Abstract
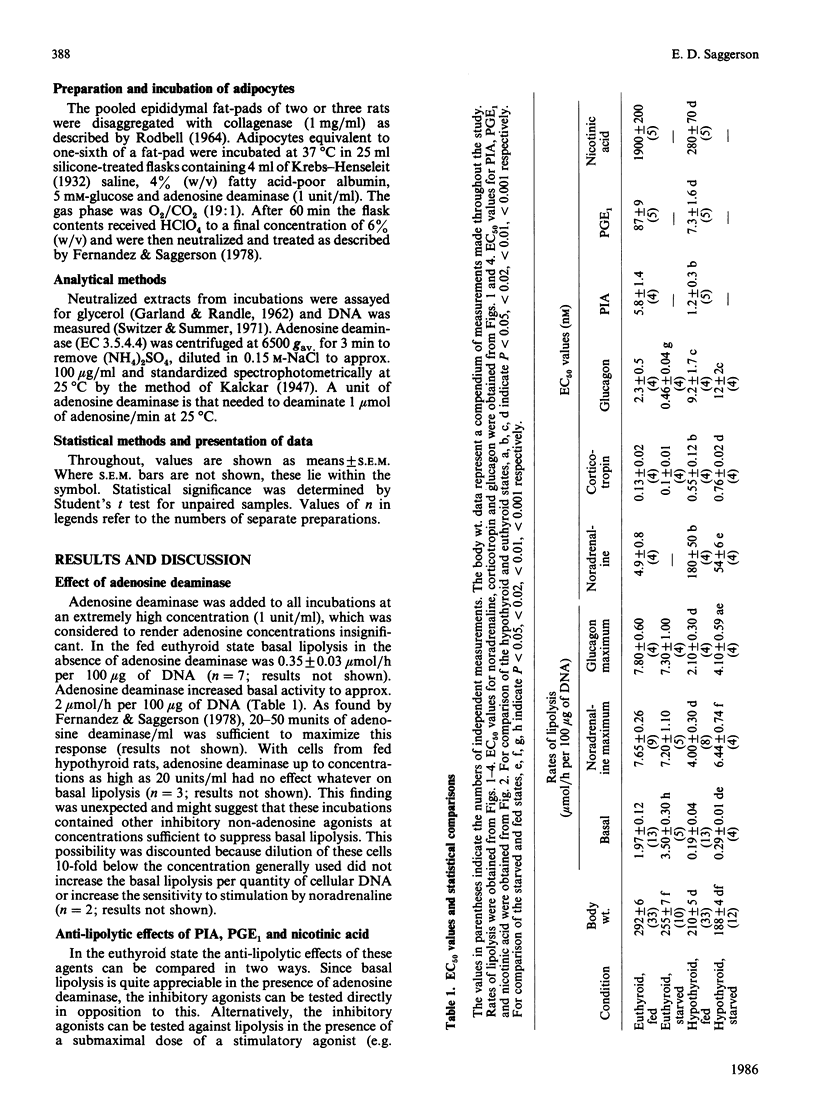
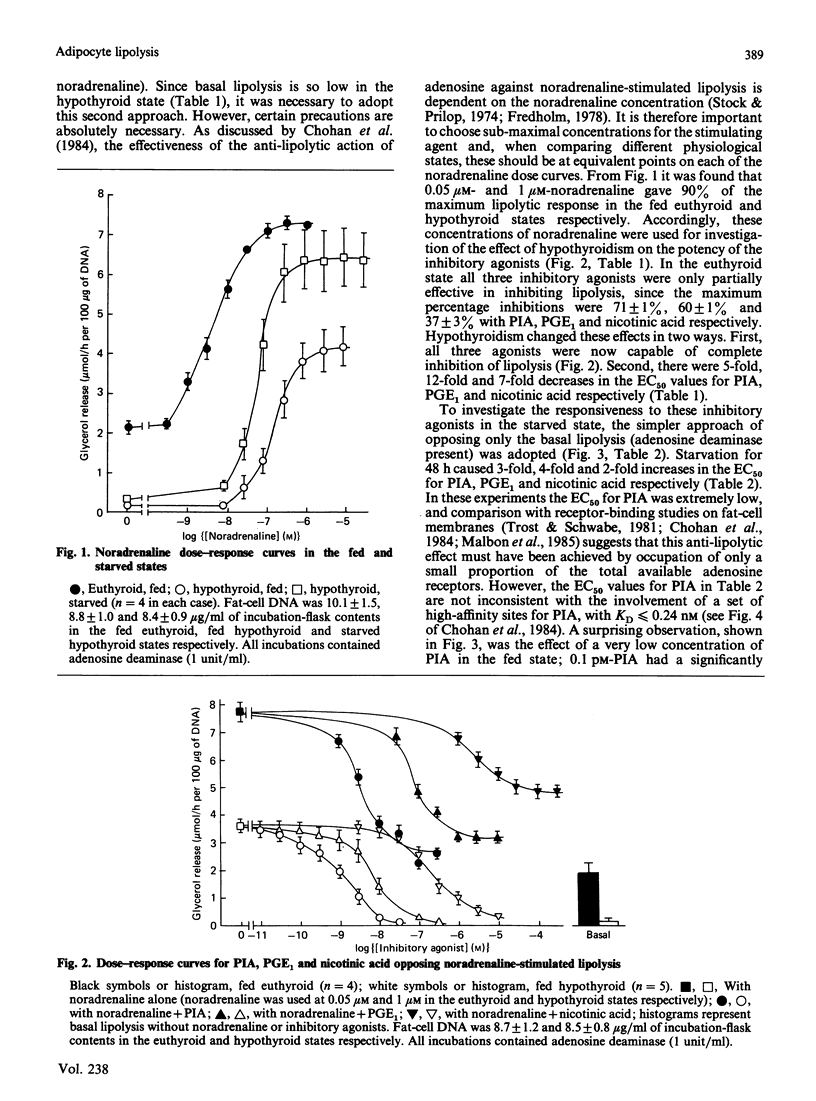
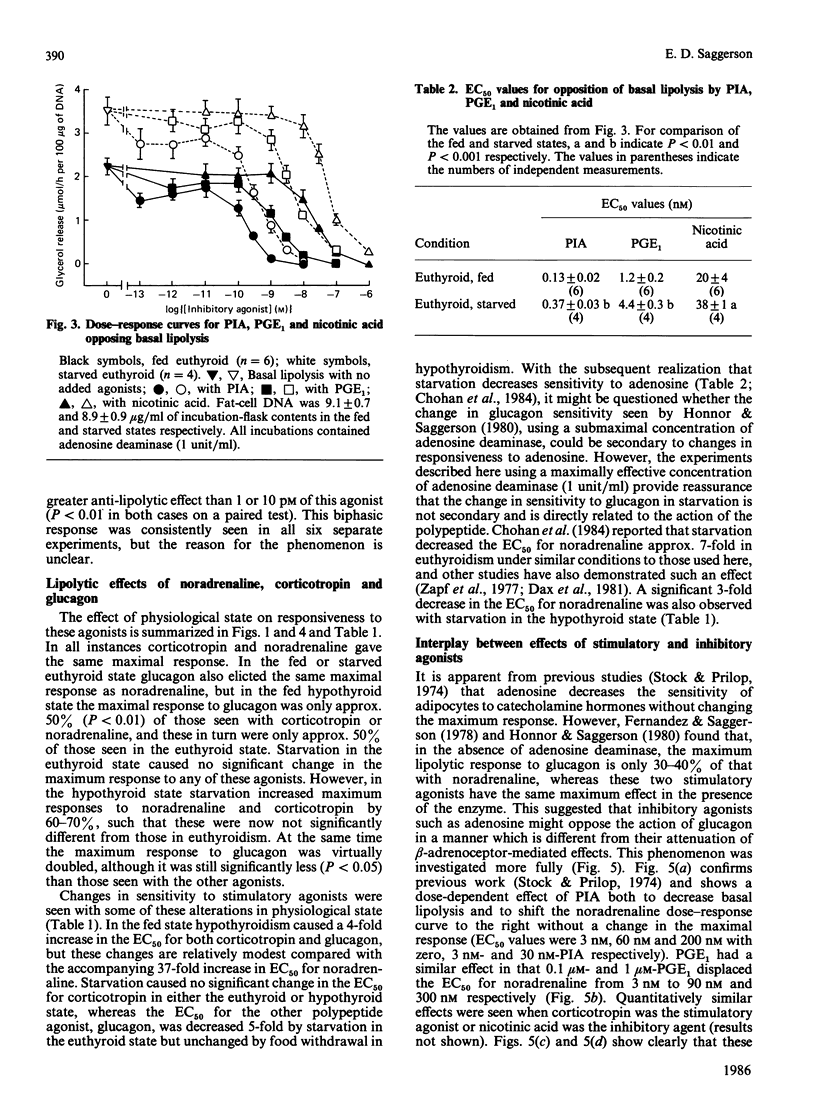
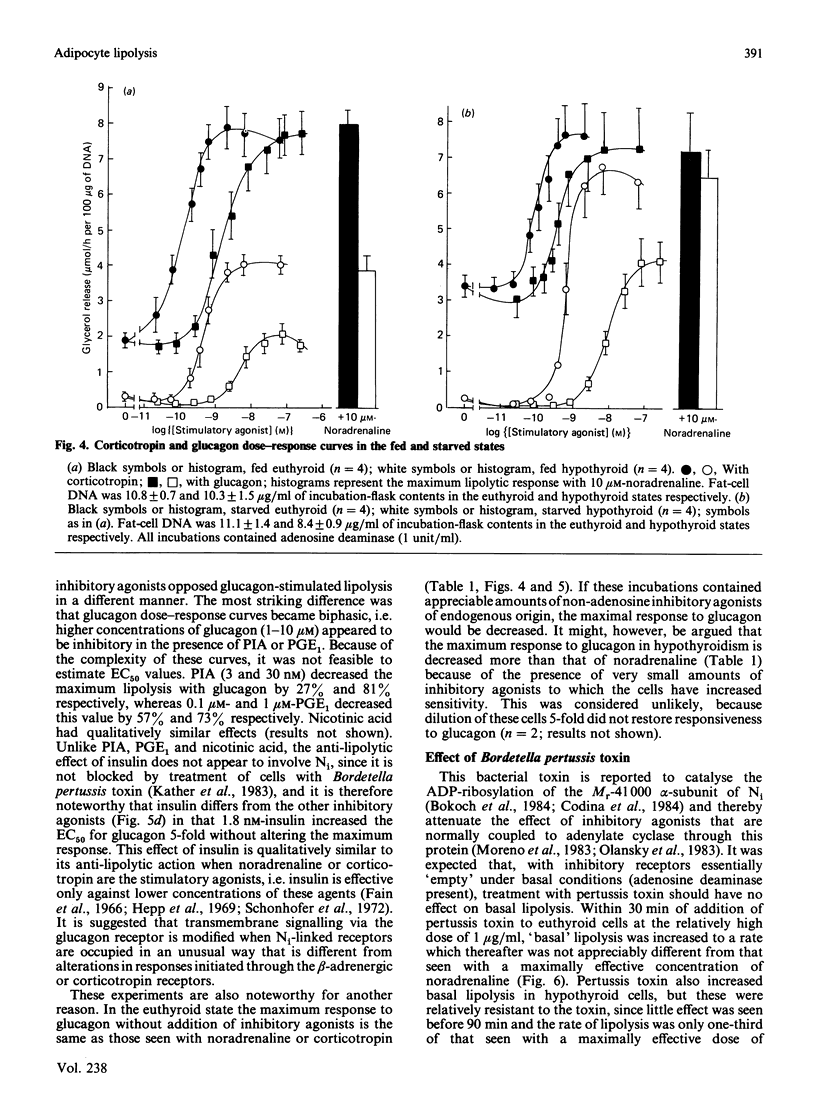
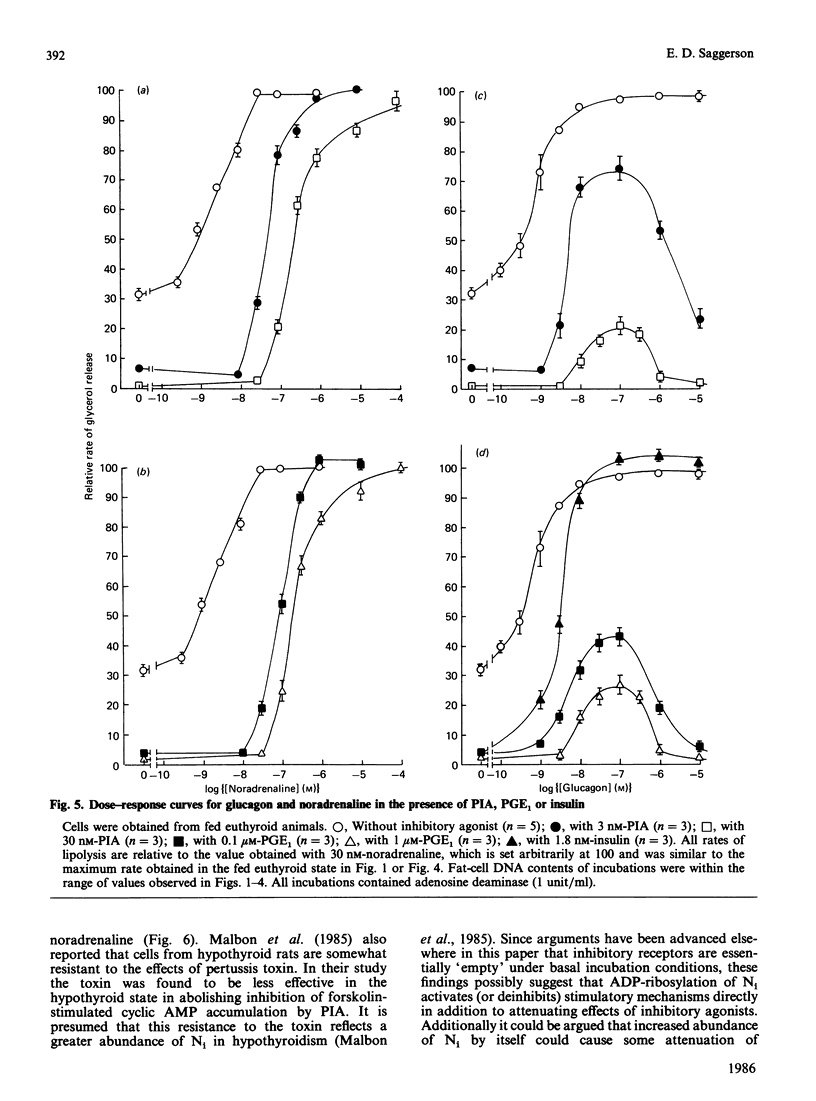
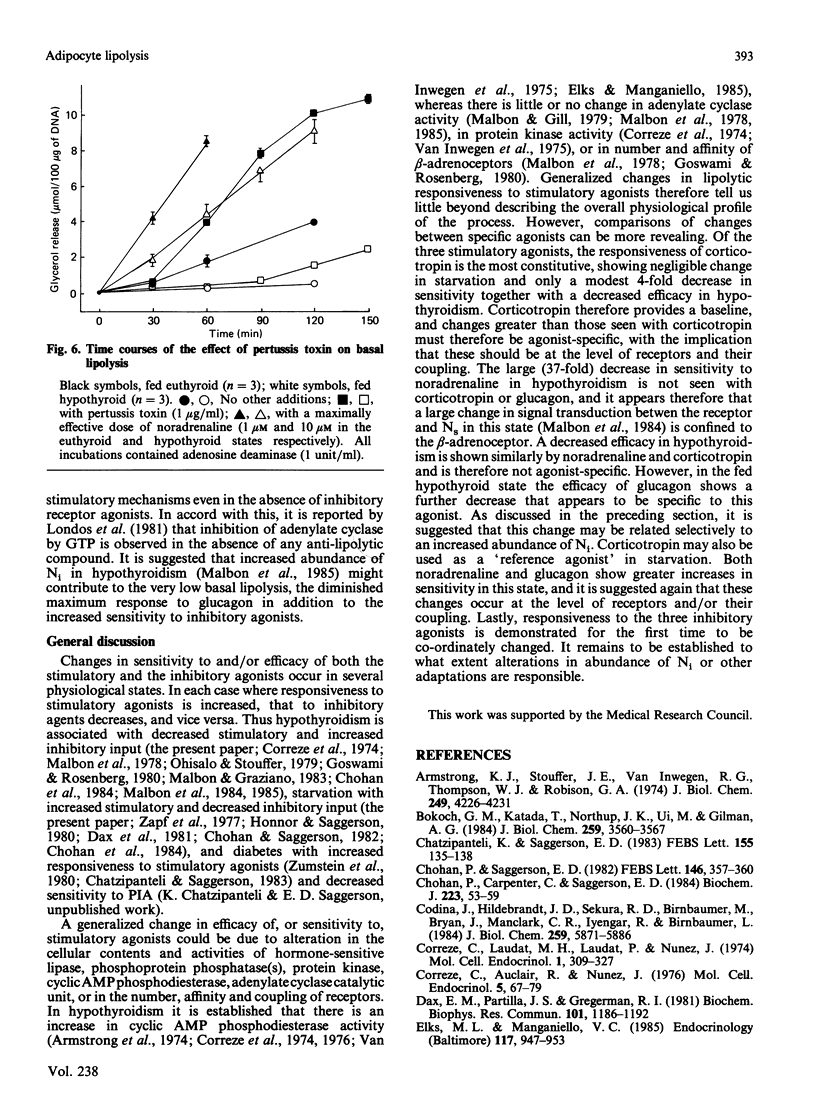
The responsiveness of lipolysis to the stimulatory agonists noradrenaline, corticotropin and glucagon and to the inhibitory agonists N6-phenylisopropyladenosine, prostaglandin E1 and nicotinic acid was investigated with rat white adipocytes incubated with a high concentration of adenosine deaminase (1 unit/ml). The cells were obtained from fed or 48 h-starved euthyroid animals or from fed or starved animals rendered hypothyroid by 4 weeks of treatment with low-iodine diet and propylthiouracil. Hypothyroidism increased sensitivity to and efficacy of all three inhibitory agonists in their opposition of noradrenaline-stimulated lipolysis. Starvation decreased sensitivity to all three inhibitory agonists when opposing basal lipolysis. Hypothyroidism decreased sensitivity to noradrenaline, glucagon and corticotropin by 37-, 4- and 4-fold respectively and decreased the maximum response to these agonists by approx. 50%, 50% and 75% respectively. Starvation reversed decreases in maximum response to these agonists in hypothyroidism. Starvation in the euthyroid state increased sensitivity to glucagon and noradrenaline, but did not alter sensitivity to corticotropin. Cells from hypothyroid rats were relatively insensitive to Bordetella pertussis toxin, which substantially increased basal lipolysis in the euthyroid state.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong K. J., Stouffer J. E., Van Inwegen R. G., Thompson W. J., Robison G. A. Effects of thyroid hormone deficiency on cyclic adenosine 3':5'-monophosphate and control of lipolysis in fat cells. J Biol Chem. 1974 Jul 10;249(13):4226–4231. [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Chatzipanteli K., Saggerson D. Streptozotocin diabetes results in increased responsiveness of adipocyte lipolysis to glucagon. FEBS Lett. 1983 May 2;155(1):135–138. doi: 10.1016/0014-5793(83)80225-7. [DOI] [PubMed] [Google Scholar]
- Chohan P., Carpenter C., Saggerson E. D. Changes in the anti-lipolytic action and binding to plasma membranes of N6-L-phenylisopropyladenosine in adipocytes from starved and hypothyroid rats. Biochem J. 1984 Oct 1;223(1):53–59. doi: 10.1042/bj2230053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan P., Saggerson D. Increased sensitivity of adipocyte adenylate cyclase to glucagon in the fasted state. FEBS Lett. 1982 Sep 20;146(2):357–360. doi: 10.1016/0014-5793(82)80952-6. [DOI] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Sekura R. D., Birnbaumer M., Bryan J., Manclark C. R., Iyengar R., Birnbaumer L. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylyl cyclases. Purification of the human erythrocyte proteins without the use of activating regulatory ligands. J Biol Chem. 1984 May 10;259(9):5871–5886. [PubMed] [Google Scholar]
- Correze C., Auclair R., Nunez J. Cyclic nucleotide phosphodiesterases, insulin and thyroid hormones. Mol Cell Endocrinol. 1976 Jun-Jul;5(1-2):67–79. doi: 10.1016/0303-7207(76)90071-x. [DOI] [PubMed] [Google Scholar]
- Correze C., Laudat M. H., Laudat P., Nunez J. Hormone-dependent lipolysis in fat-cells from thyroidectomized rats. Mol Cell Endocrinol. 1974 Oct;1(5):309–327. doi: 10.1016/0303-7207(74)90021-5. [DOI] [PubMed] [Google Scholar]
- Dax E. M., Partilla J. S., Gregerman R. I. Increased sensitivity to epinephrine stimulated lipolysis during starvation: tighter coupling of the adenylate cyclase complex. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1186–1192. doi: 10.1016/0006-291x(81)91573-4. [DOI] [PubMed] [Google Scholar]
- Elks M. L., Manganiello V. C. Effects of thyroid hormone on regulation of lipolysis and adenosine 3',5'-monophosphate metabolism in 3T3-L1 adipocytes. Endocrinology. 1985 Sep;117(3):947–953. doi: 10.1210/endo-117-3-947. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Kovacev V. P., Scow R. O. Antilipolytic effect of insulin in isolated fat cells of the rat. Endocrinology. 1966 Apr;78(4):773–778. doi: 10.1210/endo-78-4-773. [DOI] [PubMed] [Google Scholar]
- Fernandez B. M., Saggerson E. D. Alterations in response of rat white adipocytes to insulin, noradrenaline, corticotropin and glucagon after adrenalectomy. Correction of these changes by adenosine deaminase. Biochem J. 1978 Jul 15;174(1):111–118. doi: 10.1042/bj1740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B. Effect of adenosine, adenosine analogues and drugs inhibiting adenosine inactivation on lipolysis in rat fat cells. Acta Physiol Scand. 1978 Feb;102(2):191–198. doi: 10.1111/j.1748-1716.1978.tb06062.x. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Bray G. A. Role of thyroid hormones in lipolysis. Am J Physiol. 1966 May;210(5):1053–1058. doi: 10.1152/ajplegacy.1966.210.5.1053. [DOI] [PubMed] [Google Scholar]
- Goswami A., Rosenberg I. N. Thyroid hormone modulation of epinephrine-induced lipolysis in rat adipocytes: a possible role of calcium. Endocrinology. 1978 Dec;103(6):2223–2233. doi: 10.1210/endo-103-6-2223. [DOI] [PubMed] [Google Scholar]
- Hepp K. D., Menahan L. A., Wieland O., Williams R. H. Studies on the action of insulin in isolated adipose tissue cells. II. 3',5'-Nucleotide phosphodiesterase and antilipolysis. Biochim Biophys Acta. 1969 Sep 2;184(3):554–565. doi: 10.1016/0304-4165(69)90269-4. [DOI] [PubMed] [Google Scholar]
- Honnor R. C., Saggerson E. D. Altered lipolytic response to glucagon and adenosine deaminase in adipocytes from starved rats. Biochem J. 1980 Jun 15;188(3):757–761. doi: 10.1042/bj1880757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kather H., Aktories K., Schulz G., Jakobs K. H. Islet-activating protein discriminates the antilipolytic mechanism of insulin from that of other antilipolytic compounds. FEBS Lett. 1983 Sep 5;161(1):149–152. doi: 10.1016/0014-5793(83)80749-2. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Rodbell M. Receptor-mediated stimulation and inhibition of adenylate cyclases: the fat cell as a model system. Adv Cyclic Nucleotide Res. 1981;14:163–171. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C., Gill D. M. ADP-ribosylation of membrane proteins and activation of adenylate cyclase by cholera toxin in fat cell ghosts from euthyroid and hypothyroid rats. Biochim Biophys Acta. 1979 Sep 3;586(3):518–527. doi: 10.1016/0304-4165(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Graziano M. P. Adenosine deaminase normalizes cyclic AMP responses of hypothyroid rat fat cells to forskolin, but not beta-adrenergic agonists. FEBS Lett. 1983 May 2;155(1):35–38. doi: 10.1016/0014-5793(83)80203-8. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Graziano M. P., Johnson G. L. Fat cell beta-adrenergic receptor in the hypothyroid rat. Impaired interaction with the stimulatory regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 10;259(5):3254–3260. [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- Malbon C. C., Rapiejko P. J., Mangano T. J. Fat cell adenylate cyclase system. Enhanced inhibition by adenosine and GTP in the hypothyroid rat. J Biol Chem. 1985 Feb 25;260(4):2558–2564. [PubMed] [Google Scholar]
- Moreno F. J., Mills I., García-Sáinz J. A., Fain J. N. Effects of pertussis toxin treatment on the metabolism of rat adipocytes. J Biol Chem. 1983 Sep 25;258(18):10938–10943. [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Ohisalo J. J., Stouffer J. E. Adenosine, thyroid status and regulation of lipolysis. Biochem J. 1979 Jan 15;178(1):249–251. doi: 10.1042/bj1780249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olansky L., Myers G. A., Pohl S. L., Hewlett E. L. Promotion of lipolysis in rat adipocytes by pertussis toxin: reversal of endogenous inhibition. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6547–6551. doi: 10.1073/pnas.80.21.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D. Increased antilipolytic effect of the adenosine 'R-site' agonist N6-(phenylisopropyl)adenosine in adipocytes from adrenalectomized rats. FEBS Lett. 1980 Jun 16;115(1):127–128. doi: 10.1016/0014-5793(80)80741-1. [DOI] [PubMed] [Google Scholar]
- Schönhöfer P. S., Skidmore I. F., Krishna G. Effects of insulin on the lipolytic system of rat fat cells. Horm Metab Res. 1972 Nov;4(6):447–454. doi: 10.1055/s-0028-1094003. [DOI] [PubMed] [Google Scholar]
- Stock K., Prilop M. Dissociation of catecholamine-induced formation of adenosine 3'5'-monophosphate and release of glycerol in fat cells by prostaglandin E1, E2 and N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(1):15–31. doi: 10.1007/BF00647400. [DOI] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]
- Trost T., Schwabe U. Adenosine receptors in fat cells. Identification by (-)-N6-[3H]phenylisopropyladenosine binding. Mol Pharmacol. 1981 Mar;19(2):228–235. [PubMed] [Google Scholar]
- Van Inwegen R. G., Robison G. A., Thompson W. J. Cyclic nucleotide phosphodiesterases and thyroid hormones. J Biol Chem. 1975 Apr 10;250(7):2452–2456. [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Froesch E. R. Increased sensitivity of rat adipose tissue to the lipolytic action of epinephrine during fasting and its reversal during re-feeding. FEBS Lett. 1977 Apr 1;76(1):135–138. doi: 10.1016/0014-5793(77)80137-3. [DOI] [PubMed] [Google Scholar]
- Zumstein P., Zapf J., Waldvogel M., Froesch E. R. Increased sensitivity to lipolytic hormones of adenylate cyclase in fat cells of diabetic rats. Eur J Biochem. 1980 Mar;105(1):187–194. doi: 10.1111/j.1432-1033.1980.tb04488.x. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


