Abstract
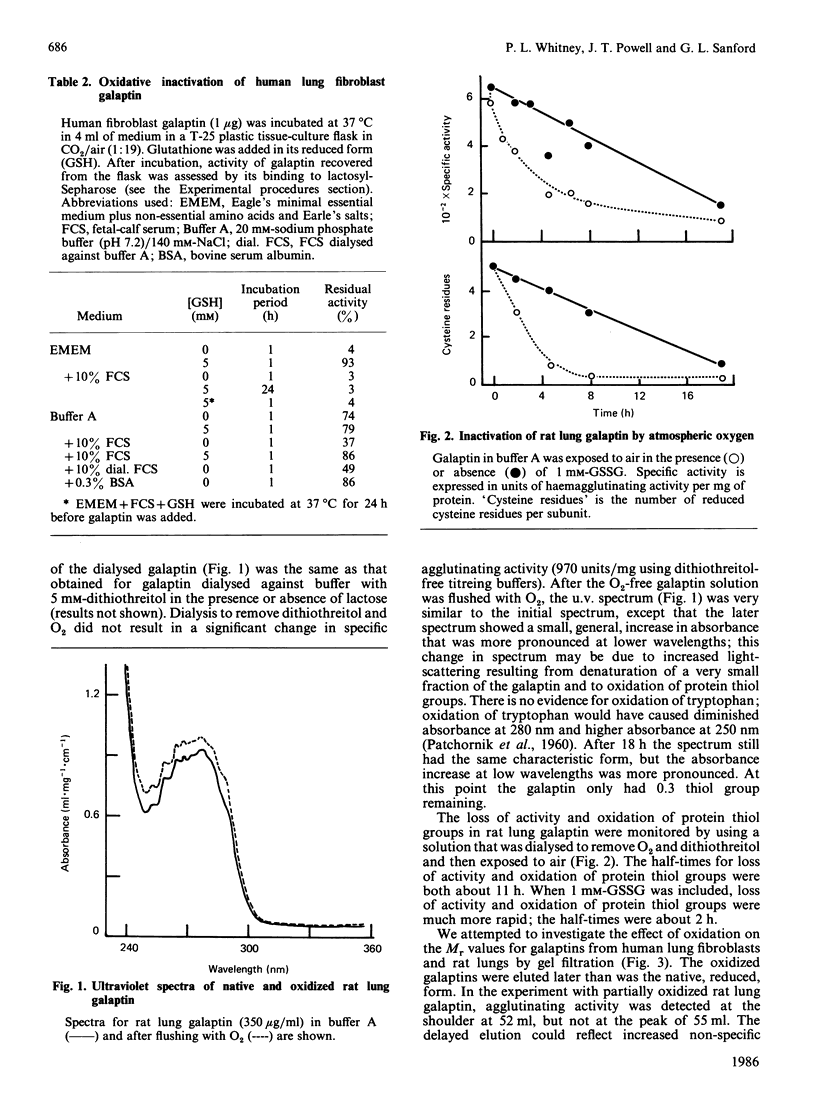
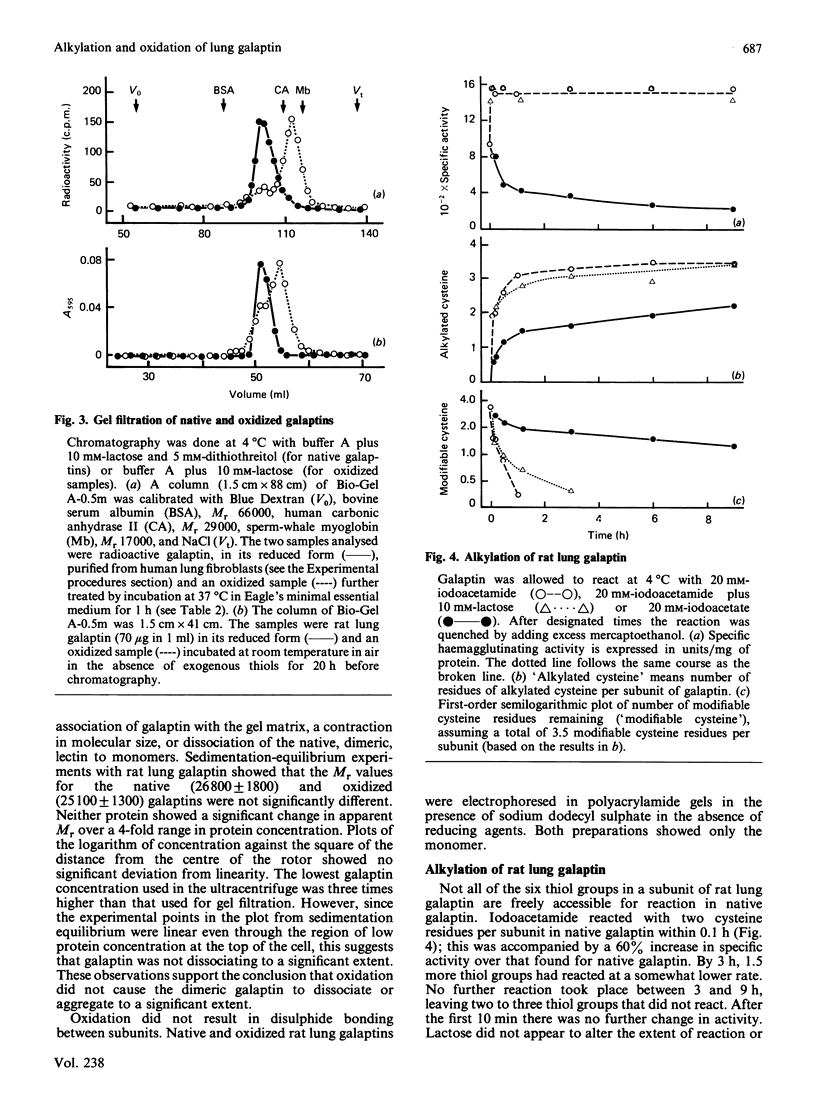
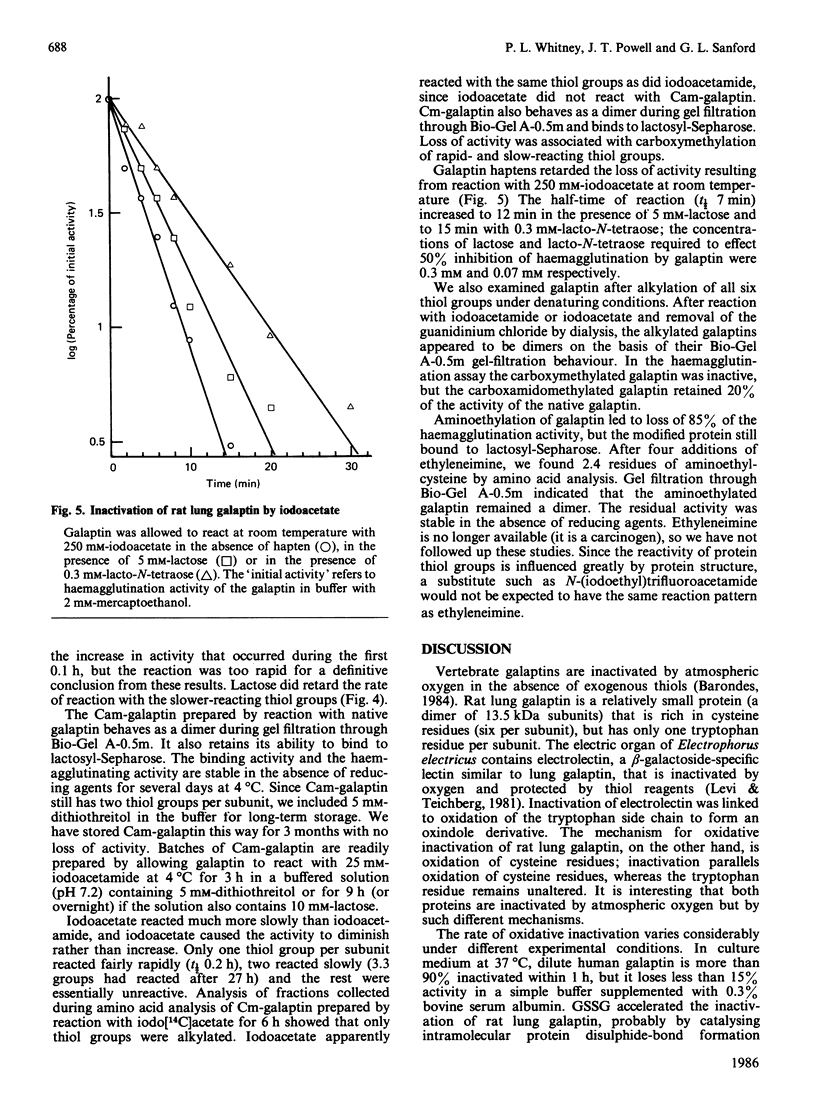
Galaptins are small, soluble, lectins with a specificity for beta-galactose residues. Many galaptins are inactivated by atmospheric oxygen and are protected by disulphide-reducing reagents. We find that each subunit of rat lung galaptin contains one residue of tryptophan and six of cysteine. Oxygen inactivates rat lung galaptin by oxidation of the cysteine residues. During oxidation, the normal dimeric structure is maintained and all disulphide bonds are formed within individual subunits. Exogenous thiols protect against inactivation, but oxidized thiols accelerate inactivation. Human lung fibroblast galaptin is almost completely inactivated within 1 h in tissue culture medium at 37 degrees C. Alkylation of native rat lung galaptin with iodoacetate or ethyleneimine causes substantial loss of activity. The dimeric galaptin structure is maintained. In contrast, alkylation with iodoacetamide yields carboxamidomethyl-galaptin, which is fully active and stable to atmospheric oxygen in the absence of disulphide-reducing reagents. This derivative is very useful for studies of galaptin properties and function.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H., Haywood-Reid P. L. Externalization of an endogenous chicken muscle lectin with in vivo development. J Cell Biol. 1981 Nov;91(2 Pt 1):568–572. doi: 10.1083/jcb.91.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes S. H. Lectins: their multiple endogenous cellular functions. Annu Rev Biochem. 1981;50:207–231. doi: 10.1146/annurev.bi.50.070181.001231. [DOI] [PubMed] [Google Scholar]
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Barondes S. H. Quantitation of two endogenous lactose-inhibitable lectins in embryonic and adult chicken tissues. J Cell Biol. 1982 Jan;92(1):23–27. doi: 10.1083/jcb.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cerra R. F., Haywood-Reid P. L., Barondes S. H. Endogenous mammalian lectin localized extracellularly in lung elastic fibers. J Cell Biol. 1984 Apr;98(4):1580–1589. doi: 10.1083/jcb.98.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Harrison F. L., Chesterton C. J. Factors mediating cell--cell recognition and adhesion. Galaptins, a recently discovered class of bridging molecules. FEBS Lett. 1980 Dec 29;122(2):157–165. doi: 10.1016/0014-5793(80)80428-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and characterization of chicken thymic electrolectin. Biochem J. 1985 Mar 1;226(2):379–384. doi: 10.1042/bj2260379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981 Jun 10;256(11):5735–5740. [PubMed] [Google Scholar]
- Podleski T. R., Greenberg I. Distribution and activity of endogenous lectin during myogenesis as measured with antilectin antibody. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1054–1058. doi: 10.1073/pnas.77.2.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T. Purification and properties of lung lectin. Rat lung and human lung beta-galactoside-binding proteins. Biochem J. 1980 Apr 1;187(1):123–129. doi: 10.1042/bj1870123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. T., Whitney P. L. Endogenous ligands of rat lung beta-galactoside-binding protein (galaptin) isolated by affinity chromatography on carboxyamidomethylated-galaptin-Sepharose. Biochem J. 1984 Nov 1;223(3):769–774. doi: 10.1042/bj2230769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford G. L., Davis L. D., Powell J. T. The subcellular localization of the beta-galactoside-binding protein of rat lung. Biochem J. 1982 Apr 15;204(1):97–102. doi: 10.1042/bj2040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P., Maxwell S., Ryan U., Massaro D. Synthesis and binding of lactose-specific lectin by isolated lung cells. Am J Physiol. 1985 Mar;248(3 Pt 1):C258–C264. doi: 10.1152/ajpcell.1985.248.3.C258. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Ziegler D. M. Role of reversible oxidation-reduction of enzyme thiols-disulfides in metabolic regulation. Annu Rev Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]


