Abstract
The M2-1 protein of human respiratory syncytial virus (hRSV) promotes processive RNA synthesis and readthrough at RSV gene junctions. It contains four highly conserved cysteines, three of which are located in the Cys3-His1 motif at the N terminus of M2-1. Each of the four cysteines, at positions 7, 15, 21, and 96, in the M2-1 protein of hRSV A2 strain was individually replaced by glycines. When tested in an RSV minigenome replicon system using β-galactosidase as a reporter gene, C7G, C15G, and C21G located in the Cys3-His1 motif showed a significant reduction in processive RNA synthesis compared to wild-type (wt) M2-1. C96G, which lies outside the Cys3-His1 motif, was fully functional in supporting processive RNA synthesis in vitro. Each of these cysteine substitutions was introduced into an infectious antigenomic cDNA clone derived from hRSV A2 strain. Except for C96G, which resulted in a viable virus, no viruses were recovered with mutations in the Cys3-His1 motif. This indicates that the Cys3-His1 motif is critical for M2-1 function and for RSV replication. The functional requirement of the C terminus of the M2-1 protein was examined by engineering premature stop codons that caused truncations of 17, 46, or 67 amino acids from the C terminus. A deletion of 46 or 67 amino acids abolished the synthesis of full-length β-galactosidase mRNA and did not result in the recovery of viable viruses. However, a deletion of 17 amino acids from the C terminus of M2-1 reduced processive RNA synthesis in vitro and was well tolerated by RSV. Relocation of the M2-1 termination codon upstream of the M2-2 initiation codons did not significantly affect the expression of the M2-2 protein. Both rA2-Tr17 and rA2-C96G did not replicate as efficiently as wt rA2 in HEp-2 cells and was restricted in replication in the respiratory tracts of cotton rats.
Human respiratory syncytial virus (hRSV) is an enveloped nonsegmented negative-strand RNA virus classified in the genus Pneumovirus of the family Paramyxoviridae (19). The genomic RNA of hRSV A2 strain is 15,222 nucleotides (nt) in length and encodes 11 proteins from 10 genes in the following gene order: 3′ NS1-NS2-N-P-M-SH-G-F-M2-L 5′. Each gene transcription unit is flanked by highly conserved gene-start and gene-stop sequences and is monocistronic except for the M2 gene, which encodes two proteins unique to pneumoviruses, M2-1 and M2-2 (6, 7, 16). As with other negative-strand RNA viruses, synthesis of viral RNA requires a genomic RNA encapsidated with the nucleoprotein (N) along with the virus-encoded phosphoprotein (P) and the large (L) polymerase protein (12, 29). In addition, the M2-1 protein is also required for synthesis of RSV RNA. The M2-1 protein is an antiterminator that prevents premature termination during transcription (6, 10, 11) and enhances read-through transcription at gene junctions (13–15).
The M2 mRNAs of all pneumoviruses encode two open reading frames (ORFs) that overlap at a similar location but with different overlapping sequences (1, 8). The M2-1 of hRSV A2 strain utilizes approximately 70% of the entire coding capacity of the M2 mRNA. The second ORF is located towards the 3′ end of the mRNA and overlaps M2-1 by 4, 8, or 10 amino acids, depending on the initiation codon(s) used for translating M2-2. It has been proposed that the translation of hRSV M2-2 occurs by a mechanism that involves reverse translocation of ribosomes terminating at the first downstream M2-1 stop codon (1). The M2-2 protein is dispensable for RSV replication, and present data indicate that M2-2 is involved in regulating the switch between viral RNA transcription and replication (3, 17).
The M2-1 protein of hRSV A2 strain is 194 amino acids in length, with a molecular weight of approximately 22,150 (6, 7). It contains a Cys3-His1 motif in the N terminus from residues 7 to 25 that is highly conserved among human, bovine, ovine, and murine strains of pneumoviruses (1, 2, 29). Nuclear magnetic resonance spectroscopy and zinc back-titration analyses of an analogous Cys3-His1 motif found in the mammalian transcription factor Nup475 indicate that the cysteines and histidine are involved in coordinating zinc (26). Replacement of cysteine 7 and 15 and histidine 25 by serine in this motif reduced the ability of M2-1 protein to enhance transcription read-through and disrupted the interaction between M2-1 and the N protein in transfected cells (14). Mutations in the Cys3-His1 motif also affected the phosphorylation of the M2-1 protein (14). In addition to the three cysteines in the Cys3-His1 motif, a fourth cysteine that is highly conserved among the M2-1 proteins of pneumoviruses is present at position 96 (9). Cysteines are often involved in intra- and intermolecular disulfide bond formations that are important for the structural and functional integrity of proteins. For example, substitutions of glycines for cysteines in the α trans-inducing factor of herpes simplex virus resulted in temperature-sensitive viruses (21). It is therefore of interest to know if the cysteine at position 96 of M2-1 is also important for protein function and for virus replication.
Sequence alignment of the M2-1 proteins from different pneumoviruses revealed heterogeneity in lengths at the C terminus of the protein (9). Among all known pneumovirus M2-1 proteins, the pneumovirus of mice (PVM) M2-1 is the shortest, differing by 17 amino acids from that of hRSV A2 strain (1, 9). Although a functional motif has been located at the N terminus of M2-1, the requirements of the C terminus for protein function and virus replication are not known.
In this study we investigated the role of the cysteine residues in M2-1 function by replacing each of the four cysteines individually with glycine. The functional requirement of the C terminus of M2-1 was also examined by deleting different numbers of amino acids from the C terminal end of the protein. Alterations engineered in the M2-1 protein were analyzed for their effects on processive RNA synthesis in vitro. Furthermore, these changes were introduced into an infectious antigenomic cDNA clone to evaluate their effects on virus recovery and virus replication.
MATERIALS AND METHODS
Cells and viruses.
Monolayers of Vero and HEp-2 cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. Modified vaccinia virus Ankara (MVA) expressing T7 RNA polymerase, MVA-T7, was provided by Bernard Moss (23, 28). MVA-T7 was propagated in CEK cells (SPAFAS).
Construction of RSV protein expression plasmids and minigenome.
The protein expression plasmids that contained the N, P, or L gene under the control of a T7 promoter in pCITE2a vector (Novagen) were described previously (18). The M2-1 ORF was cloned into pCITE2a under the control of the T7 promoter (pM2-1). The cysteine-to-glycine changes and tandem stop codons introduced into M2-1 are shown in Fig. 1. Mutations were introduced into pM2-1 using a QuikChange Site-Directed Mutagenesis Kit (Stratagene), and the sequence of the entire M2-1 gene was confirmed by DNA sequence analysis.
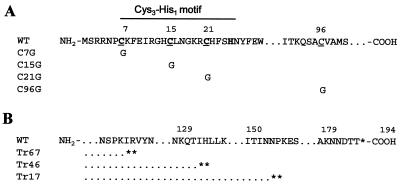
FIG. 1.
(A) Cysteine scanning mutagenesis of the M2-1 gene. The underlined cysteine residues at position 7, 15, 21, and 96 were changed to glycine, and the mutants are denoted C96G, C15G, C21G, and C96G. (B) Three C-terminal-end truncations were engineered by the introduction of tandem stop codons, indicated by asterisks. Mutants with a truncation of 67, 46, or 17 amino acids are designated Tr67, Tr46, or Tr17.
RSV minigenome, pRSVLacZ, was engineered to encode a negative-sense β-galactosidase gene under the control of the T7 promoter. The β-galactosidase gene contains the RSV gene-start and gene-stop sequences flanked by the RSV trailer and leader sequences. The RSV leader sequences are followed by the hepatitis delta virus ribozyme and the T7 polymerase terminator sequences. The cDNA encoding β-galactosidase was amplified by PCR using a pair of primers containing the NheI and NsiI restriction enzyme sites at its 5′ and 3′ ends, respectively. The NheI-to-NsiI restriction fragment was then used to replace the chloramphenicol acetyltransferase (CAT) gene in the pRSVCAT minigenome through the XbaI and PstI restriction sites. The NheI and NsiI restriction sites are compatible with the XbaI and PstI sites, respectively.
Construction of RSV antigenomic cDNA containing mutations in the M2-1 gene.
To introduce M2-1 mutations into the full-length RSV antigenomic A2 cDNA clone, each mutation was first individually engineered into an RSV subclone, pET-S/B. pET-S/B contained a SacI-to-BamHI restriction fragment bearing RSV sequences from nt 4477 to 8499, which included the entire M2 gene. The cysteine-to-glycine changes and the premature tandem stop codons were separately introduced into pET-S/B by using a QuikChange Site-Directed Mutagenesis Kit (Stratagene). The SacI-to-BamHI restriction fragments containing the M2-1 mutations were then used to replace the corresponding region in pRSVC4G (18). pRSVC4G contained a C-to-G change in the antigenomic sense in the RSV leader. All the M2-1 mutations introduced into the full-length antigenomic cDNA clones were confirmed by DNA sequencing.
Transfection and measurement of reporter gene expression.
RSV RNA transcription and replication programmed by an RNA minigenome replicon (pRSVLacZ) was analyzed by transfection following infection with a recombinant vaccinia virus expressing T7 polymerase. Subconfluent HEp-2 cells were infected with MVA-T7 at a multiplicity of infection (MOI) of 1 PFU/cell, and virus was allowed to adsorb at room temperature for 1 h. The MVA-T7-infected cells were then transfected with 0.2 μg of pN, 0.2 μg of pP, 0.1 μg of pL, 0.2 μg of pRSVLac Z, and 0.3 μg of pM2-1 or its derivatives unless otherwise indicated. The transfection was performed using lipofectACE (Life Technology) according to the manufacturer's protocol. The transfected cells were incubated at 33°C for 36 h, and cell extracts were prepared by lysis in cell permeabilization buffer that contained 0.5% NP-40 and 20 mM β-mercaptoethanol. Cell lysates were clarified by centrifugation at 2,500 rpm for 5 min at 4°C in an Eppendorf 5415C microcentrifuge and analyzed for β-galactosidase activity using the substrate chlorophenol red-β-d-galactopyranoside (CPRG; Roche Molecular Biochemicals). Aliquots of the clarified lysates were incubated with the detection buffer containing 5 mM CPRG in a microtiter plate at room temperature for various amounts of time. The change in optical density at 550 nm (OD550) was measured with SPECTRAmax, a 340PC microplate spectrophotometer using SOFTmax software (Molecular Devices). The assay was shown to be linearly responsive up to an OD550 of 3.0.
Recovery of infectious RSV bearing mutations in the M2-1 gene.
Transfection and recovery of infectious RSV bearing mutations in the M2-1 gene were performed as described previously for rescue of recombinant RSV (5, 18). Briefly, subconfluent HEp-2 cells infected with MVA-T7 at 1 PFU/cell were transfected with 0.4 μg of pN, 0.4 μg of pP, 0.2 μg of pL, and 0.4 μg of an RSV antigenomic cDNA clone containing the engineered mutations in the M2-1 gene. For some rescue experiments, 0.4 μg of pM2-1 was also included in the transfection reaction. Following transfection, cells were incubated at 33°C for 3 days and the cell culture supernatants were used to infect fresh Vero cells to amplify rescued viruses. The mutations introduced into the virus were confirmed by sequencing the cDNA of the M2-1 gene obtained by reverse transcription (RT)-PCR of viral genomic RNA. Virus recovered from cDNA was plaque purified three times and amplified in Vero cells. Virus stocks were stabilized with SPG (0.2 M sucrose, 3.8 mM KH2 PO4, 7.2 mM K2HPO4, and 5.4 mM monosodium glutamate) and stored at −80°C.
Growth of viruses in cell culture.
HEp-2 and Vero cells were used for multiple-cycle growth analyses. Cell monolayers in 6-cm-diameter dishes were infected with virus at an MOI of 0.1 PFU/cell. After 1 h of adsorption, the infected cells were washed twice with phosphate-buffered saline and incubated with 2 ml of OptiMEM at 35°C. At 24-h intervals 180 μl of the culture supernatant was removed, stabilized with SPG and stored at −80°C prior to titration, and 180 μl of fresh OptiMEM was added back to each well. For titration, Vero cells in 12-well plates were infected with 10-fold serially diluted virus samples and overlaid with L-15 medium containing 1% methylcellulose and 2% fetal bovine serum. After 6 days of incubation at 35°C, plaques were enumerated following immunostaining with a primary goat anti-RSV antibody (Biogenesis) and a secondary rabbit anti-goat immunoglobulin G conjugated with horseradish peroxidase. The amount of input viruses at the start of each experiment was verified by titration on Vero cells.
RNA expression by Northern blot analyses.
To examine RNA expression, total cellular RNA was prepared from virus-infected or DNA-transfected cells by using Trizol reagents (Life Technologies). The RNA was further extracted once with phenol-chloroform and precipitated with ethanol. RNA pellets were resuspended in diethyl pyrocarbonate-treated water and stored at −80°C. Equal amounts of total RNA were separated on 1% agarose gels containing 1% formaldehyde and were transferred to nylon membranes (Amersham Pharmacia Biotech) by using a Turboblotter apparatus (Schleicher & Schuell). The blots were hybridized with digoxigenin (DIG)–UTP-labeled riboprobes synthesized by in vitro transcription using a DIG RNA labeling kit (Roche Molecular Biochemicals). Hybridization was carried out at 68°C for 12 h in Express Hyb solution (Clontech). The blots were washed at 68°C twice with 2× SSC (1× SSC is 0.015 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) followed by one wash with 1× SSC–0.1% SDS and a final wash with 0.1× SSC–0.1% SDS. Signals from the hybridized probes were detected by using a DIG-Luminescent detection kit (Roche Molecular Biochemicals) and visualized by exposure to BioMax ML film (Kodak).
Analyses of RSV proteins by Western blotting and immunoprecipitation.
For Western blot analyses, HEp-2 or Vero cells were infected with virus at an MOI of 0.1 PFU/cell and total cell lysates were harvested at 48 h postinfection. Cells were disrupted in Laemmli buffer (Bio-Rad), and equal amount of lysates were separated on 15% polyacrylamide gels containing 0.1% SDS and were transferred to nylon membranes (Amersham Pharmacia Biotech). The blots were incubated with guinea pig anti-M2-1 antibody (provided by Jeyesh Meanger) or anti-N monoclonal antibody (provided by Jose Melero). The membrane was then incubated with a secondary antibody conjugated with horseradish peroxidase and the protein bands were developed using the ECL substrate (Amersham Pharmacia Biotech). For immunoprecipitation, HEp-2 or Vero cells were infected at an MOI of 1 PFU/cell. At 15 to 18 h postinfection, cells were incubated with Dulbecco's modified Eagle medium deficient in methionine and cysteine (ICN) for 30 min and then were exposed to (100 μCi/ml) [35S]methionine and [35S]cysteine (Promix; Amersham Pharmacia Biotech) for 4 h. Immunoprecipitation of RSV-specific proteins from cytoplasmic extracts was performed with goat anti-RSV A2 serum (Biogenesis), guinea pig anti-M2-1 serum, or rabbit anti-M2-2 serum (17). Immunoprecipitated polypeptides were separated on 17.5% polyacrylamide gels containing 0.1% SDS and 4 M urea or 15% polyacrylamide gels containing 0.1% SDS and were detected by autoradiography.
Replication of M2-1 mutant viruses in cotton rats.
Replication of M2-1 mutants was compared with the wt recombinant A2 RSV (rA2) in 4- to 6-week-old respiratory pathogen-free cotton rats (Sigmodon hispidus). Cotton rats in groups of five were inoculated with 106 PFU of virus in 0.1 ml OptiMEM intranasally under light methoxyflurane anesthesia. Four days after infection cotton rats were sacrificed by CO2 asphyxiation and their nasal turbinates and lungs were harvested. Tissues were homogenized and virus titers were determined by plaque assay on Vero cells.
RESULTS
Effects of M2-1 mutations on processive RNA transcription in vitro.
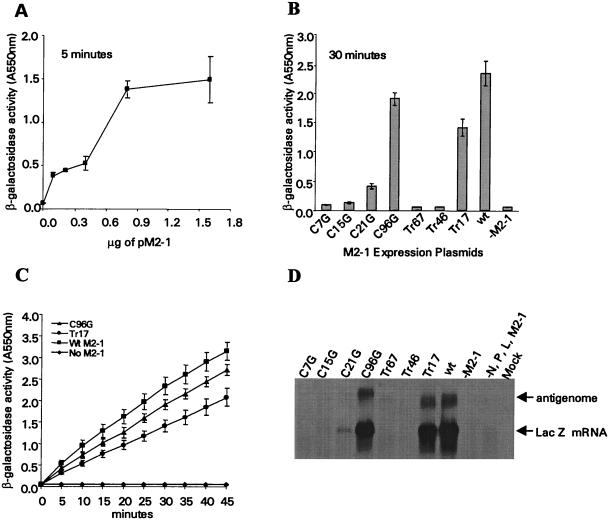
To examine the effects of the engineered mutations on M2-1 function, mutant M2-1 proteins were analyzed for their ability to promote processive RNA synthesis of a minigenome replicon supported by the recombinant vaccinia virus-T7 expression system. The requirement of M2-1 protein for the synthesis of full-length RNA is greater for transcripts longer than 1.3 kb (10); therefore, the 3.1-kb β-galactosidase gene was selected as a reporter gene for this analysis. Transcription of the pRSVLacZ minigenome by T7 RNA polymerase generates an antisense β-galactosidase RNA. Production of the β-galactosidase mRNA and expression of the enzyme were dependent on M2-1 in addition to the RSV N, P, and L proteins. To determine the amount of wt pM2-1 needed to produce β-galactosidase activity within the responsive range of the assay, MVA-T7-infected HEp-2 cells were transfected with pRSVLacZ and plasmids expressing RSV N, P, and L proteins along with various amounts of wt pM2-1. In the absence of pM2-1, no β-galactosidase activity was detected (Fig. 2A). The level of β-galactosidase activity increased with increasing amounts of pM2-1, and peak activity was observed at 0.8 μg of pM2-1 plasmid. No further increase in β-galactosidase activity was detected with larger amounts of pM2-1. As shown in Fig. 2A, transfection with 0.3 μg of wt pM2-1 expression plasmid produced a signal within the responsive range of the β-galactosidase assay and was used to compare the processive function of each M2-1 mutant protein with that of wt M2-1 protein.
FIG. 2.
(A) Expression of β-galactosidase is dependent on the M2-1 protein. The indicated amount of pM2-1 expression plasmid was transfected into MVA-T7-infected HEp-2 cells together with 0.2 μg of pN, 0.2 μg of pP, 0.2 μg of pL, and 0.2 μg of pRSVLacZ minigenome. Thirty-six hours after transfection, the β-galactosidase activity was measured by incubating the cell lysates with 5 mM CPRG for 5 min. An average of three transfection experiments is shown. (B) Comparison of β-galactosidase activity from cells transfected with wt and mutant M2-1 expression plasmids. Cells were transfected with 0.3 μg of wt M2-1 plasmid, and the β-galactosidase activity following 30 min of incubation with CPRG is shown. (C) β-Galactosidase activities from cells transfected with wt, C96G, or Tr17 M2-1. The enzymatic activity was monitored for 45 min after incubation of the cell lysates with CPRG. The signal was linearly responsive up to an OD550 of 3.0. (D) Northern blot of total RNA extracted from HEp-2 cells transfected with wt and mutant M2-1 expression plasmids. The blot was hybridized with a riboprobe specific for β-galactosidase mRNA. The LacZ antigenome is also indicated.
The level of β-galactosidase activity in the presence of different M2-1 mutants was compared to that for wt M2-1 (Fig. 2B). Substitutions of glycine for C7, C15, and C21 in the Cys3-His1 motif and truncation of 67 or 46 amino acids from the C terminus of M2-1 (Tr67 and Tr46) drastically decreased the level of β-galactosidase activity. C7G, Tr67, and Tr46 reduced the amount of β-galactosidase activity to almost background levels. However, C15G and C21G did not completely abolish the ability of M2-1 to support processive RNA synthesis. β-galactosidase activity at a level of approximately 20% that of wt M2-1 was produced in cells expressing C21G M2-1. C96G and Tr17 were able to support processive RNA synthesis at a level slightly less than that of wt M2-1. The β-galactosidase activity from cells expressing C96G and Tr17 was approximately 80 and 60% that of wt M2-1, respectively. Figure 2C shows that the optical density reflecting the β-galactosidase enzymatic activity increased linearly over a period of 45 min. Less β-galactosidase activity was detected in cells expressing C96G and Tr17 M2-1 compared to that of wt M2-1, and the relative differences were similar throughout the entire period of the assay.
The level of β-galactosidase RNA expression in transfected cells was further analyzed by Northern blot analysis (Fig. 2D). β-Galactosidase mRNAs were readily detected in cells expressing wt M2-1 protein. A negligible amount of full-length β-galactosidase mRNA was produced when pM2-1 was omitted. The β-galactosidase mRNA detected in cells transfected with C7G, Tr67, and Tr46 was comparable to that obtained in the absence of wt M2-1. The amount of β-galactosidase mRNA detected in cells expressing C15G and C21G was less than 10% of that detected in cells expressing wt M2-1. The level of β-galactosidase RNA detected for C96G and Tr17 M2-1 mutants was similar to that observed in wt pM2-1 transfected cells. Comparable amounts of M2-1 mRNAs were detected in cells transfected with wt pM2-1 and all its derivatives (data not shown). Thus, the level of β-galactosidase mRNA expression obtained by Northern blotting was consistent with the observed enzymatic level of β-galactosidase.
Recovery of recombinant RSV with mutations in M2-1.
To examine the effect of the M2-1 mutations on virus replication, all the cysteine-to-glycine changes and premature stop codons were introduced into the M2-1 gene of a full-length RSV antigenomic cDNA derived from the A2 strain. As expected from their in vitro activity, recombinant viruses expressing C96G or Tr17 M2-1 were recovered from HEp-2 cells. The wt pM2-1 expression plasmid was not required to rescue these recombinants. Multiple attempts were made to recover the other M2-1 mutants, but no viruses were obtained even when the wt pM2-1 was included in the transfection reactions. Therefore, consistent with the in vitro minigenome analysis, mutations that significantly reduced the processivity function of M2-1 also disabled its ability to regenerate infectious virus. The two viable M2-1 RSV mutants recovered contained M2-1 mutations that did not severely reduce the ability of M2-1 to support processive RNA synthesis in vitro.
Replication of rA2-C96G and rA2-Tr17 in HEp-2 and Vero cells.
rA2-C96G and rA2-Tr17 were plaque purified and amplified in Vero cells, and the replication of these two viruses was compared to that of wt rA2 in cell culture. In HEp-2 cells, rA2-C96G and rA2-Tr17 exhibited small plaque morphology, with plaque size reduction of approximately 30% relative to that of wt rA2. The plaque size of both viruses in Vero cells was similar to that of rA2. The plaque forming efficiency of rA2-C96G and rA2-Tr17 at 33 and 39°C was examined in HEp-2 and Vero cells. No significant reduction in titer was observed for either virus at 39°C compared to that at 33°C. The titer of rA2-C96G was reduced by approximately 10-fold at 39°C, but this difference was only observed in HEp-2 cells.
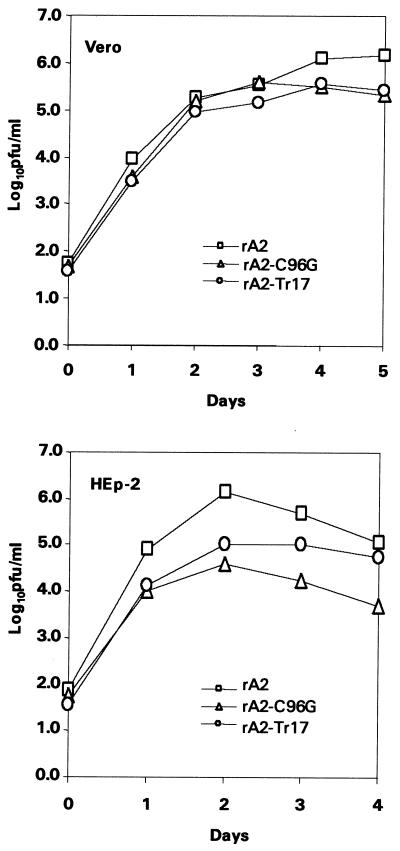
Multiple-step replication cycles of rA2-C96G and rA2-Tr17 were performed in HEp-2 and Vero cells infected at an MOI of 0.1 PFU/cell. As shown in Fig. 3, the growth kinetics of both rA2-C96G and rA2-Tr17 were similar to that of rA2 in Vero cells, but both viruses showed a reduction in peak titer of approximately 0.5 log10 relative to rA2. In HEp-2 cells, rA2-C96G and rA2-Tr17 reached peak titers that were reduced by 2.0 log10 and 1.5 log10, respectively, relative to wt rA2.
FIG. 3.
Multiple-step replication cycles in Vero and HEp-2 cells. Vero cells (top) or HEp-2 cells (bottom) were infected with rA2, rA2-Tr17, or rA2-C96G at an MOI of 0.1 PFU/cell. Aliquots of culture medium in duplicates were harvested at 24-h intervals, and the virus was quantitated by plaque assay on Vero cells.
Viral RNA expression.
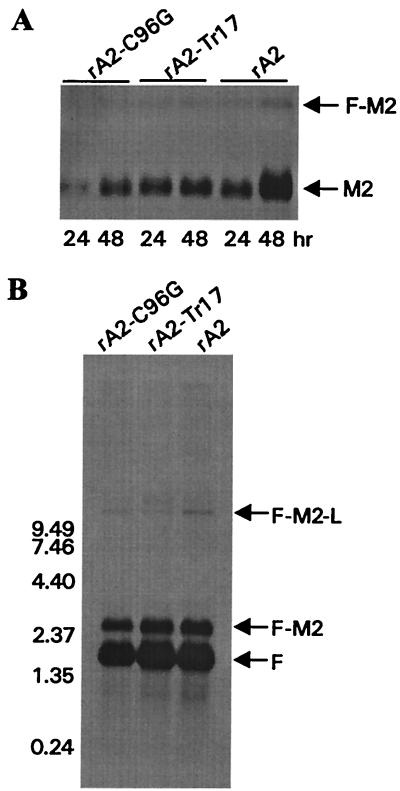
Viral mRNA accumulation and production of read-through transcripts generated by rA2-C96G and rA2-Tr17 were examined by Northern blotting. HEp-2 cells were infected with wt rA2, rA2-C96G, or rA2-Tr17 at 0.1 PFU/cell, and total cellular RNA was prepared at 24 and 48 h postinfection. The Northern blot was hybridized with an M2-specific riboprobe. A smaller amount of M2 mRNA was detected in rA2-C96G-infected cells than in wt rA2 and rA2-Tr17 at 24 h postinfection (Fig. 4A). Both rA2-C96G and rA2-Tr17 produced less M2 mRNA than rA2 at 48 h postinfection. Transcription read-through at the gene junction between the F and M2 genes (F-M2) is known to be highly dependent on M2-1 and has been postulated to have a role in the regulation of M2-1 expression during RSV infection (13). Northern blotting was used to compare the amount of read-through transcripts at the F-M2 gene junction produced by the M2-1 mutants and wt rA2. Total cellular RNA prepared at 48 h postinfection was probed with an F-specific riboprobe to detect the F mRNA and its read-through transcripts. Figure 4B shows the F mRNA, F-M2, and F-M2-L read-through transcripts generated by both M2-1 mutants and wt rA2. Less F mRNA was detected for rA2-C96G, but the ratio of F to F-M2 or F-M2-L appeared to be similar to that of wt rA2 and rA2-Tr17. Thus, M2-1 bearing a C96G change or a truncation of 17 amino acids did not show substantial alterations in their ability to support viral RNA transcription and read-through at gene junctions during RSV replication.
FIG. 4.
Northern blot analyses of viral RNA expression. HEp-2 cells were infected with rA2, rA2-Tr17, or rA2-C96G at an MOI of 0.1 PFU/ml. Total cellular RNA was extracted at 24 or 48 h postinfection (A) or at 48 h postinfection (B). The RNA blots were hybridized with DIG-UTP-labeled riboprobe specific to M2 (A) or F (B) mRNAs. The mRNA and read-through transcripts are indicated.
Viral protein expression of rA2-C96G and rA2-Tr17.
Western blotting and immunoprecipitation were performed to compare the level of viral protein expression in cells infected with rA2-C96G, rA2-Tr17, or wt A2 at an MOI of 0.1 PFU/cell. Cell extracts were harvested at 48 h postinfection. Western blots were probed with anti-N and anti-M2-1 antibodies. As shown in Fig. 5A, a similar level of viral protein expression was observed in Vero cells infected with the three viruses. In HEp-2 cells, the amount of M2-1 and N was less than that in Vero cells, and M2-1 of rA2-Tr17 was reduced by approximately twofold compared to that of wt rA2. The M2-1 protein of rA2-Tr17 migrated faster on polyacrylamide gels, confirming the smaller size of the prematurely terminated Tr17 M2-1 protein. No detectable full-length M2-1 protein was observed in cells infected with rA2-Tr17, indicating that the tandem stop codons engineered in the M2-1 gene of rA2-Tr17 terminated translation of M2-1 efficiently.
FIG. 5.
(A) Western blot of cell lysates infected with rA2, rA2-C96G, or rA2-Tr17. Total cellular proteins from infected cells were separated on 15% polyacrylamide gels containing 0.1% SDS, and the blots were hybridized separately with anti-M2-1 or anti-N antibodies. (B) Immunoprecipitation of labeled RSV polypeptides from infected HEp-2 or Vero cells. HEp-2 or Vero cells were infected with each virus as indicated, and the proteins were labeled with [35S]methionine and [35S]cysteine at 16 h postinfection. The labeled proteins were immunoprecipitated with anti-RSV or anti-M2-2 antibodies. The F1 protein from RSV-infected HEp-2 and Vero cells showed different gel mobilities, and less F1 was produced in rA2-Tr17-infected Vero cells in this particular experiment.
The introduction of the premature stop codons in the M2 gene of rA2-Tr17 abolished the translational overlap between M2-1 and the downstream M2-2 ORF. Since this genetic alteration could affect the expression of M2-2, the amount of M2-2 protein produced by rA2-Tr17 was compared to that expressed by wt rA2. The electrophoretic pattern of RSV polypeptides immunoprecipitated from rA2-Tr17- and rA2-C96G-infected cells were similar to that of wt rA2 (Fig. 5B), but the amount of these viral proteins was reduced relative to that of rA2. It was noted that in this experiment, less F1 protein was produced in rA2-Tr17-infected Vero cells, but the reduction of the F protein was not reproducible. M2-2 was detected in Vero and HEp-2 cells infected with rA2-Tr17 and rA2-C96G. However, the amount of M2-2 expressed by both M2-1 mutants was also slightly lower than that of rA2. This might be due to the overall reduction in the level of virus replication exhibited by the M2-1 mutant viruses.
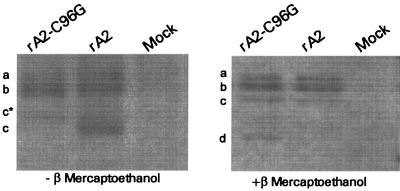
Electrophoretic mobility of C96G M2-1 protein from rA2-C96G-infected cells.
Previously it was reported that multiple forms of M2-1 protein could be distinguished by differences in their electrophoretic mobilities on SDS-polyacrylamide gels (22). Recently it was also shown that both phosphorylated and nonphosphorylated forms of the M2-1 protein migrated with different gel mobilities under reducing conditions (14). Since cysteine residues are often involved in the formation of disulfide bonds, it is of interest to examine whether the C96G substitution in M2-1 resulted in any covalent bond disruptions that would affect the conformation of M2-1. The M2-1 proteins immunoprecipitated from rA2-C96G- or rA2-infected cells were resolved on SDS-polyacrylamide gels following denaturation in the presence or absence of a reducing reagent (Fig. 6). Under nonreducing conditions three major bands, indicated as a, b, and c, were detected in wt rA2-infected cells. Two major species (a, b) with gel mobilities similar to those of wt M2-1 were detected in rA-C96G-infected cells. A minor species, denoted c*, that migrated slower than wt M2-1 (c) was also detected in rA2-C96G-infected cells. Under reducing conditions the M2-1 protein from rA2-C96G-infected cells exhibited three major species similar to that of wt M2-1. In addition, a fourth species (d) was also observed for C96G M2-1 under reducing condition (Fig. 6). The difference in the number of major M2-1 species detected under reducing conditions indicated that replacement of cysteine at position 96 by glycine might have altered the conformation of M2-1 and/or affected the modification of the protein. The different electrophoretic migration of the third species in the wt (c) and C96G (c*) M2-1 under nonreducing condition suggested that C96 might be involved in the formation of covalent disulfide linkages that were disrupted by the glycine substitution. Although the C96G M2-1 protein showed distinct electrophoretic differences from the wt M2-1 protein, these differences did not appear to disrupt its interactions with N, since the N protein was readily coimmunoprecipitated with C96G M2-1 in rA2-C96G-infected cells (data not shown).
FIG. 6.
Immunoprecipitation of the M2-1 proteins from infected cells. HEp-2 cells were infected with rA2, rA2T17, or rA2C96G at an MOI of 1 PFU/cell. The labeled M2-1 proteins were immunoprecipitated with anti-M2-1 antibody and denatured in the presence (right panel) or absence (left panel) of β-mercaptoethanol. Immunoprecipitated M2-1 proteins were resolved by SDS–-15% polyacrylamide gel electrophoresis. The different forms of M2-1 on the SDS-polyacrylamide gel are indicated as a, b, c, c*, or d.
Genetic stability of rA2-C96G and rA2-Tr17.
One of the rA2-Tr17 isolates was found to express a P protein with a retarded electrophoretic mobility (data not shown). DNA sequence analysis of the P gene from this viral isolate revealed three alterations at nt 2482, 2627, and 2637. T2482C and T2637C resulted in amino acid changes at position 46 from I to T and at position 98 from F to L. rA2-Tr17 with wt P or mutant P had similar growth kinetics in vitro, and the significance of these amino acid changes detected in P require further investigation. The propensity of C96G and the tandem stop codons to revert following multiple passages in Vero cells was then examined. DNA sequence analyses of M2-1 RT-PCR product from viruses that had undergone multiple rounds of amplification in Vero cells did not reveal any genetic reversions at position 96 of rA2-C96G or at the premature stop codons of rA2-Tr17. This suggests that the C96G substitution and the introduced premature stop codons (Tr17) are genetically stable in tissue culture.
Replication of rA2-C96G and rA2-Tr17 in cotton rats.
rA2-C96G and rA2-Tr17 were evaluated for their abilities to replicate in the respiratory tracts of cotton rats. Cotton rats were inoculated with 106 PFU of rA2, rA2-C96G, or rA2-Tr17 intranasally, and virus replication in the lungs was measured by plaque assays on Vero cells. The replication of rA2-C96G was reduced by approximately 1.3 log10 compared to that of rA2. The replication of rA2-Tr17 was slightly more restricted, and its titer in the lungs was reduced by about 1.5 log10 compared to that of rA2.
DISCUSSION
The M2-1 protein of human RSV is an antiterminator that is required for processive RNA synthesis by the viral polymerase (5, 6) and transcription read-through at gene junctions (13, 15). These M2-1 functions may allow a greater number of polymerase molecules to access genes distal from the polymerase entry site and counteract RNA transcription attenuation during RSV replication. In this study we showed that the first three cysteines in the M2-1 protein were essential for supporting processive RNA synthesis in vitro, and no virus was recovered when any of these cysteines were replaced with glycine. C96 located in the center of M2-1 was not critical for virus replication. We also demonstrated that the last 17 amino acids could be removed from the C terminus of M2-1 without severely impairing the function of M2-1 in vitro and in vivo.
The cysteines in the highly conserved Cys3-His1 motif are predicted to coordinate zinc (26). When C7 or C15 of hRSV M2-1 was changed to serine, the ability of M2-1 to promote transcription read-through at RSV gene junctions was drastically impaired (14). Disruption of the Cys3-His1 motif also altered the ratio between phosphorylated and nonphosphorylated forms of M2-1 and decreased the physical interaction between M2-1 and the N protein (14). M2-1 has also been demonstrated to bind RNA, and the RNA binding domain was mapped to a region between residues 59 and 85. Furthermore, the RNA binding capacity of M2-1 was found to be dependent on the phosphorylation status of the protein (9). The effects of the M2-1 mutations generated in this study were first examined by an assay that measured M2-1-dependent processive RNA synthesis in vitro. Although replacement of the three cysteines in the Cys3-His1 motif of M2-1 greatly reduced their ability to promote full-length β-galactosidase mRNA synthesis, the level of reduction for each replacement was different. C7G completely abolished the ability of M2-1 to support production of full-length β-galactosidase mRNA, whereas C15G and C21G M2-1 generated full-length reporter gene RNA at a level 10 to 20% that of wt M2-1. This implied that the three cysteines in the Cys3-His1 motif might play different structural roles in the maintenance of M2-1 function. Even though a low level of β-galactosidase RNA was detected for C15G and C21G, these mutations did not result in virus recovery, supporting the previous report by Hardy and Wertz (14) that the Cys3-His1 motif is critical for M2-1 function. Replacement of the fourth highly conserved cysteine residue at position 96, which lies outside the conserved cysteine-rich motif, was not lethal to M2-1 function. The replication of rA2-C96G, however, was less efficient in HEp-2 cells and was restricted in the respiratory tracts of cotton rats. M2-1 carrying this mutation showed no detectable differences in their physical interaction with the N protein in rA2-C96G-infected cells. As reported previously, several forms of M2-1 with distinct electrophoretic mobilities are present in RSV-infected cells (22). Under reducing conditions two major species of M2-1 proteins can be detected, and the slower migrating form was found to be phosphorylated (14). Although the M2-1 protein from rA2-C96G showed a band that migrated differently from wt M2-1, it exhibited two major species identical to those of wt M2-1, of which the slowest migrating band likely represented the phosphorylated form. This indicated that the C96G change did not affect the phosphorylation of M2-1. Under nonreducing conditions, C96G M2-1 also showed species with different gel mobilities from that of wt M2-1, suggesting that cysteine 96 is involved in the formation of a disulfide bond. The disruption of this covalent bond may have altered the conformation of M2-1 and may account for the less efficient replication of rA2-C96G.
The M2-1 proteins of pneumoviruses are heterogeneous in their sequences and lengths at the C terminus, suggesting a less critical role for the C terminus. We made three M2-1 truncations by deleting 67, 46, or 17 amino acids from the end of the C terminus. The shortest truncation did not significantly disrupt M2-1 function, and an infectious virus bearing this truncation was obtained. Tr17 M2-1 coincided with the length of the PVM M2-1 protein. The M2-1 protein of PVM was less efficient at supporting processive RSV RNA synthesis than the hRSV M2-1 protein (unpublished results). In vitro analysis showed that the full-length β-galactosidase mRNA produced from cells expressing Tr17 M2-1 was reduced by about 50 to 60% relative to that of wt M2-1. The recovered recombinant virus, rA2-Tr17, replicated less efficiently in cell culture and in the lungs of cotton rats than wt rA2. These results indicate that although the last 17 amino acids of M2-1 are dispensable for virus growth, they are required for efficient virus replication. The mutations identified in the P gene of a rA2-Tr17 virus were T-to-C changes, which probably resulted from biased hypermutation (4, 24). Sequencing of the P genes from several other rA2-Tr17 isolates and from rA2-Tr17 after several in vitro passages did not reveal any mutations in the P gene. This suggests that the mutations in P are probably not compensatory mutations for overcoming the defect in the M2-1 function of rA2-Tr17.
The ORF carrying M2-1 overlaps with that of M2-2, and the introduction of the premature stop codons in the M2-1 gene of rA2-Tr17 abolished the overlap between the two M2 ORFs. Recently it was shown that the location of the termination codon of M2-1 played a crucial role in directing translation of M2-2 from the upstream initiation codons (1). It was found that when the initiation codon of M2-2 was relocated downstream of the M2-1 termination codon in a synthetic template, expression of the second ORF was abolished. We also constructed a synthetic DNA template in which the M2-2 ORF was replaced by the CAT gene. The expression of the CAT protein from the nonoverlapping structure was similar to that of the overlapping structure (data not shown). The expression of the M2-2 protein in rA2-Tr17-infected cells indicates that ribosomes are able to access the AUGs located downstream of the engineered premature stop codons to direct the translation of M2-2. The difference between our result and that of Ahmadian et al. (1) might be due to differences in the sequences and/or structures of the RNAs used in the two studies. Expression of the second ORF from a single mRNA has been shown to be less efficient, irrespective of whether the two ORFs overlapped or not (20). The overlapping structure between M2-1 and M2-2 might be important for regulating the level of M2-2 expression. M2-2 is a transcriptional regulator involved in viral RNA replication and transcription. Removal of the M2-2 gene significantly reduced the amount of accumulated genomic and antigenomic RNA and resulted in a virus that replicated less efficiently in HEp-2 cells and in cotton rats (3, 17). Since the M2-2 protein was expressed in rA2-Tr17-infected cells and the minigenome analysis showed that Tr17 M2-1 had reduced processivity compared to that of wt M2-1, it is most likely that the less efficient replication of rA2-Tr17 is a result of the impaired M2-1 function rather than any changes in M2-2 protein expression.
Previously, a single mutation (T7605C) in the gene start signal of the M2 gene was found to be a major determinant specifying the temperature-sensitive phenotype of a live attenuated RSV vaccine candidate, cpts 248/404 (25). Phenotypic reversion of cpts 248/404 was detected in virus isolated from immunized infants in a clinical study (27). Our finding that both C96G and Tr17 mutations in the M2-1 protein compromised the efficiency of RSV replication in vitro and in vivo provides additional evidence that the M2-1 protein has an essential role in RSV replication.
ACKNOWLEDGMENTS
We thank Aviron's animal facility for assistance with the cotton rat experiments, the tissue culture facility for supplying cells, Robert Brazas for discussions and construction of the pRSVlacZ minigenome, Helen Zhou and Mary Munoz for technical assistance, and George Kemble and Bin Lu for critical review of the manuscript. We are grateful to Jayesh Meanger and Jose Melero for providing antibodies.
This work was supported in part by National Institutes of Health SBIR grants (2R44A145267-01/02).
REFERENCES
- 1.Ahmadian G, Randhawa J S, Easton A J. Expression of the ORF-2 protein of the human respiratory syncytial M2 gene is initiated by a ribosomal termination-dependent reinitiation mechanism. EMBO J. 2000;19:2681–2689. doi: 10.1093/emboj/19.11.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alansari H, Potgieter L N D. Molecular cloning and sequence analysis of the phosphoprotein, nucleocapsid protein, matrix protein and 22K (M2) protein of the ovine respiratory syncytial virus. J Gen Virol. 1994;75:3597–3601. doi: 10.1099/0022-1317-75-12-3597. [DOI] [PubMed] [Google Scholar]
- 3.Bermingham A, Collins P L. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci USA. 1999;96:11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo R. Biased (A→I) hypermutation of animal RNA virus genomes. Curr Opin Genet Dev. 1994;4:895–900. doi: 10.1016/0959-437x(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 5.Collins P L, Hill M G, Camargo E, Grosfeld H, Chanock R M, Murphy B R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA. 1995;92:11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins P L, Hill M G, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci USA. 1996;93:81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins P L, Hill M G, Johnson P R. The two open reading frames of the 22K mRNA of human respiratory syncytial virus: sequence comparison of antigenic subgroups A and B and expression in vitro. J Gen Virol. 1990;71:3015–3020. doi: 10.1099/0022-1317-71-12-3015. [DOI] [PubMed] [Google Scholar]
- 8.Collins P L, Wertz G W. The envelope-associated 22K protein of human respiratory syncytial virus: nucleotide sequence of the mRNA and a related polytranscript. J Virol. 1985;54:65–71. doi: 10.1128/jvi.54.1.65-71.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuesta I, Geng X, Asenjo A, Villanueva N. Structural phosphoprotein M2-1 of the human respiratory syncytial virus is an RNA binding protein. J Virol. 2000;74:9858–9867. doi: 10.1128/jvi.74.21.9858-9867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fearns R, Collins P L. Model for polymerase access to the overlapped L gene of respiratory syncytial virus. J Virol. 1999;73:388–397. doi: 10.1128/jvi.73.1.388-397.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearns R, Collins P L. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J Virol. 1999;73:5852–5864. doi: 10.1128/jvi.73.7.5852-5864.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosfeld H, Hill M G, Collins P L. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L protein; transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J Virol. 1995;69:5677–5686. doi: 10.1128/jvi.69.9.5677-5686.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy R W, Harmon S B, Wertz G W. Diverse gene junctions of respiratory syncytial virus modulate the efficiency of transcription termination and respond differently to M2-mediated antitermination. J Virol. 1999;73:170–176. doi: 10.1128/jvi.73.1.170-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy R W, Wertz G W. The Cys3-His1 motif of the respiratory syncytial virus M2-1 protein is essential for protein function. J Virol. 2000;74:5880–5885. doi: 10.1128/jvi.74.13.5880-5885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy R W, Wertz G W. The product of the respiratory syncytial virus M2 gene ORF1 enhances readthrough of intergenic junctions during viral transcription. J Virol. 1998;72:520–526. doi: 10.1128/jvi.72.1.520-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y T, Collins P L, Wertz G W. Characterization of the 10 proteins of human respiratory syncytial virus: identification of a fourth envelope-associated protein. Virus Res. 1985;2:157–173. doi: 10.1016/0168-1702(85)90246-1. [DOI] [PubMed] [Google Scholar]
- 17.Jin H, Cheng X, Zhou H Z, Li S, Seddiqui A. Respiratory syncytial virus that lacks open reading frame 2 of the M2 gene (M2-2) has altered growth characteristics and is attenuated in rodents. J Virol. 2000;74:74–82. doi: 10.1128/jvi.74.1.74-82.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin H, Clarke D, Zhou Z-Y H, Cheng X, Coelingh K, Bryant M, Li S. Recombinant human respiratory syncytial virus (RSV) from cDNA and construction of subgroup A and B chimeric RSV. Virology. 1998;251:206–214. doi: 10.1006/viro.1998.9414. [DOI] [PubMed] [Google Scholar]
- 19.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, editor. Fields virology. 4th ed. Phildelphia, Pa: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 20.Peabody D S, Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986;6:2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poon A P W, Roizman B. The phenotype in vitro and in infected cells of herpes simplex virus 1 alpha trans-inducing factor (VP16) carrying temperature-sensitive mutations introduced by substitution of cysteines. J Virol. 1995;69:7658–7667. doi: 10.1128/jvi.69.12.7658-7667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Routledge E G, Willcocks M M, Morgan L, Samson A C R, Scott R, Toms G L. Heterogeneity of the respiratory syncytial virus 22K protein revealed by western blotting with monoclonal antibodies. J Gen Virol. 1987;68:1209–1215. doi: 10.1099/0022-1317-68-4-1209. [DOI] [PubMed] [Google Scholar]
- 23.Sutter G, Ohlmann M, Erfle V. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 1995;371:9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]
- 24.Teng M N, Collins P L. Altered growth characteristics of recombinant respiratory syncytial viruses which do not produce NS2 protein. J Virol. 1999;73:466–473. doi: 10.1128/jvi.73.1.466-473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitehead S S, Firestone C Y, Collins P L, Murphy B R. A single nucleotide substitution in the transcription start signal of the M2 gene of respiratory syncytial virus vaccine candidate cpts248/404 is the major determinant of the temperature-sensitive and attenuation phenotypes. Virology. 1998;247:232–239. doi: 10.1006/viro.1998.9248. [DOI] [PubMed] [Google Scholar]
- 26.Worthington M T, Amann B T, Nathans D, Berg J M. Metal binding properties and secondary structure of the zinc-binding domain of Nup475. Proc Natl Acad Sci USA. 1996;93:13754–13759. doi: 10.1073/pnas.93.24.13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright P F, Karron R A, Belshe R B, Thompson J, Crowe J E, Jr, Boyce T G, Halburnt L L, Reed G W, Whitehead S S, Anderson E L, Wittek A E, Casey R, Eichelberger M, Thumar B, Randolph V B, Udem S A, Chanock R M, Murphy B R. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis. 2000;182:1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 28.Wyatt L S, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]
- 29.Yu Q, Hardy R W, Wertz G W. Functional cDNA clones of the human respiratory syncytial (RS) virus N, P, and L proteins support replication of RS virus genomic RNA analogs and define minimal trans-acting requirements for RNA replication. J Virol. 1995;69:2412–2419. doi: 10.1128/jvi.69.4.2412-2419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]