Abstract
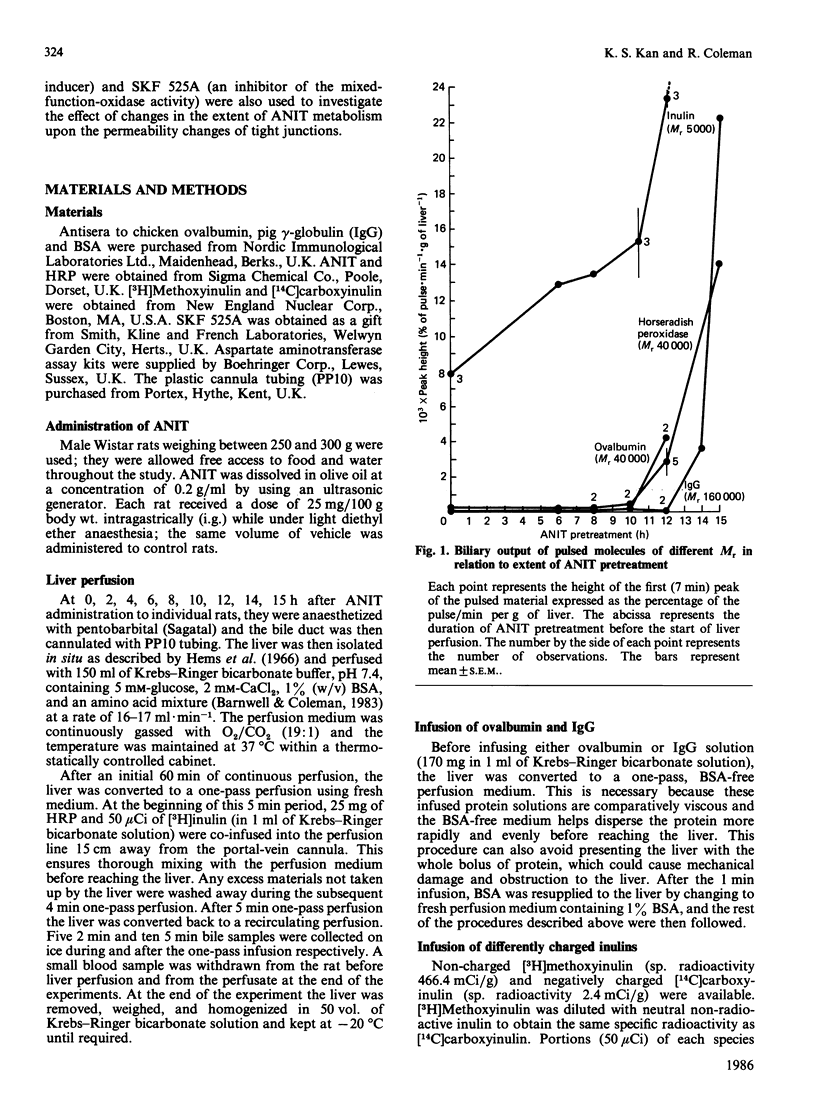
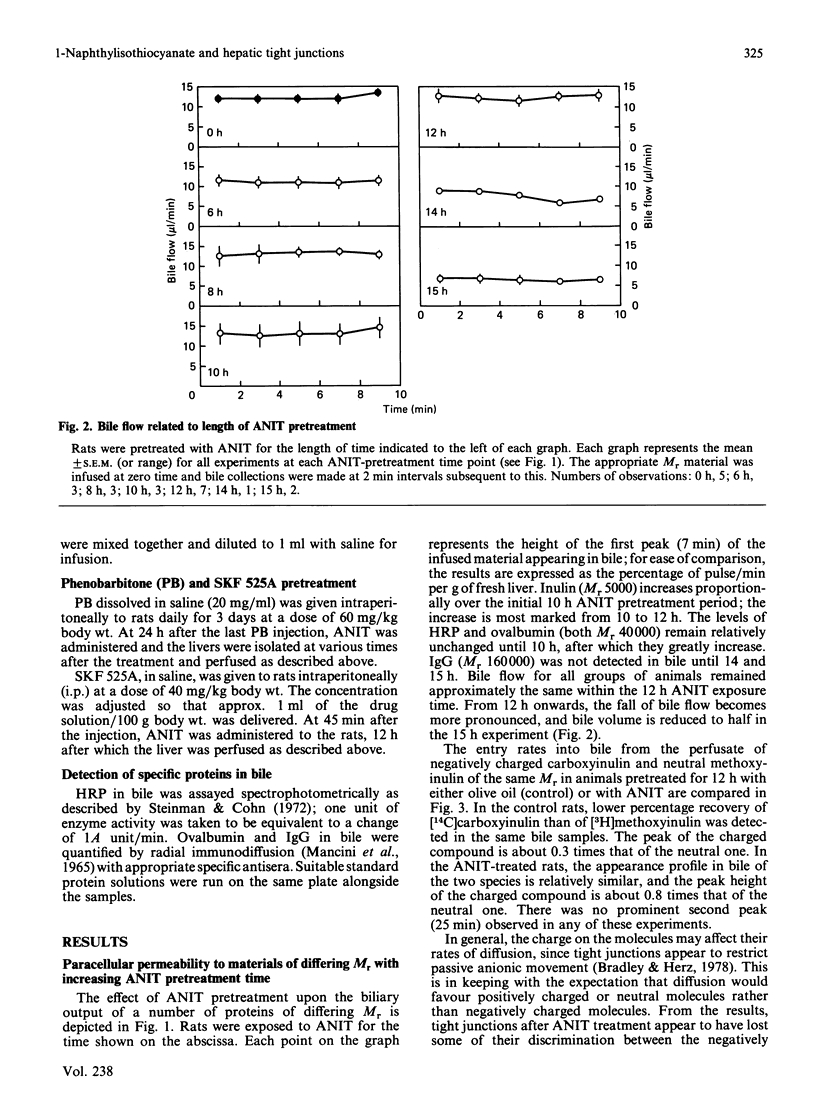
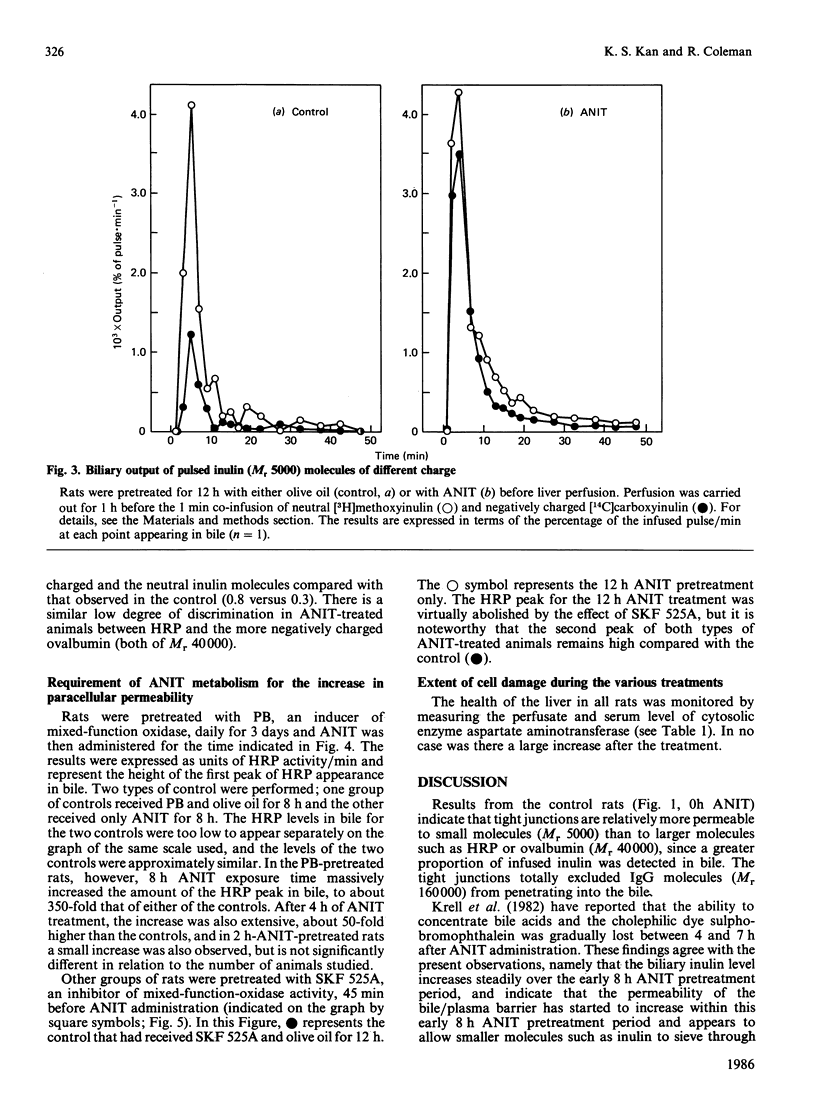
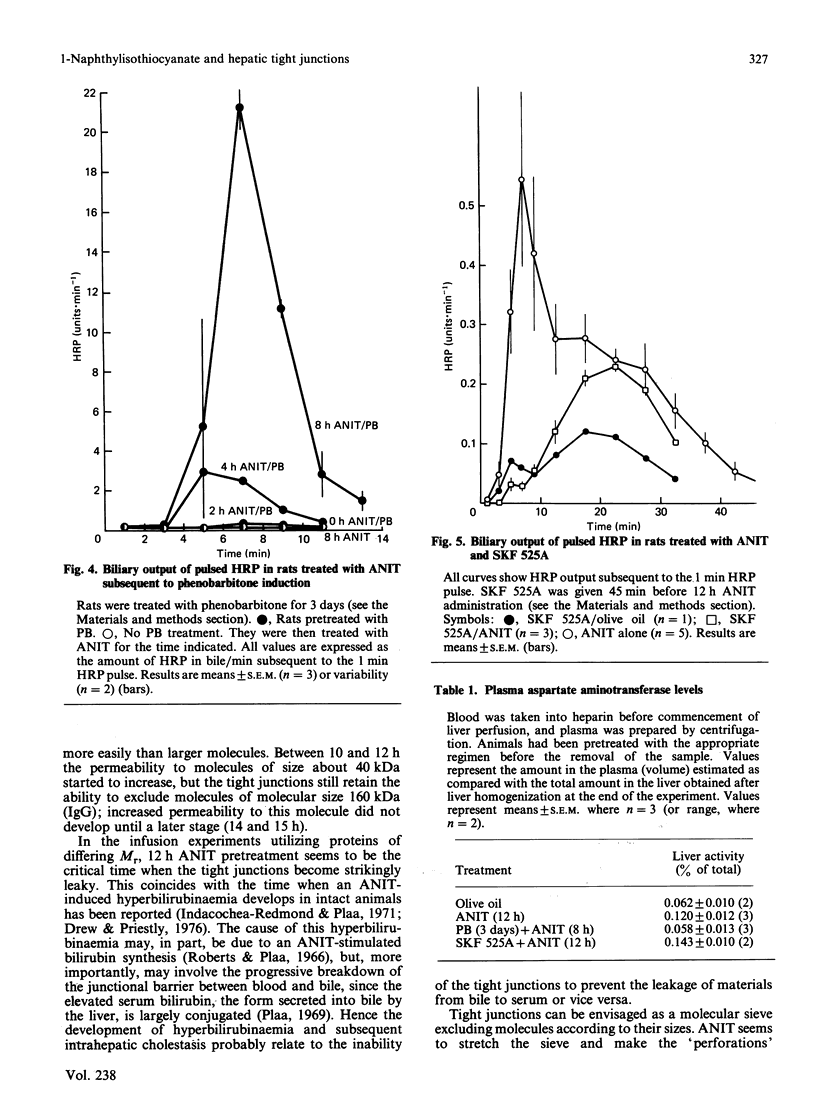
We have studied the early action of 1-naphthylisothiocyanate (ANIT) in relation to its effect on the permeability barrier formed by hepatic tight junctions. Materials having different Mr values [inulin (5000), horseradish peroxidase (HRP) (40,000), ovalbumin (also 40,000) and pig gamma-globulin (IgG) (160,000)] were individually pulsed, within 1 min, into perfused rat livers operating under single-pass conditions. In untreated rats, a small peak of HRP and ovalbumin and a comparatively larger peak of inulin were observed in the bile at 7 min. In rats treated with ANIT, with increasing duration of ANIT treatment the inulin peak increased proportionally, whereas the HRP and ovalbumin peaks remained unchanged until after 10 h of ANIT exposure; gamma-globulin was not detected in the 7 min bile sample until after 14 h of ANIT treatment. Bile flow in all rats remained approximately the same until after 14 h of ANIT pretreatment, when substantial bile-flow reduction was observed. Phenobarbitone pretreatment increased the effect of ANIT and massively elevated the first HRP peak; it also shortened the time (to 4 h) at which the increase in permeability to this protein was observed. In contrast, the first HRP peak was virtually abolished in rats that had received the mixed-function-oxidase inhibitor SKF 525A. These experiments suggest that (i) ANIT progressively increased the permeability of the junctional barrier before the reduction in bile flow, (ii) the ANIT-increased permeability change seems to be inversely dependent upon the Mr of the infused proteins, and (iii) metabolites of ANIT were involved in the development of the junctional permeability change.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnwell S. G., Coleman R. Abnormal secretion of proteins into bile from colchicine-treated isolated perfused rat livers. Biochem J. 1983 Nov 15;216(2):409–414. doi: 10.1042/bj2160409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. L., Elias E., Layden T. J. The paracellular pathway and bile formation. Yale J Biol Med. 1979 Jan-Feb;52(1):61–67. [PMC free article] [PubMed] [Google Scholar]
- Bradley S. E., Herz R. Permselectivity of biliary canalicular membrane in rats: clearance probe analysis. Am J Physiol. 1978 Nov;235(5):E570–E576. doi: 10.1152/ajpendo.1978.235.5.E570. [DOI] [PubMed] [Google Scholar]
- Dive C., Heremans J. F. Nature and origin of the proteins of bile. I. A comparative analysis of serum and bile proteins in man. Eur J Clin Invest. 1974 Aug;4(4):235–239. doi: 10.1111/j.1365-2362.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Dive C., Nadalini R. A., Vaerman J. P., Heremans J. F. Origin and nature of the proteins of bile. II. A comparative analysis of serum, hepatic lymph and bile proteins in the dog. Eur J Clin Invest. 1974 Aug;4(4):241–246. doi: 10.1111/j.1365-2362.1974.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Drew R., Priestly B. G. Microsomal drug metabolism during alpha-naphthylisothiocyanate-induced cholestasis. Toxicol Appl Pharmacol. 1976 Mar;35(3):491–499. doi: 10.1016/0041-008x(76)90072-7. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Kremmmer T., Culvenor J. G. Role of membranes in bile formation. Comparison of the composition of bile and a liver bile-canalicular plasma-membrane subfraction. Biochem J. 1976 Mar 15;154(3):589–595. doi: 10.1042/bj1540589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. G., Gyure L. A., Payne A. W. Comparative aspects of the transport of immunoglobulin A from blood to bile. Immunology. 1980 Dec;41(4):899–902. [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton R. H., Dobrota M., Mullock B. M. Haptoglobin-mediated transfer of haemoglobin from serum into bile. FEBS Lett. 1980 Apr 7;112(2):247–250. doi: 10.1016/0014-5793(80)80190-6. [DOI] [PubMed] [Google Scholar]
- Indacochea-Redmond N., Plaa G. L. Functional effects of -naphthylisothiocyanate in various species. Toxicol Appl Pharmacol. 1971 May;19(1):71–80. doi: 10.1016/0041-008x(71)90191-8. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Krell H., Pfaff E. No increase of biliary permeability in ethinylestradiol-treated rats. Gastroenterology. 1983 Oct;85(4):808–814. [PubMed] [Google Scholar]
- John W. G., Mullock B. M., Hinton R. H. Proteins of guinea-pig bile: selective resorption in the gall bladder. Biosci Rep. 1983 Apr;3(4):389–394. doi: 10.1007/BF01122904. [DOI] [PubMed] [Google Scholar]
- Kakis G., Yousef I. M. Protein composition of rat bile. Can J Biochem. 1978 May;56(5):287–290. doi: 10.1139/o78-044. [DOI] [PubMed] [Google Scholar]
- Krell H., Höke H., Pfaff E. Development of intrahepatic cholestasis by alpha-naphthylisothiocyanate in rats. Gastroenterology. 1982 Mar;82(3):507–514. [PubMed] [Google Scholar]
- Krell H., Jäschke H., Höke H., Pfaff E. Bile secretion in hemoglobin-free perfused rat liver. Hoppe Seylers Z Physiol Chem. 1984 Sep;365(9):1115–1122. doi: 10.1515/bchm2.1984.365.2.1115. [DOI] [PubMed] [Google Scholar]
- Lowe P. J., Kan K. S., Barnwell S. G., Sharma R. K., Coleman R. Transcytosis and paracellular movements of horseradish peroxidase across liver parenchymal tissue from blood to bile. Effects of alpha-naphthylisothiocyanate and colchicine. Biochem J. 1985 Jul 15;229(2):529–537. doi: 10.1042/bj2290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McLEAN M. R., REES K. R. Hyperplasia of bile-ducts induced by alpha-naphthyl-iso-thiocyanate: experimental biliary cirrhosis free from biliary obstruction. J Pathol Bacteriol. 1958 Jul;76(1):175–188. doi: 10.1002/path.1700760120. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Dobrota M., Hinton R. H. Sources of the proteins of rat bile. Biochim Biophys Acta. 1978 Nov 1;543(4):497–507. doi: 10.1016/0304-4165(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H. The relation of bile proteins to serum and liver plasma membrane [proceedings]. Biochem Soc Trans. 1978;6(1):274–276. doi: 10.1042/bst0060274. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Shaw L. J., Fitzharris B., Peppard J., Hamilton M. J., Simpson M. T., Hunt T. M., Hinton R. H. Sources of proteins in human bile. Gut. 1985 May;26(5):500–509. doi: 10.1136/gut.26.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlans E., Peppard J. V., Payne A. W., Fitzharris B. M., Mullock B. M., Hinton R. H., Hall J. G. Comparative aspects of the hepatobiliary transport of IgA. Ann N Y Acad Sci. 1983 Jun 30;409:411–427. doi: 10.1111/j.1749-6632.1983.tb26886.x. [DOI] [PubMed] [Google Scholar]
- Plaa G. L. Functional aspects of the cholestatic response induced by -naphthylisothiocyanate in mice and rats. Agents Actions. 1969 Nov;1(2):22–27. doi: 10.1007/BF01977662. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Plaa G. L. Potentiation and inhibition of alpha-naphthylisothiocyanate-induced hyperbilirubinemia and cholestasis. J Pharmacol Exp Ther. 1965 Dec;150(3):499–506. [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. Studies on the mechanisms of biliary excretion of circulating glycoproteins. The carcinoembryonic antigen. Biochem J. 1980 Dec 15;192(3):837–843. doi: 10.1042/bj1920837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Toth C. A., Zamcheck N. The mechanism of biliary excretion of alpha 1-acid glycoprotein in the rat: evidence for a molecular weight-dependent, nonreceptor-mediated pathway. Hepatology. 1982 Nov-Dec;2(6):800–803. doi: 10.1002/hep.1840020610. [DOI] [PubMed] [Google Scholar]


