Abstract
The influence and fate of the 3'-substituent of a series of cephalosporins on their interaction with the DD-transpeptidase/carboxypeptidase of Streptomyces R61 were investigated. Good 3'-leaving groups are eliminated from the acyl-enzyme before deacylation, and hence cephalosporins with such substituents generate a common covalent intermediate. The rate of this elimination reaction in the two cases studied in detail was much lower than from the corresponding cephalosporoates in free solution. Cephalosporins without good 3'-leaving groups yield acyl-enzymes that appear to hydrolyse, leading to enzyme re-activation, faster than those from cephalosporins with leaving groups.
Full text
PDF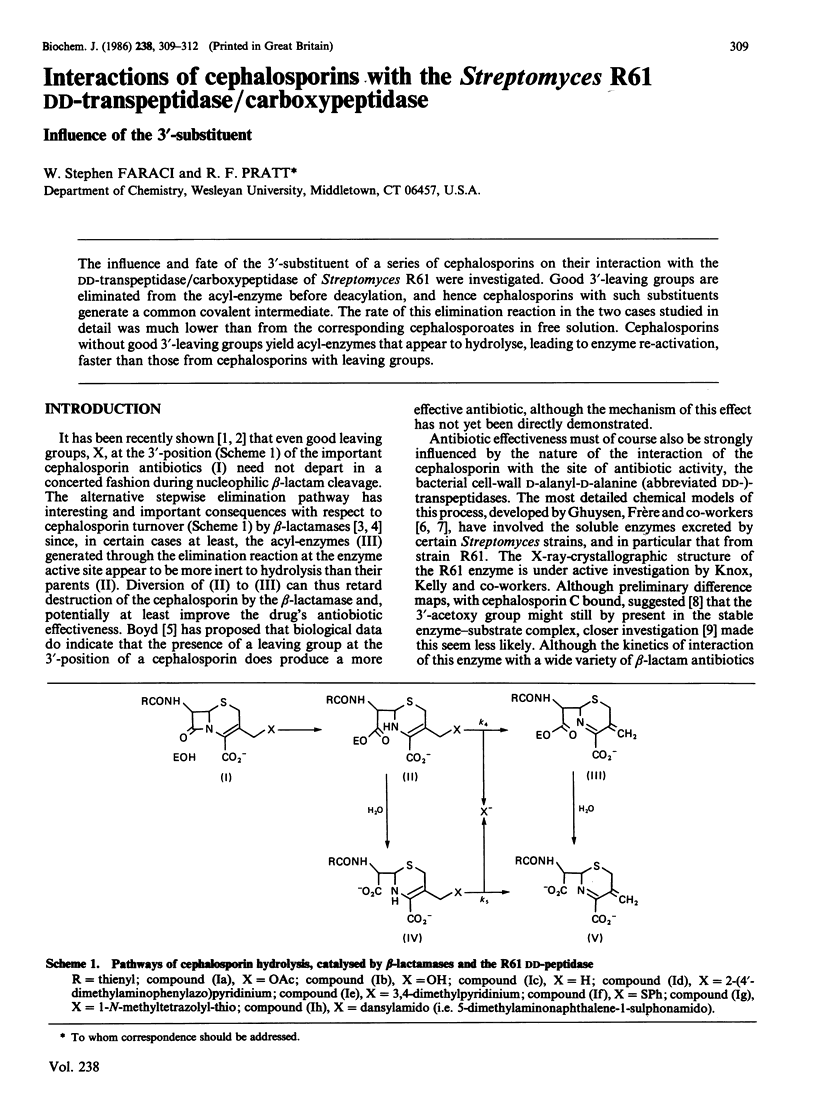



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. G., Pratt R. F. Pre-steady state beta-lactamase kinetics. The trapping of a covalent intermediate and the interpretation of pH rate profiles. J Biol Chem. 1983 Nov 10;258(21):13120–13126. [PubMed] [Google Scholar]
- Boyd D. B. Electronic structures of cephalosporins and penicillins. 15. Inductive effect of the 3-position side chain in cephalosporins. J Med Chem. 1984 Jan;27(1):63–66. doi: 10.1021/jm00367a012. [DOI] [PubMed] [Google Scholar]
- Boyd D. B. Electronic structures of cephalosporins and penicillins. 15. Inductive effect of the 3-position side chain in cephalosporins. J Med Chem. 1984 Jan;27(1):63–66. doi: 10.1021/jm00367a012. [DOI] [PubMed] [Google Scholar]
- Boyd D. B., Lunn W. H. Electronic structures of cephalosporins and penicillins. 9. Departure of a leaving group in cephalosporins. J Med Chem. 1979 Jul;22(7):778–784. doi: 10.1021/jm00193a006. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Faraci W. S., Pratt R. F. Mechanism of inhibition of RTEM-2 beta-lactamase by cephamycins: relative importance of the 7 alpha-methoxy group and the 3' leaving group. Biochemistry. 1986 May 20;25(10):2934–2941. doi: 10.1021/bi00358a030. [DOI] [PubMed] [Google Scholar]
- Faraci W. S., Pratt R. F. Mechanism of inhibition of the PC1 beta-lactamase of Staphylococcus aureus by cephalosporins: importance of the 3'-leaving group. Biochemistry. 1985 Feb 12;24(4):903–910. doi: 10.1021/bi00325a014. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Coyette J., Dusart J., Nguyen-Distèche M. Use of model enzymes in the determination of the mode of action of penicillins and delta 3-cephalosporins. Annu Rev Biochem. 1979;48:73–101. doi: 10.1146/annurev.bi.48.070179.000445. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M., Frère J. M., Leyh-Bouille M., Perkins H. R., Nieto M. The active centres in penicillin-sensitive enzymes. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):285–301. doi: 10.1098/rstb.1980.0046. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Knox J. R., Moews P. C., Hite G. J., Bartolone J. B., Zhao H., Joris B., Frère J. M., Ghuysen J. M. 2.8-A Structure of penicillin-sensitive D-alanyl carboxypeptidase-transpeptidase from Streptomyces R61 and complexes with beta-lactams. J Biol Chem. 1985 May 25;260(10):6449–6458. [PubMed] [Google Scholar]
- Kelly J. A., Moews P. C., Knox J. R., Frère J. M., Ghuysen J. M. Penicillin target enzyme and the antibiotic binding site. Science. 1982 Oct 29;218(4571):479–481. doi: 10.1126/science.7123246. [DOI] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Frère J. M., Ghuysen J. M. Fluorescence and circular dichroism studies on the Streptomyces R61 DD-carboxypeptidase-transpeptidase. Penicillin binding by the enzyme. Biochem J. 1973 Nov;135(3):493–505. doi: 10.1042/bj1350493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Ways to overcome cephalosporinase-mediated beta-lactam resistance in Enterobacter cloacae. Chemioterapia. 1985 Feb;4(1):83–89. [PubMed] [Google Scholar]


