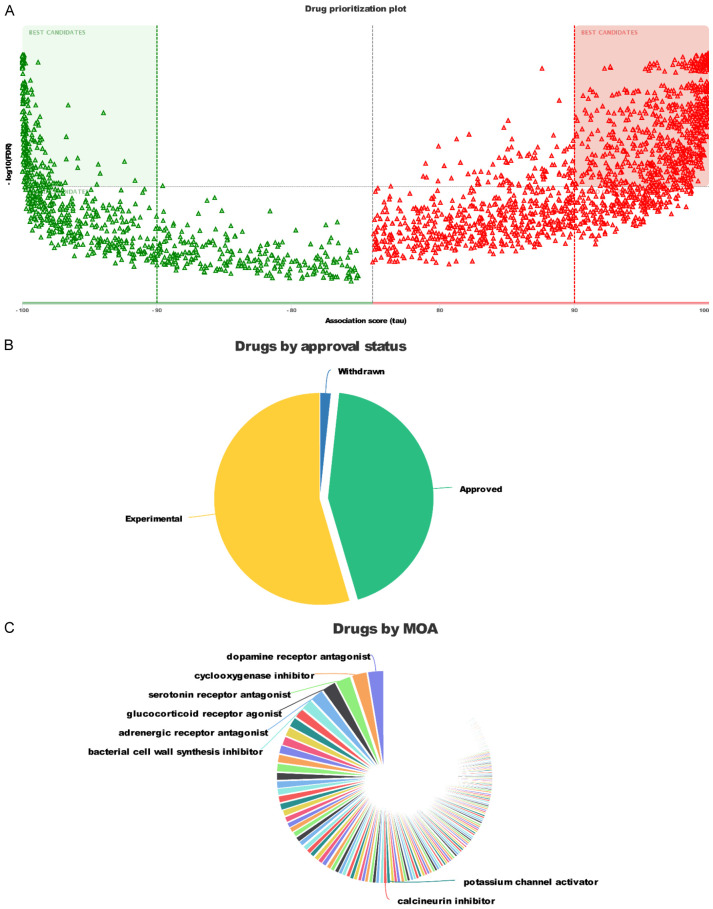
Therapeutic targets and mechanisms of drugs
Volcano plots showed the priorities of different drugs in the DREAMT database and after batch correction (Figure 7A). Then we analyzed the marketing status of all oncology drugs (Figure 7B). A large proportion of experimental drugs are approved, mainly including dopamine receptor antagonists, cyclooxygenase inhibitors, serotonin receptor antagonists, glucocorticoid receptor antagonists, adrenergic receptor agonist, adrenergic receptor antagonists, and bacterial cell wall synthesis inhibitor (Figure 7C). Further analyses screened nine drugs: cefuroxime benzocaine, benzocaine, cefazolin, methotrexate, tacrolimus, rimantadine, doxorubicin, cefuroxime, guanethidine (Table 5).
Figure 7.
The therapeutic targets and mechanisms of drugs. A. Volcano plots shows the priorities of different medicines found in the DREIMT database. B. The mechanism of action of tumor drugs on the market. C. Classification of the medications for treating tumors according to their approval status.
Table 5.
Common tumor treatment drugs on the market
| Drug_name | Drug_pubchm_id | Summary | FDR | tau | Drug specificity_score | Drug_source_db | Drug_source_name | Drug_status | Drug_moa | Drug_target_gene_names | Drug_target_gene_ids |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefuroxime | 5361202 | Cefuroxime boosts case type compared to reference type | 0.001652893 | 99.98135372 | LINCS | BRD-K63641886 | APPROVED | Bacterial cell wall synthesis inhibitor | |||
| Calcifediol | Calcifediol boosts case type compared to reference type | 0.001788909 | 99.98135372 | 0.55427037 | LINCS | BRD-K77175907 | APPROVED | Vitamin D receptor agonist | VDR | 7421 | |
| Benzocaine | 2337 | Benzocaine boosts case type compared to reference type | 0.001841621 | 99.98135372 | LINCS | BRD-K75466013 | APPROVED | Sodium channel blocker | SCN10A | 6336 | |
| Cefazolin | Cefazolin boosts case type compared to reference type | 0.001851852 | 99.98135372 | LINCS | APPROVED | Bacterial cell wall synthesis inhibitor | PON1 | 5444 | |||
| Mesoridazine | 4078 | Mesoridazine boosts case type compared to reference type | 0.001901141 | 99.98135372 | 0.6460514 | LINCS | BRD-A14395271 | APPROVED | Dopamine receptor antagonist | HTR2A, DRD2 | 3356, 1813 |
| Tacrolimus | 445643 | Tacrolimus boosts case type compared to reference type | 0.002028398 | 99.98135372 | 0.61548928 | LINCS | BRD-K65261396 | APPROVED | Calcineurin inhibitor | FKBP1A | 2280 |
| Rimantadine | 5071 | Rimantadine boosts case type compared to reference type | 0.002325581 | 99.98135372 | LINCS | APPROVED | Antiviral, RNA synthesis inhibitor | ||||
| Doxorubicin | 31703 | Doxorubicin boosts case type compared to reference type | 0.002421308 | 99.98135372 | 0.69624245 | LINCS | BRD-A52530684 | APPROVED | Topoisomerase inhibitor | TOP2A | 7153 |
| Cefuroxime | 5361202 | Cefuroxime boosts case type compared to reference type | 0.001623377 | 99.96270744 | LINCS | APPROVED | Bacterial cell wall synthesis inhibitor | ||||
| Guanethidine | 3518 | Guanethidine boosts case type compared to reference type | 0.001766784 | 99.96270744 | LINCS | BRD-M18219129 | APPROVED | Adrenergic inhibitor | SLC6A2 | 6530 |
Drug sensitivity experiments of doxorubicin on LOXL2 expression profile
To further substantiate the predicted drug response to LOXL2 inhibition, we selected doxorubicin, the agent displaying the strongest correlation with our target gene, for subsequent experimental validation. We initially assessed the expression levels of LOXL2 across three commonly used non-small cell lung cancer (NSCLC) cell lines: PC9, A549, and H1299. These expression profiles were quantified via quantitative PCR (qPCR) analysis. As illustrated in the Figure, the H1299 cell line, exhibiting robust LOXL2 expression, and the PC9 cell line, characterized by relatively low LOXL2 expression, were chosen for our drug sensitivity studies (Figure 8A). We then employed a Cell Counting Kit-8 (CCK-8) assay to determine the half-maximal inhibitory concentration (IC50) of doxorubicin at various dose gradients in both the H1299 and PC9 cell lines (Figure 8B, 8C). The IC50 of H1299 was determined to be 2.248 µg/ml. Furthermore, the IC50 of PC9 was 6.026 µg/ml (P<0.05), indicating that the high LOXL2-expressing cell line H1299 exhibited greater sensitivity to doxorubicin than the low LOXL2-expressing cell line PC9.
Figure 8.
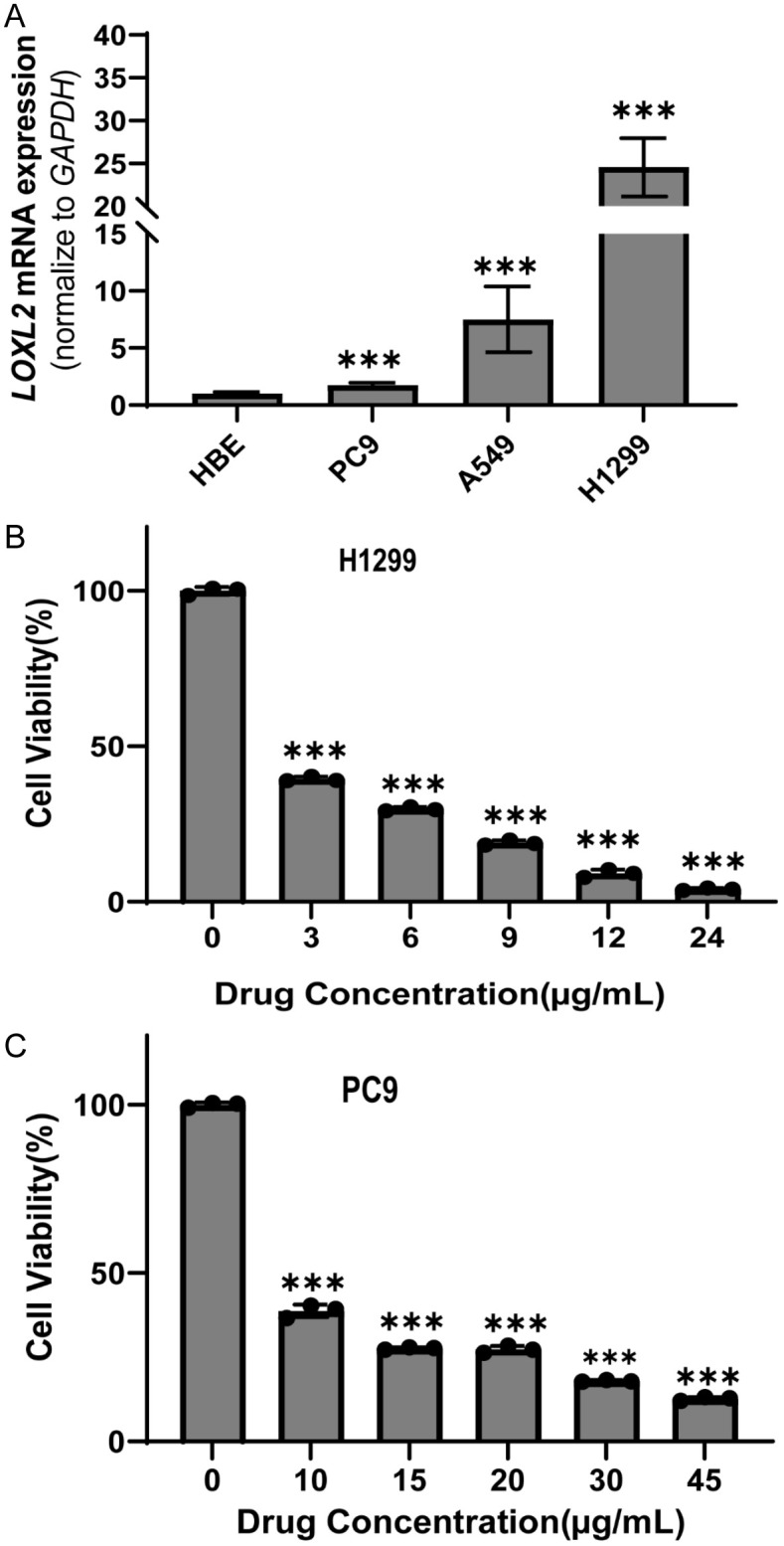
Drug sensitivity experiments of doxorubicin on LOXL2 expression profile. A. The expression of LOXL2 mRNA in NSCLC assessed using RT-qPCR (n=12, ***P<0.001). B, C. Cell Counting Kit-8 assay was employed to ascertain the half maximal inhibitory concentration (IC50) of doxorubicin at varying concentration gradients on the H1299 cell line, exhibiting robust LOXL2 expression and PC9 cell line, characterized by relatively low LOXL2 expression (n=3, ***P<0.001).
Discussion
Many patients with non-small cell lung cancer are diagnosed at an advanced stage, and those diagnosed in an early stage often relapse and develop metastatic lesions, despite recent advances in treatment [19]. Tumor immunotherapy has developed rapidly and has attracted increasing attention due to its effectiveness [20-22]. In light of this, our study aims to discover new targets for immunotherapy and predictive indicators for NSCLC patients. We began by the intersecting 500 differentially expressed genes (DEGs) found in both lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC), then perform survival curve analysis on the 11 genes shared between the two groups. Additionally, we compared the expression levels of these 11 genes in para-cancerous and lung cancerous tissues, ultimately identifying LOXL2 as a promising biomarker.
The lysyl oxidase (LOX) family, currently comprising LOXL1, LOXL2, LOXL3, and LOXL4, has been demonstrated to enhance extracellular matrix stability by cross-linking elastin and collagen in the outer matrix [23,24]. Recent studies have demonstrated that LOX also facilitates tumor cell migration and invasion through a number of mechanisms, including the promotion of epithelial-mesenchymal transition (EMT) [25], the activation of the p-FAK/p-paxillin/YAP signaling pathway [26] and the involvement in the formation of a pre-metastatic microenvironment [27]. This has been demonstrated to be a crucial factor in the process of tumorigenesis and metastasis [26,28]. It is anticipated that this will prove to be a promising target for tumor therapy. Among these, lysyl oxidase-like 2 (LOXL2) has been identified as a gene that upregulation promotes tumor infiltration and metastasis [29]. The aberrant expression of LOXL2 in various tumors has been associated with several adverse outcomes, including epithelial-mesenchymal transition (EMT), metastasis, poor prognosis, chemo-radiotherapy resistance, and tumor progression [29,30]. Previous study demonstrated that LOX and LOXL2 are directly regulated by the miR-00/ZEB1 axis, and that LOXL2 might serve as a new target for lung cancer therapy [31]. Subsequently, a number of studies demonstrated a correlation between elevated LOXL2 expression and reduced overall survival, as well as worsening of clinicopathological features of tumors [32]. Furthermore, our findings indicate that LOXL2 is highly expressed in non-small cell lung cancer and is associated with a poor prognosis. Consequently, it is postulated that LOXL2 may represent a novel therapeutic target for the treatment of NSCLC.
Consequently, we calculated the immune, matrix, and tumor purity of LUAD and LUSC and confirmed that LOXL2 was associated with the immune response of NSCLC. The relationship between LOXL2 and T-reg was identified through the analysis of the degree of immune infiltration and the proportion of immune T cells. A positive correlation was observed between LOXL2 and T-reg. Moreover, regulatory T cells (Tregs) have been shown to promote immune suppression in malignant tumors by suppressing the immune response to cancer cells [33]. Treg cells plays a pivotal role in maintaining peripheral tolerance in vivo through the active suppression of self-reactive T-cell activation and expansion. This helps to prevent autoimmune diseases and restrain chronic inflammatory conditions [34]. In patients with early-stage NSCLC, an increased number of circulating and tumor-infiltrating regulatory Tregs are associated with a poorer prognosis and a higher risk of recurrence [35]. In light of these findings, LOXL2 may serve as a promising novel marker for the treatment of LUAD and LUSC, as well as for prognostication.
To further confirmed the prognostic indicative role of LOXL2 in patients with non-small cell lung cancer, we searched for the related molecules of LOXL2 and performed multi-factor Cox survival analysis to identify two prognostic factors SMEATA and VEGFC. SEMA7A, a glycosylphosphatidylinositol-anchored (GPI-anchored) glycoprotein on the plasma membrane. Recent research has shown that FUT8-mediated aberrant N-glycosylation of SEMA7A promotes head and neck squamous cell carcinoma progression [36]. It is a possible therapeutic target for patients with EGFR-TKI-resistant lung adenocarcinoma [37]. VEGFC, a member of the vascular endothelial growth factor/platelet-derived growth factor family, promotes endothelial cell proliferation and angiogenesis. VEGF family consists of seven members, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, placental growth factor (PlGF), and non-human genome encoded VEGF-E and svVEGF [38]. VEGFC promotes tumor growth and metastasis through lymphangiogenesis and lymphatic metastasis, which is mediated by VEGFR-3 [39]. Blocking this pathway leads to apoptosis of lymphatic endothelial cells and disruption of the lymphatic network. Thus, VEGFC is involved in lymphatic metastasis of tumor, which is a feature of poor tumor prognosis [40,41]. These two prognostic factors are further evidence for the prognostic role of LOXL2 in NSCLC.
The “oncoPredict” is an R package for predicting drug responses. It integrates three approaches to (1) correct for overall drug sensitivity for drug-specific biomarker discovery, (2) predict a patient’s clinical drug response, and (3) correlate these predictions with clinical features for in vivo drug biomarker discovery. This new “oncoPredict” R software package can be applied to a variety of in vitro and in vivo drug and biomarker discovery settings [42]. We use the “oncoPredict” R package to analyze the batch-corrected prioritization of different drugs in the DREAMT database. Nine drugs are tested: cefuroxime benzocaine, benzocaine, cefazolin, methotrexate, tacrolimus, rimantadine, doxorubicin, cefuroxime and guanethidine. In order to investigate the relationship between LOXL2 expression and drug sensitivity, we proceeded to identify the compounds with the strongest correlation with LOXL2 levels, which were then subjected to subsequent experiments. The results demonstrated that the H1299 cell line, which exhibited higher LOXL2 expression, exhibited greater sensitivity to doxorubicin compared to the PC9 cell line, which exhibited lower LOXL2 expression. These findings illustrate the potential role of LOXL2 in predicting drug response and emphasize the importance of considering gene expression levels when selecting compounds for therapeutic intervention.
This study has some limitations. Our study is a retrospective, not a prospective analysis, and we analyze the role of LOXL2 in NSCLC by bioinformatics analysis without exploring the mechanism. In conclusion, our results indicate a prognostic role of LOXL2 in NSCLC patients. The analysis of the tumor immune microenvironment suggests the possibility of LOXL2 as a new target for immunotherapy of NSCLC. The results of molecular interactions reveal that SEMA7A and VEGFC may be prognostic factors in NSCLC.
Acknowledgements
This study was supported by research grants from the National Natural Science Foundation of China under grant 82171576, and Jiangsu Province Capability Improvement Project through Science, Technology and Education under grant No. CXZX202228 to Jianqing Wu.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20:624–639. doi: 10.1038/s41571-023-00798-3. [DOI] [PubMed] [Google Scholar]
- 2.Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943–e955. doi: 10.1016/S2468-2667(23)00211-6. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster HL, Heuvelmans MA, Oudkerk M. Low-dose computed tomography lung cancer screening: clinical evidence and implementation research. J Intern Med. 2022;292:68–80. doi: 10.1111/joim.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna NH, Schneider BJ, Temin S, Baker S Jr, Brahmer J, Ellis PM, Gaspar LE, Haddad RY, Hesketh PJ, Jain D, Jaiyesimi I, Johnson DH, Leighl NB, Phillips T, Riely GJ, Robinson AG, Rosell R, Schiller JH, Singh N, Spigel DR, Stabler JO, Tashbar J, Masters G. Therapy for Stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J. Clin. Oncol. 2020;38:1608–1632. doi: 10.1200/JCO.19.03022. [DOI] [PubMed] [Google Scholar]
- 5.Al Bakir M, Huebner A, Martinez-Ruiz C, Grigoriadis K, Watkins TBK, Pich O, Moore DA, Veeriah S, Ward S, Laycock J, Johnson D, Rowan A, Razaq M, Akther M, Naceur-Lombardelli C, Prymas P, Toncheva A, Hessey S, Dietzen M, Colliver E, Frankell AM, Bunkum A, Lim EL, Karasaki T, Abbosh C, Hiley CT, Hill MS, Cook DE, Wilson GA, Salgado R, Nye E, Stone RK, Fennell DA, Price G, Kerr KM, Naidu B, Middleton G, Summers Y, Lindsay CR, Blackhall FH, Cave J, Blyth KG, Nair A, Ahmed A, Taylor MN, Procter AJ, Falzon M, Lawrence D, Navani N, Thakrar RM, Janes SM, Papadatos-Pastos D, Forster MD, Lee SM, Ahmad T, Quezada SA, Peggs KS, Van Loo P, Dive C, Hackshaw A, Birkbak NJ, Zaccaria S RACERx Consortium. Jamal-Hanjani M, McGranahan N, Swanton C. The evolution of non-small cell lung cancer metastases in TRACERx. Nature. 2023;616:534–542. doi: 10.1038/s41586-023-05729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hynds RE, Huebner A, Pearce DR, Hill MS, Akarca AU, Moore DA, Ward S, Gowers KHC, Karasaki T, Al Bakir M, Wilson GA, Pich O, Martinez-Ruiz C, Hossain ASMM, Pearce SP, Sivakumar M, Ben Aissa A, Gronroos E, Chandrasekharan D, Kolluri KK, Towns R, Wang K, Cook DE, Bosshard-Carter L, Naceur-Lombardelli C, Rowan AJ, Veeriah S, Litchfield K, Crosbie PAJ, Dive C, Quezada SA, Janes SM, Jamal-Hanjani M, Marafioti T TRACERx Consortium. McGranahan N, Swanton C. Representation of genomic intratumor heterogeneity in multi-region non-small cell lung cancer patient-derived xenograft models. Nat Commun. 2024;15:4653. doi: 10.1038/s41467-024-47547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Yu Q, Song T, Wang Z, Song L, Yang Y, Shao J, Li J, Ni Y, Chao N, Zhang L, Li W. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Signal Transduct Target Ther. 2022;7:289. doi: 10.1038/s41392-022-01130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang W, Zhao M, Cui L, Ren Y, Zhang J, Chen J, Jia L, Zhang J, Yang J, Chen G, Ashby CR Jr, Wu C, Chen ZS, Wang L. Characterization of a novel HDAC/RXR/HtrA1 signaling axis as a novel target to overcome cisplatin resistance in human non-small cell lung cancer. Mol Cancer. 2020;19:134. doi: 10.1186/s12943-020-01256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allaeys T, Berzenji L, Lauwers P, Yogeswaran SK, Hendriks JMH, Billiet C, De Bondt C, Van Schil PE. Multimodality treatment including surgery related to the type of N2 involvement in locally advanced non-small cell lung cancer. Cancers (Basel) 2022;14:1656. doi: 10.3390/cancers14071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Zhou L, Guan Q, Hou X, Wang C, Liu L, Wang J, Yu X, Li W, Liu H. Skp2-mediated MLKL degradation confers cisplatin-resistant in non-small cell lung cancer cells. Commun Biol. 2023;6:805. doi: 10.1038/s42003-023-05166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Yang X, Sun Y, Li Z, Yang W, Ju B, Easton J, Pei D, Cheng C, Lee S, Pui CH, Yu J, Chi H, Yang JJ. Impact of T-cell immunity on chemotherapy response in childhood acute lymphoblastic leukemia. Blood. 2022;140:1507–1521. doi: 10.1182/blood.2021014495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–1136. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Paul MK. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40. doi: 10.1186/s12943-023-01740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 15.Chow A, Perica K, Klebanoff CA, Wolchok JD. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat Rev Clin Oncol. 2022;19:775–790. doi: 10.1038/s41571-022-00689-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Qiao M, Zhou C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol Immunol. 2021;18:279–293. doi: 10.1038/s41423-020-00577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannone G, Ghisoni E, Genta S, Scotto G, Tuninetti V, Turinetto M, Valabrega G. Immuno-metabolism and microenvironment in cancer: key players for immunotherapy. Int J Mol Sci. 2020;21:4414. doi: 10.3390/ijms21124414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Jiang W, Chen C, Gao H, Shi J, Wang D. CEBPA facilitates LOXL2 and LOXL3 transcription to promote BCL-2 stability and thus enhances the growth and metastasis of lung carcinoma cells in vitro. Exp Cell Res. 2024;435:113937. doi: 10.1016/j.yexcr.2024.113937. [DOI] [PubMed] [Google Scholar]
- 19.Su CC, Wu JT, Choi E, Myall NJ, Neal JW, Kurian AW, Stehr H, Wood D, Henry SM, Backhus LM, Leung AN, Wakelee HA, Han SS. Overall survival among patients with de novo stage iv metastatic and distant metastatic recurrent non-small cell lung cancer. JAMA Netw Open. 2023;6:e2335813. doi: 10.1001/jamanetworkopen.2023.35813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Shi M, Ren Y, Xu H, Weng S, Ning W, Ge X, Liu L, Guo C, Duo M, Li L, Li J, Han X. Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy. Mol Cancer. 2023;22:35. doi: 10.1186/s12943-023-01738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira G, Wu CJ. Dynamics and specificities of T cells in cancer immunotherapy. Nat Rev Cancer. 2023;23:295–316. doi: 10.1038/s41568-023-00560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng C, Chu Y, Zhang N, Jia T, Li Y, Jiang T, Sun J. Pan-cancer analysis of the LOx family reveals that LOX affects tumor prognosis by affecting immune infiltration. Crit Rev Eukaryot Gene Expr. 2024;34:87–100. doi: 10.1615/CritRevEukaryotGeneExpr.2023049049. [DOI] [PubMed] [Google Scholar]
- 24.Liburkin-Dan T, Toledano S, Neufeld G. Lysyl oxidase family enzymes and their role in tumor progression. Int J Mol Sci. 2022;23:6249. doi: 10.3390/ijms23116249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso-Nocelo M, Ruiz-Canas L, Sancho P, Gorgulu K, Alcala S, Pedrero C, Vallespinos M, Lopez-Gil JC, Ochando M, Garcia-Garcia E, David Trabulo SM, Martinelli P, Sanchez-Tomero P, Sanchez-Palomo C, Gonzalez-Santamaria P, Yuste L, Wormann SM, Kabacaoglu D, Earl J, Martin A, Salvador F, Valle S, Martin-Hijano L, Carrato A, Erkan M, Garcia-Bermejo L, Hermann PC, Algul H, Moreno-Bueno G, Heeschen C, Portillo F, Cano A, Sainz B Jr. Macrophages direct cancer cells through a LOXL2-mediated metastatic cascade in pancreatic ductal adenocarcinoma. Gut. 2023;72:345–359. doi: 10.1136/gutjnl-2021-325564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Li J, Yang X, Li X, Kong J, Qi D, Zhang F, Sun B, Liu Y, Liu T. Carcinoma-associated fibroblast-derived lysyl oxidase-rich extracellular vesicles mediate collagen crosslinking and promote epithelial-mesenchymal transition via p-FAK/p-paxillin/YAP signaling. Int J Oral Sci. 2023;15:32. doi: 10.1038/s41368-023-00236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urooj T, Wasim B, Mushtaq S, Shah SNN, Shah M. Cancer cell-derived secretory factors in breast cancer-associated lung metastasis: their mechanism and future prospects. Curr Cancer Drug Targets. 2020;20:168–186. doi: 10.2174/1568009620666191220151856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umezaki N, Nakagawa S, Yamashita YI, Kitano Y, Arima K, Miyata T, Hiyoshi Y, Okabe H, Nitta H, Hayashi H, Imai K, Chikamoto A, Baba H. Lysyl oxidase induces epithelial-mesenchymal transition and predicts intrahepatic metastasis of hepatocellular carcinoma. Cancer Sci. 2019;110:2033–2043. doi: 10.1111/cas.14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cano A, Eraso P, Mazon MJ, Portillo F. LOXL2 in cancer: a two-decade perspective. Int J Mol Sci. 2023;24:14405. doi: 10.3390/ijms241814405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chitty JL, Yam M, Perryman L, Parker AL, Skhinas JN, Setargew YFI, Mok ETY, Tran E, Grant RD, Latham SL, Pereira BA, Ritchie SC, Murphy KJ, Trpceski M, Findlay AD, Melenec P, Filipe EC, Nadalini A, Velayuthar S, Major G, Wyllie K, Papanicolaou M, Ratnaseelan S, Phillips PA, Sharbeen G, Youkhana J, Russo A, Blackwell A, Hastings JF, Lucas MC, Chambers CR, Reed DA, Stoehr J, Vennin C, Pidsley R, Zaratzian A, Da Silva AM, Tayao M, Charlton B, Herrmann D, Nobis M, Clark SJ, Biankin AV, Johns AL, Croucher DR, Nagrial A, Gill AJ, Grimmond SM Australian Pancreatic Cancer Genome Initiative (APGI); Australian Pancreatic Cancer Matrix Atlas (APMA) Pajic M, Timpson P, Jarolimek W, Cox TR. A first-in-class pan-lysyl oxidase inhibitor impairs stromal remodeling and enhances gemcitabine response and survival in pancreatic cancer. Nat Cancer. 2023;4:1326–1344. doi: 10.1038/s43018-023-00614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng DH, Ungewiss C, Tong P, Byers LA, Wang J, Canales JR, Villalobos PA, Uraoka N, Mino B, Behrens C, Wistuba II, Han RI, Wanna CA, Fahrenholtz M, Grande-Allen KJ, Creighton CJ, Gibbons DL. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene. 2017;36:1925–1938. doi: 10.1038/onc.2016.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deshpande H. Levoleucovorin inhibits LOXL2 (lysyl oxidase like-2) to control breast cancer proliferation: a repurposing approach. J Biomol Struct Dyn. 2024;42:5104–5113. doi: 10.1080/07391102.2023.2224894. [DOI] [PubMed] [Google Scholar]
- 33.Dwivedi M, Tiwari S, Kemp EH, Begum R. Implications of regulatory T cells in anti-cancer immunity: from pathogenesis to therapeutics. Heliyon. 2022;8:e10450. doi: 10.1016/j.heliyon.2022.e10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schloder J, Shahneh F, Schneider FJ, Wieschendorf B. Boosting regulatory T cell function for the treatment of autoimmune diseases - That’s only half the battle! Front Immunol. 2022;13:973813. doi: 10.3389/fimmu.2022.973813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Principe DR, Chiec L, Mohindra NA, Munshi HG. Regulatory T-cells as an emerging barrier to immune checkpoint inhibition in lung cancer. Front Oncol. 2021;11:684098. doi: 10.3389/fonc.2021.684098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Z, Meng X, Zhang Y, Sun J, Tang X, Zhang Z, Liu L, He Y. FUT8-mediated aberrant N-glycosylation of SEMA7A promotes head and neck squamous cell carcinoma progression. Int J Oral Sci. 2024;16:26. doi: 10.1038/s41368-024-00289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passaro A, Janne PA, Mok T, Peters S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat Cancer. 2021;2:377–391. doi: 10.1038/s43018-021-00195-8. [DOI] [PubMed] [Google Scholar]
- 38.Dakowicz D, Zajkowska M, Mroczko B. Relationship between VEGF family members, their receptors and cell death in the neoplastic transformation of colorectal cancer. Int J Mol Sci. 2022;23:3375. doi: 10.3390/ijms23063375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Chen C, Luo Y, Yu M, He W, An M, Gao B, Kong Y, Ya Y, Lin Y, Li Y, Xie K, Huang J, Lin T. Tumor-derived exosomal BCYRN1 activates WNT5A/VEGF-C/VEGFR3 feedforward loop to drive lymphatic metastasis of bladder cancer. Clin Transl Med. 2021;11:e497. doi: 10.1002/ctm2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan Y, Liu L, Cheng Y, Yu J, Feng Y. Amplified LncRNA PVT1 promotes lung cancer proliferation and metastasis by facilitating VEGFC expression. Biochem Cell Biol. 2020;98:676–682. doi: 10.1139/bcb-2019-0435. [DOI] [PubMed] [Google Scholar]
- 41.Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. 2023;8:198. doi: 10.1038/s41392-023-01460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeser D, Gruener RF, Huang RS. oncoPredict: an R package for predicting in vivo or cancer patient drug response and biomarkers from cell line screening data. Brief Bioinform. 2021;22:bbab260. doi: 10.1093/bib/bbab260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.