Abstract
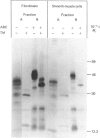
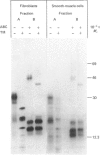
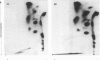
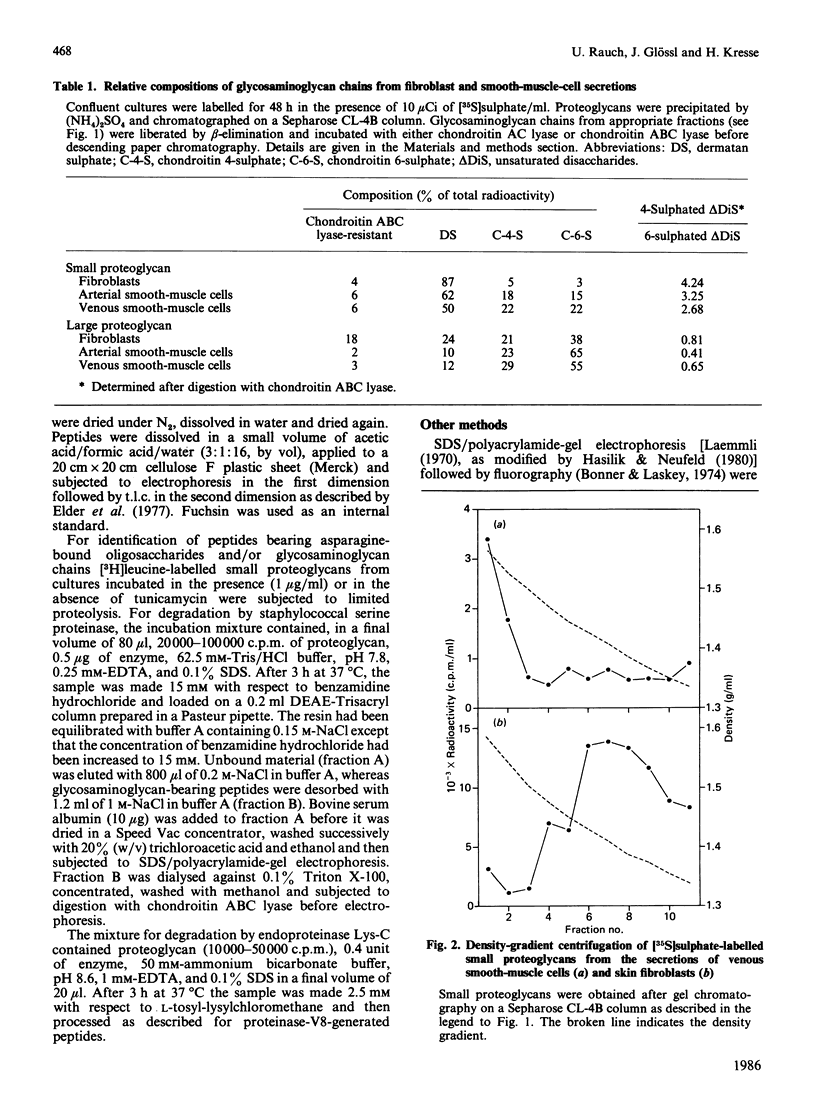
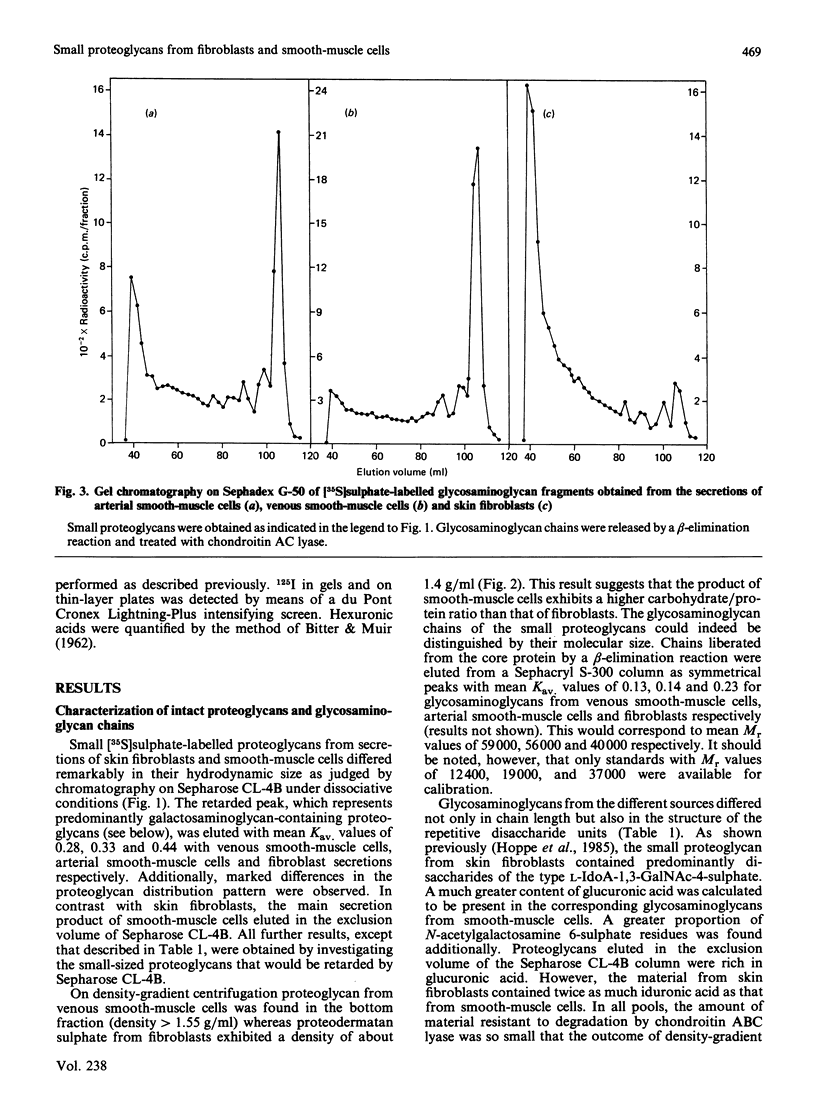
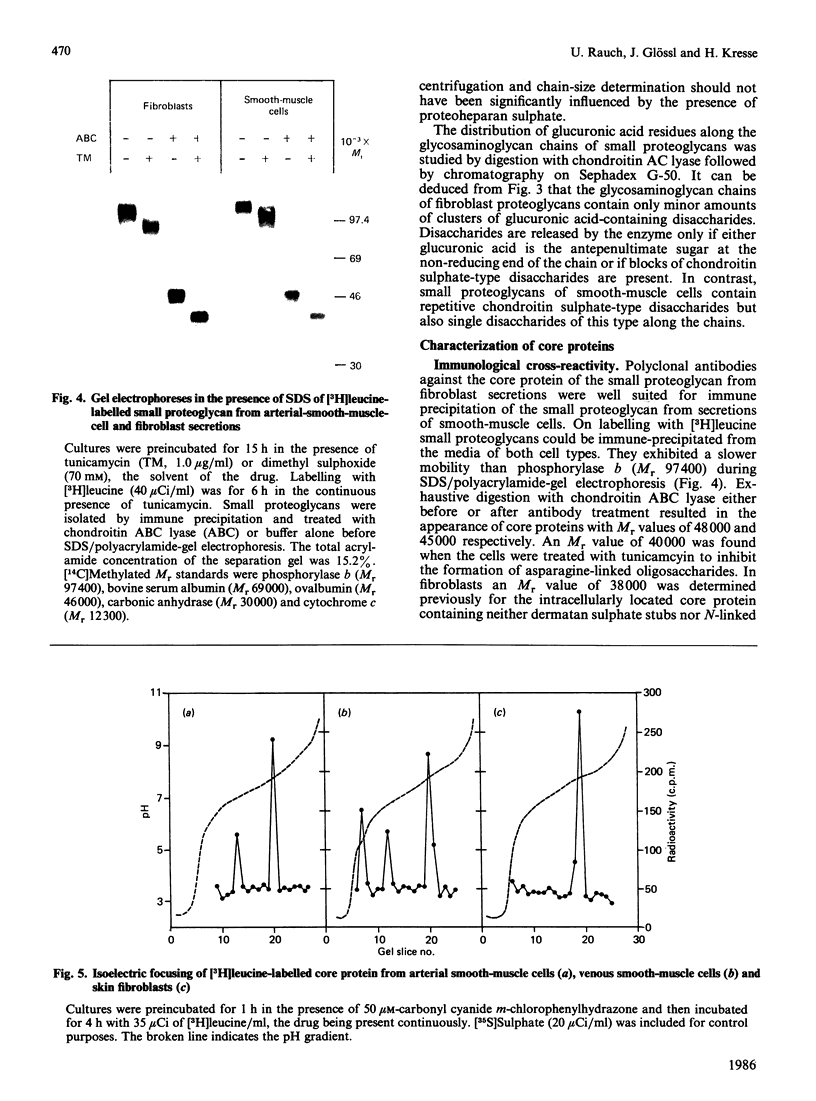
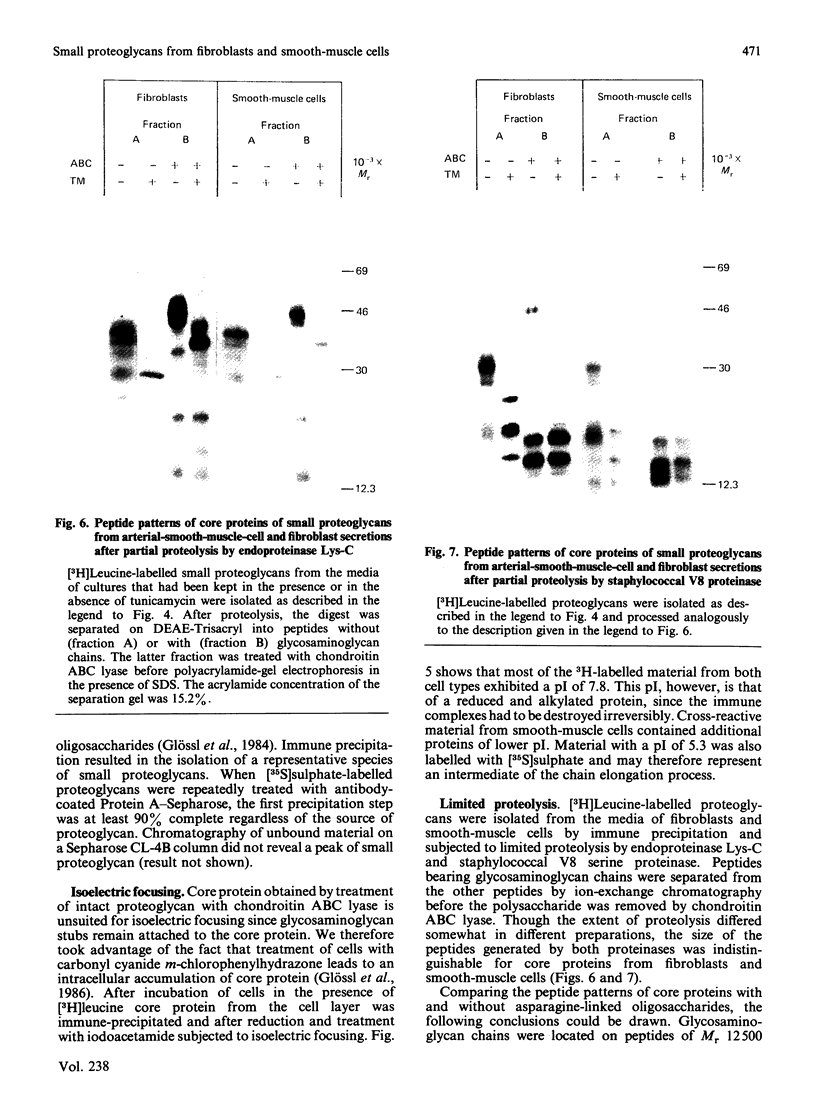
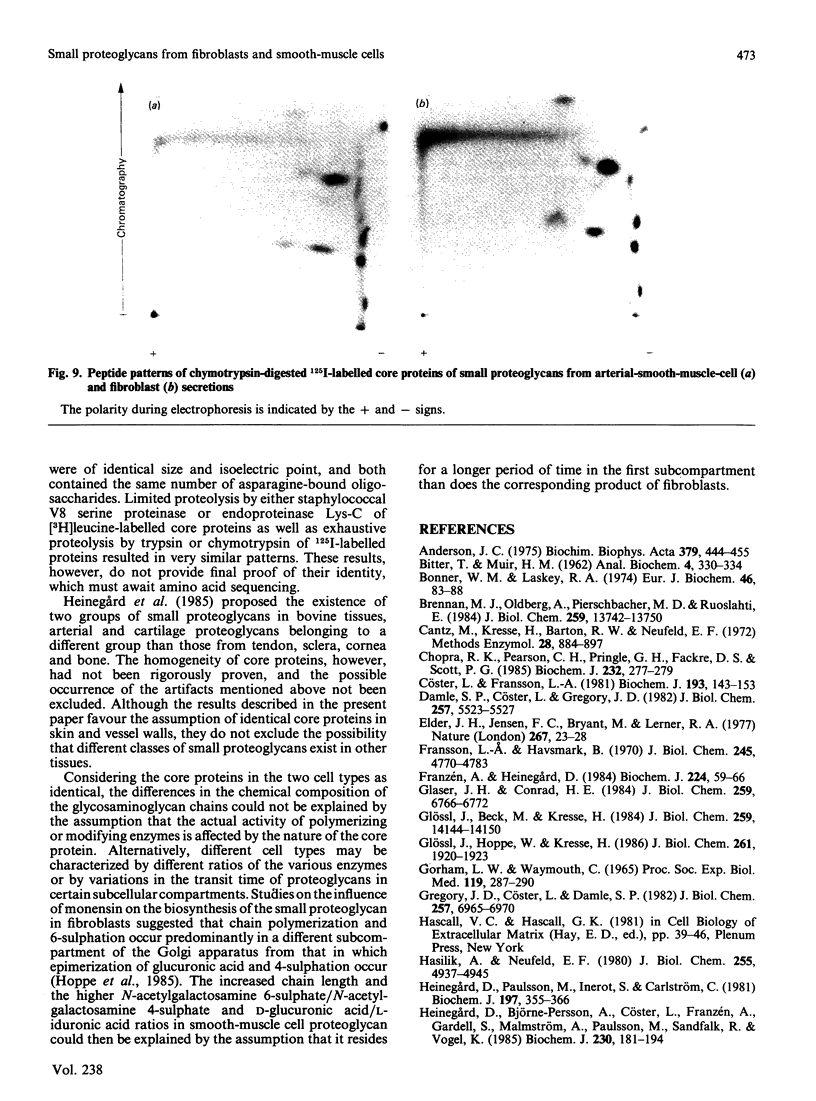
Physicochemical and chemical properties of small proteoglycans containing galactosaminoglycan chains from cultured human skin fibroblasts and human smooth-muscle cells were compared to determine the extent of structural similarity. The proteoglycan secreted by smooth-muscle cells was of larger molecular size and of higher buoyant density, due to longer glycosaminoglycan chains, than the secretion product of skin fibroblasts. Additionally, both proteoglycans differed in the ratio of iduronic acid and glucuronic acid residues. On the other hand, degradation of secreted [3H]leucine-labelled proteoglycans with chondroitin ABC lyase followed by SDS/polyacrylamide-gel electrophoresis resulted in the appearance of core protein bands of identical size (Mr 48,000 and 45,000, depending on the number of asparagine-bound oligosaccharides). An Mr value of 40,000 was determined for the core protein of cells pretreated with tunicamycin. An antibody against the core protein from fibroblast secretions was cross-reactive with the core protein from smooth-muscle cells. Core protein accumulating intracellularly after treatment with carbonyl cyanide m-chlorophenylhydrazone exhibited, on reduction and alkylation, an isoelectric point of 7.8 in both cell types. Limited proteolysis by staphylococcal V8 serine proteinase or endoproteinase Lys-C led in both instances to the formation of peptides of identical size. Peptides bearing asparagine-bound oligosaccharides were free of glycosaminoglycan chains. Similar peptide patterns were obtained when 125I-labelled core proteins were digested with either trypsin or chymotrypsin. Thus small proteoglycans from fibroblasts and smooth-muscle cells can be differentiated by their glycosaminoglycan moieties but not by the nature of their core proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. C. Isolation of a glycoprotein and proteodermatan sulphate from bovine achilles tendon by affinity chromatography on concanavalin A-Sepharose. Biochim Biophys Acta. 1975 Feb 27;379(2):444–455. doi: 10.1016/0005-2795(75)90151-8. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Oldberg A., Pierschbacher M. D., Ruoslahti E. Chondroitin/dermatan sulfate proteoglycan in human fetal membranes. Demonstration of an antigenically similar proteoglycan in fibroblasts. J Biol Chem. 1984 Nov 25;259(22):13742–13750. [PubMed] [Google Scholar]
- Chopra R. K., Pearson C. H., Pringle G. A., Fackre D. S., Scott P. G. Dermatan sulphate is located on serine-4 of bovine skin proteodermatan sulphate. Demonstration that most molecules possess only one glycosaminoglycan chain and comparison of amino acid sequences around glycosylation sites in different proteoglycans. Biochem J. 1985 Nov 15;232(1):277–279. doi: 10.1042/bj2320277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cöster L., Fransson L. A. Isolation and characterization of dermatan sulphate proteoglycans from bovine sclera. Biochem J. 1981 Jan 1;193(1):143–153. doi: 10.1042/bj1930143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle S. P., Cöster L., Gregory J. D. Proteodermatan sulfate isolated from pig skin. J Biol Chem. 1982 May 25;257(10):5523–5527. [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Havsmark B. Structure of dermatan sulfate. VII. The copolymeric structure of dermatan sulfate from horse aorta. J Biol Chem. 1970 Sep 25;245(18):4770–4783. [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Characterization of proteoglycans from the calcified matrix of bovine bone. Biochem J. 1984 Nov 15;224(1):59–66. doi: 10.1042/bj2240059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORHAM L. W., WAYMOUTH C. DIFFERENTIATION IN VITRO OF EMBRYONIC CARTILAGE AND BONE IN A CHEMICALLY-DEFINED MEDIUM. Proc Soc Exp Biol Med. 1965 May;119:287–290. doi: 10.3181/00379727-119-30160. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Conrad H. E. Properties of chick embryo chondrocytes grown in serum-free medium. J Biol Chem. 1984 Jun 10;259(11):6766–6772. [PubMed] [Google Scholar]
- Glössl J., Beck M., Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984 Nov 25;259(22):14144–14150. [PubMed] [Google Scholar]
- Glössl J., Hoppe W., Kresse H. Post-translational phosphorylation of proteodermatan sulfate. J Biol Chem. 1986 Feb 5;261(4):1920–1923. [PubMed] [Google Scholar]
- Gregory J. D., Cöster L., Damle S. P. Proteoglycans of rabbit corneal stroma. Isolation and partial characterization. J Biol Chem. 1982 Jun 25;257(12):6965–6970. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Paulsson M., Inerot S., Carlström C. A novel low-molecular weight chondroitin sulphate proteoglycan isolated from cartilage. Biochem J. 1981 Aug 1;197(2):355–366. doi: 10.1042/bj1970355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe W., Glössl J., Kresse H. Influence of monensin on biosynthesis, processing and secretion of proteodermatan sulfate by skin fibroblasts. Eur J Biochem. 1985 Oct 1;152(1):91–97. doi: 10.1111/j.1432-1033.1985.tb09167.x. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Wight T. N. Isolation and characterization of proteoglycans synthesized by human colon and colon carcinoma. J Biol Chem. 1982 Sep 25;257(18):11135–11144. [PubMed] [Google Scholar]
- Kapoor R., Phelps C. F., Cöster L., Fransson L. A. Bovine aortic chondroitin sulphate- and dermatan sulphate-containing proteoglycans. Isolation, fractionation and chemical characterization. Biochem J. 1981 Aug 1;197(2):259–268. doi: 10.1042/bj1970259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Ebert M. Protein variations associated with Lesch-Nyhan syndrome. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6471–6475. doi: 10.1073/pnas.78.10.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H. Proteoglycans as organizers of the intercellular matrix. Biochem Soc Trans. 1983 Dec;11(6):613–622. doi: 10.1042/bst0110613. [DOI] [PubMed] [Google Scholar]
- Pearson C. H., Gibson G. J. Proteoglycans of bovine periodontal ligament and skin. Occurrence of different hybrid-sulphated galactosaminoglycans in distinct proteoglycans. Biochem J. 1982 Jan 1;201(1):27–37. doi: 10.1042/bj2010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schmidt A., Prager M., Selmke P., Buddecke E. Isolation and properties of proteoglycans from bovine aorta. Eur J Biochem. 1982 Jun 15;125(1):95–101. doi: 10.1111/j.1432-1033.1982.tb06655.x. [DOI] [PubMed] [Google Scholar]
- Strålfors P., Belfrage P. Electrophoretic elution of proteins from polyacrylamide gel slices. Anal Biochem. 1983 Jan;128(1):7–10. doi: 10.1016/0003-2697(83)90336-6. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Vassalli P. Plasma cell immunoglobulin M molecules. Their biosynthesis, assembly, and intracellular transport. J Cell Biol. 1979 Nov;83(2 Pt 1):284–299. doi: 10.1083/jcb.83.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldbjerg N., Malmström A., Ekman G., Sheehan J., Ulmsten U., Wingerup L. Isolation and characterization of dermatan sulphate proteoglycan from human uterine cervix. Biochem J. 1983 Feb 1;209(2):497–503. doi: 10.1042/bj2090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W. D., Rowe H. A., Connor J. R. Biochemical characteristics of dissociatively isolated aortic proteoglycans and their binding capacity to hyaluronic acid. J Biol Chem. 1983 Sep 25;258(18):11136–11142. [PubMed] [Google Scholar]
- Wight T. N., Hascall V. C. Proteoglycans in primate arteries. III. Characterization of the proteoglycans synthesized by arterial smooth muscle cells in culture. J Cell Biol. 1983 Jan;96(1):167–176. doi: 10.1083/jcb.96.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. II. Synthesis and secretion of glycosaminoglycans by arterial smooth muscle cells in culture. J Cell Biol. 1975 Dec;67(3):675–686. doi: 10.1083/jcb.67.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagishita M., Rodbard D., Hascall V. C. Isolation and characterization of proteoglycans from porcine ovarian follicular fluid. J Biol Chem. 1979 Feb 10;254(3):911–920. [PubMed] [Google Scholar]