Abstract
Background
Although cisplatin plus gemcitabine and other combinations have improved the survival of advanced biliary tract cancer (BTC), high unmet medical needs remain. This study aimed to assess the efficacy and safety of nivolumab plus lenvatinib in the second-line treatment for advanced BTC.
Patients and methods
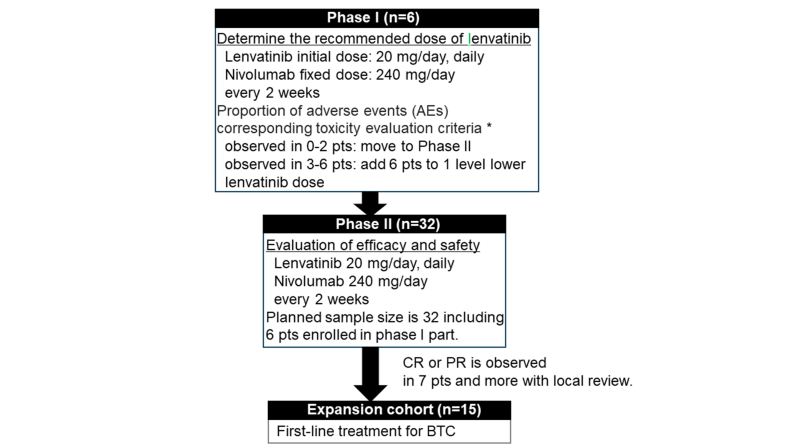
Nivolumab (240 mg) was administered biweekly. Phase I determined the recommended phase II dose of lenvatinib (20 mg or 14 mg). In phase II, the primary endpoint was the objective response rate (ORR). Secondary endpoints were disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety. The planned sample size was 32 patients with a power of 80%, a one-sided alpha error of 5%, threshold ORR of 10%, and expected ORR of 30%.
Results
In phase I, the recommended dose of lenvatinib was determined to be 20 mg in six patients, with one dose-limiting toxicity (myocarditis). In phase II, we enrolled 26 patients. ORR, DCR, and median OS and PFS were 9.4% [90% confidence interval (CI) 2.6% to 22.5%], 53.1% (95% CI 34.7% to 70.9%), and 6.4 months (95% CI 4.9-9.7 months) and 2.5 months (95% CI 1.5-4.1 months), respectively. No response was observed in patients with the usage of antibiotics. The grade 3 or 4 adverse events were hypertension (59.4%) and biliary tract infection (37.5%). Rash (28.1%) and hypothyroidism (21.9%) were observed as immune-mediated adverse events of any grade.
Conclusions
Nivolumab plus lenvatinib had a manageable safety in advanced BTC, but its efficacy in the second-line treatment was limited.
Key words: biliary tract cancer, nivolumab, lenvatinib, biliary tract infection, immune checkpoint inhibitor
Highlights
-
•
Nivolumab plus lenvatinib had a manageable safety profile for BTC treatment.
-
•
Nivolumab plus lenvatinib had limited efficacy as a second-line BTC treatment.
-
•
Efficacy may depend on the presence or absence of biliary tract infections and a history of antibiotic use.
Introduction
Biliary tract cancer (BTC) includes cancers in the intrahepatic bile duct (IHBD), extrahepatic bile duct (EHBD), gall-bladder (GB), and ampulla of Vater (AV). It causes 2.3 deaths per 100 000 population globally and is most common in Asia.1 BTC is a lethal disease and is generally diagnosed at an advanced stage.2 Since 2010, gemcitabine plus cisplatin (GC) has been the standard chemotherapy treatment for advanced/recurrent BTC.3 In second-line chemotherapy, ABC-06 showed the superiority of FOLFOX compared with active symptom control, and NIFTY showed the superiority of Nal-IRI plus fluorouracil and leucovorin.4,5 As targeted agents based on next-generation sequencing, inhibitors of fibroblast growth factor receptor (FGFR) aberrations, such as pemigatinib and futibatinib, showed promising activities in the IHBD.6,7 In addition, dabrafenib plus trametinib for BRAF V600E was reported, and drug developments are continued to be made in the research on human epidermal growth factor receptor 2 (HER2) gene abnormalities.8,9 Moreover, immune checkpoint inhibitors (ICIs) are being actively developed. However, in second-line treatment, the efficacy of a single ICI was limited,10, 11, 12 and attention was focused on combining it with GC therapy in first-line treatment or with a molecular targeted therapy as a combined immunotherapy in second-line treatment. Chen et al. reported that the cancer immunity cycle, including the release of cancer antigens, presentation of cancer antigens to T cells, priming of T cells, and transport of T cells to the tumor, plays an important role in enhancing the effects of ICIs.13 In previous studies, angiogenesis inhibitors were expected to improve the tumor microenvironment through the maturation of dendritic cells, priming of T cells, normalization of tumor vasculature for T-cell trafficking, and reduction of myeloid-derived suppressor cells and regulatory T cells (Tregs).14, 15, 16 The combination of ramucirumab and pembrolizumab, an antiangiogenic antibody drug that had already been reported at that time, showed a response rate of 4%.17 Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1-3, FGFRs 1-4, platelet-derived growth factor receptorα, rearranged during transfection, and KIT.18, 19, 20 It was very promising as a potentiator of ICIs in BTC, not only because of its potentiation of ICIs via the inhibition of angiogenesis and fibroblast growth factor (FGF)21 but also because, as a single agent, it showed a response rate of 11.5% in the second-line treatment of BTC.22 In addition, high response rates were reported for the combination of lenvatinib and an ICI in renal and gynecologic cancers.23,24
Therefore, we decided to investigate the efficacy and safety of the combination of nivolumab, an ICI, and lenvatinib, an angiogenesis inhibitor, in the second-line treatment of BTC.
Patients and methods
Study design and patients
This multicenter, single-arm, phase I/II study was conducted at five centers and in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines. The study protocol was approved by the ethics committee or institutional review board of each participating center. All patients provided written informed consent before study entry. This study is registered in the Japan Registry for Clinical Trials as jRCT2091220436. The main eligibility criteria for inclusion were as follows: clinical diagnosis of BTC; unresectable or recurrent disease with a measurable lesion per RECIST version 1.1; age above 20 years; histologically or cytologically confirmed diagnosis of adenocarcinoma; disease progression or treatment failure following one prior gemcitabine-based chemotherapy regimen (in combination with cisplatin or other platinum agent/fluoropyrimidine agent); ability to maintain sufficient food intake; an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, in addition to adequate organ function; and no receipt of any anticancer treatment within 21 days before the first dose of the study drug. Patients with interstitial pneumonia, lung fibrosis, or watery diarrhea were excluded.
Procedures
Patients received lenvatinib orally once daily in 14-day cycles. The nivolumab dose was fixed at 240 mg once every 2 weeks. Treatment continued until the development of an unacceptable toxicity, disease progression, or withdrawal of consent. In phase I, the initial lenvatinib dose was set at 20 mg daily. Safety and tolerability were observed for 28 days (two cycles). In phase II, patients started at 20 mg or 14 mg of lenvatinib, which were the doses determined in phase I. The criteria for intolerable toxicities were defined as any of the following events occurring during the first cycle of treatment: hematological toxicities, which included febrile neutropenia, grade 4 neutropenia for 7 days, grade 4 decrease in platelet count, grade 3 decrease in platelet count for 7 days or with bleeding, and grade 4 anemia; and non-hematological toxicities, which included grade 4 adverse events (AEs), grade 3 gastrointestinal perforation, thromboembolic event, uveitis, pneumonitis, bronchospasm, allergic reaction, infusion-related reaction, wound dehiscence needing treatment, grade 3 AEs for 3 days after proper treatment, grade 2 uveitis, eye pain, blurred vision that was treated locally and not improved to grade 1 during the re-administration period or needed systematic treatment, and adverse reactions requiring lenvatinib discontinuation for >8 days every 2 weeks.
Outcomes
In phase I, the recommended dose of lenvatinib was determined. With six patients, if the occurrence of AEs meeting the criteria for intolerable toxicities ranged from 0 to 2 patients, we proceeded to the phase II part with a recommended dose of 20 mg/day. If not, the dosage of lenvatinib was reduced by one level to 14 mg/day, and an additional six cases were enrolled for safety assessment. In phase II, the efficacy and safety of lenvatinib and nivolumab were evaluated. If efficacy was expected in phase II, we planned to initiate a first-line expansion cohort (n = 15). In phase II, the primary endpoint was objective response rate (ORR). Secondary endpoints included overall survival (OS), progression-free survival (PFS), disease control rate (DCR), and safety. Tumor assessments were carried out by a blinded independent central review every 6 weeks until week 24 and then every 12 weeks thereafter using RECIST version 1.1. Complete response (CR) and partial response (PR) required subsequent confirmation of the responses ≥4 weeks later. ORR was defined as the proportion of patients with a CR plus those with a PR. DCR was defined as the overall proportion of patients with CR, PR, or stable disease for 4 weeks or longer. The safety profile was assessed by monitoring and recording all AEs, including all the Common Terminology Criteria for Adverse Events, version 5.0, grades. Toxicity was managed with supportive medications, treatment interruption, dose reduction, and/or treatment discontinuation in accordance with the protocol’s prespecified dose-modification guidelines.
Statistical analysis
In the phase II part including patients who received the dose recommended in the phase I part, the threshold for ORR (under the null hypothesis) was set as 10%, and the expected ORR (under the alternative hypothesis) was set at 30% based on results of previous studies,25 which provided an 80% power for the primary endpoint with a one-sided alpha error of 5%. A total sample size of at least 32 patients was estimated to be required. In the phase II part, if there are 7 or more patients showing efficacy out of 32 patients, it is possible to reject the null hypothesis.
The PFS and OS were estimated by the Kaplan–Meier method. The confidence interval (CI) for ORR and DCR was estimated by the Clopper–Pearson method. All statistical analyses were carried out with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Patient characteristics
At the five centers, six patients were enrolled in phase I between August and October 2019, and a total of 32 patients were enrolled in phases I and II, which ended in November 2020, as shown in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103919. The recommended dose of lenvatinib was determined to be 20 mg in the six patients in phase I, with one AE corresponding to the toxicity evaluation criteria of myocarditis. The trial was completed without a first-line expansion cohort because the efficacy in phase II was limited. Primary tumor locations included the GB (n = 7), EHBD (n = 5), IHBD (n = 15), and AV (n = 5). Most patients were male (71.9%), had an ECOG PS score of 0 (71.9%), and had an unresectable disease (78.1%). Additionally, data regarding prior chemotherapy, microsatellite instability status, biliary drainage, and usage of antibiotics within 1 month before the start of treatment in this trial are shown in Table 1. For 32 patients, the median number of treatment courses was 4 (range 1-25). The breakdown of reasons for protocol treatment discontinuation included 28 patients (87.5%) due to disease progression, 2 patients (6.3%) due to toxicity, 1 patient due to patient refusal, and 1 case due to other reasons.
Table 1.
Patient characteristics (N = 32)
| Median age, years (range) | 63 (44-78) |
|---|---|
| Sex, n (%) | |
| Male | 23 (71.9) |
| Female | 9 (28.1) |
| ECOG PS, n (%) | |
| 0 | 23 (71.9) |
| 1 | 9 (28.1) |
| Primary site, n (%) | |
| Gall-bladder | 7 (21.9) |
| Extrahepatic bile duct | 5 (15.6) |
| Intrahepatic bile duct | 15 (46.9) |
| Ampulla of Vater | 5 (15.6) |
| Extent of disease, n (%) | |
| Unresectable | 25 (78.1) |
| Recurrent | 7 (21.9) |
| Primary chemotherapy (first line), n (%) | |
| GEM + CDDP | 23 (71.9) |
| GEM + CDDP + S-1 | 9 (28.1) |
| Microsatellite instability status, n (%) | |
| Stable | 27 (84.4) |
| High | 0 (0.0) |
| Unknown | 5 (15.6) |
| Biliary drainage, n (%) | |
| Presence | 14 (43.8) |
| Absence | 18 (56.3) |
| Use of antibiotics, n (%)a | |
| Yes | 9 (28.1) |
| No | 23 (71.9) |
CDDP, cisplatin; ECOG PS, Eastern Cooperative Oncology Group performance status; GEM, gemcitabine.
Within 1 month before the start of treatment.
Efficacy
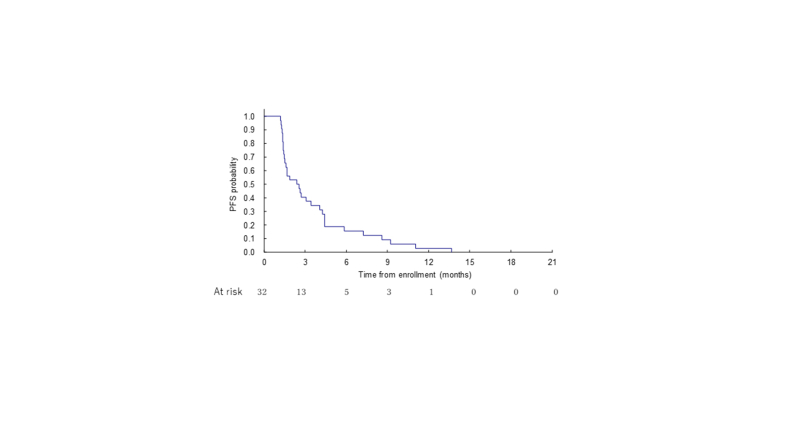
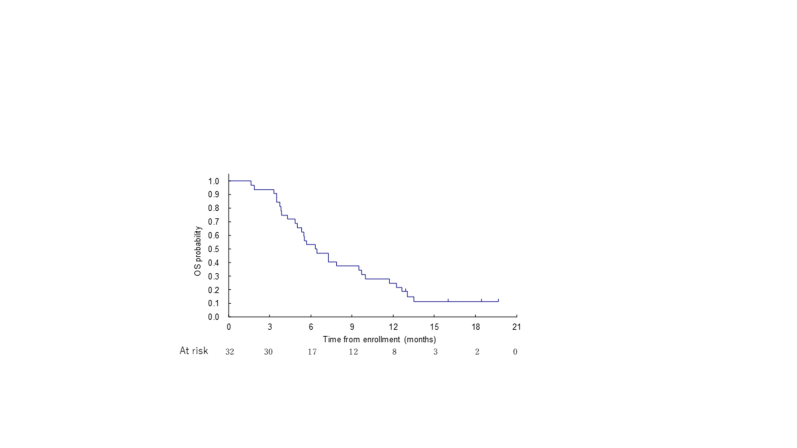
The ORR following nivolumab plus lenvatinib was 9.4% (90% CI 2.6% to 22.5%); 3 patients (9.4%) experienced a PR, and the disease became stable in 14 patients (43.8%; Table 2). With median follow-up of 6.4 months for 32 patients, the median PFS was 2.5 months (95% CI 1.5-4.1 months; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103919). The median OS was 6.4 months (95% CI 4.9-9.7 months; Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103919). In the overall response rate subgroup analysis, tumor response was observed only in the GB (28.6%) and AV (20.0%) and without the usage of antibiotics within 1 month before the start of treatment (13.0%). No tumor response was observed with the usage of antibiotics (Table 3).
Table 2.
Best overall response (N = 32)
| n (%) | |
|---|---|
| Complete response | 0 (0.0) |
| Partial response | 3 (9.4) |
| Stable disease | 14 (43.8) |
| Progressive disease | 14 (43.8) |
| Not evaluated | 1 (3.1) |
| ORR (90% CI) | 3 (9.4; 2.6-22.5) |
| DCR (95% CI) | 17 (53.1; 34.7-70.9) |
CI, confidence interval; DCR, disease control rate; ORR, objective response rate.
Table 3.
Subgroup analysis of objective response rate
| Factor | ORR (%) (95% CI) |
|---|---|
| MSI status | |
| Stable | 7.4 (0.9-24.3) |
| Unknown | 20.0 (0.5-71.6) |
| Primary site | |
| Gall-bladder | 28.6 (3.7-71.0) |
| Extrahepatic bile duct | 0.0 (0.0-52.2) |
| Intrahepatic bile duct | 0.0 (0.0-21.8) |
| Ampullary | 20.0 (0.5-71.6) |
| Biliary drainage | |
| Presence | 7.1 (0.2-33.9) |
| Absence | 11.1 (1.4-34.7) |
| Use of antibioticsa | |
| Yes | 0.0 (0.0-33.6) |
| No | 13.0 (2.8-33.6) |
CI, confidence interval; ORR, objective response rate; MSI, microsatellite instability.
Within 1 month before the start of treatment.
Safety
AEs are shown in Table 4. The most common AEs were hypertension (78.1%), proteinuria (62.5%), anorexia (53.1%), platelet count decreased (50.0%), hoarseness (43.8%), biliary tract infection (40.6%), and malaise (40.6%). The most common AEs of ≥grade 3 were hypertension (59.4%) and biliary tract infection (37.5%). No treatment-related deaths occurred. AEs led to nivolumab discontinuation in 9.4% of patients and to lenvatinib discontinuation in 21.9% of patients. However, most AEs were manageable: 62.5% of patients required a lenvatinib dose reduction, 93.8% of patients required a lenvatinib dose interruption, and 81.3% of patients required a nivolumab dose interruption. Immune-mediated adverse events (IMAEs; considered causally related to nivolumab) are shown in Table 5. The most common IMAEs were rash (28.1%), hypothyroidism (21.9%), malaise (18.8%), fever (12.5%), and anorexia (12.5%). AEs and IMAEs of the phase I part, respectively, are shown in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2024.103919.
Table 4.
Adverse events (N = 32)
| CTCAE ver. 5.0 | Any grade, n (%) | Grade 3/4, n (%) |
|---|---|---|
| Hypertension | 25 (78.1) | 19 (59.4) |
| Proteinuria | 20 (62.5) | 2 (6.3) |
| Anorexia | 17 (53.1) | 2 (6.3) |
| Platelet count decreased | 16 (50.0) | 3 (9.4) |
| Hoarseness | 14 (43.8) | 0 |
| Biliary tract infection | 13 (40.6) | 12 (37.5) |
| Malaise | 13 (40.6) | 1 (3.1) |
| Rash | 12 (37.5) | 2 (6.3) |
| Fever | 11 (34.4) | 1 (3.1) |
| Hypothyroidism | 11 (34.4) | 0 |
| AST increased | 9 (28.1) | 0 |
| Lymphocyte count decreased | 9 (28.1) | 3 (9.4) |
| Diarrhea | 9 (28.1) | 1 (3.1) |
| Nausea | 9 (28.1) | 0 |
| Hypoalbuminemia | 9 (28.1) | 2 (6.3) |
| Weight loss | 8 (25.0) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 8 (25.0) | 0 |
| Vomiting | 7 (21.9) | 0 |
| ALT increased | 6 (18.8) | 1 (3.1) |
| Fatigue | 6 (18.8) | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
Table 5.
Immune-mediated adverse events (N = 32)
| CTCAE ver. 5.0 | Any grade, n (%) | Grade 3/4, n (%) |
|---|---|---|
| Rash | 9 (28.1) | 1 (3.1) |
| Hypothyroidism | 7 (21.9) | 0 |
| Malaise | 6 (18.8) | 1 (3.1) |
| Fever | 4 (12.5) | 0 |
| Anorexia | 4 (12.5) | 0 |
| Diarrhea | 3 (9.4) | 1 (3.1) |
| Blood corticotrophin increased | 3 (9.4) | 0 |
| Hyperthyroidism | 2 (6.3) | 0 |
| ALT increased | 2 (6.3) | 0 |
| Mucositis oral | 2 (6.3) | 1 (3.1) |
| Pneumonitis | 2 (6.3) | 0 |
| Pruritus | 2 (6.3) | 0 |
| Increase in blood thyroid-stimulating hormone | 2 (6.3) | 0 |
| Atrial fibrillation | 2 (6.3) | 0 |
| Infusion reaction | 2 (6.3) | 0 |
ALT, alanine aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
Discussion
In recent years, ICIs and combined immunotherapies have demonstrated activity in multiple cancers. However, nivolumab plus lenvatinib did not show sufficient efficacy for BTC in this study.
The combination of GC and ICIs has proven to be superior in the first-line treatment of BTC.26,27 On the other hand, after second-line treatment, neither ICIs alone nor in combination have shown efficacy. In this study, however, the patients were not necessarily in poor condition, as 70% of them had an ECOG PS of 0, but the prognosis was limited, with a median survival of 6.4 months, which may have limited the efficacy of the ICI.
The use of antibiotics has recently been reported to alter the intestinal microbiota, negatively affecting the effectiveness of ICIs. While there are reports of a negative impact of antibiotic administration on treatment efficacy defined as administration within 1 month before the start of treatment,28 no such difference was observed when an ICI was used in combination with GC therapy in the first-line treatment of BTC.29 The impact of antibiotics may be not so large when an ICI is combined with chemotherapy. The combination in this study did not include chemotherapy but instead included a molecular targeted therapy and this may have had a greater impact. As a result, no response was observed in the group that had a history of antibiotic use within 1 month before the start of treatment. This corresponds to 28.1% of the patients in this study, so the impact was not small. Furthermore, during the treatment course, biliary tract infections were observed in 40.6% of cases, which suggests that antibiotics were used. This is also important as one of the potential reasons for the poor outcomes in this study.
Compared with a previous phase II trial of lenvatinib for second-line treatment of BTC,22 there was no increase in the response rate; rather, it tended to be lower. The dose of lenvatinib was set at 24 mg daily in the previous lenvatinib study, whereas it was set at 20 mg in our study. On the other hand, grade 3/4 hypertension occurred at a higher rate, suggesting that a sufficient dose intensity may not have been maintained due to dose interruption and dose reduction. In phase I of this study, the dose of lenvatinib was considered acceptable in only one case of intolerable toxicities; however, it was also considered important to select appropriate subjects for this combination therapy, such as those with no history of hypertension.
In another study, the combination of lenvatinib and ICI proved to be superior to chemotherapy regimens for endometrial cancer. The response rate in a group with mismatch repair-proficient endometrial cancer was 30.3%.30 This study was a second-line treatment like ours, but the response rates were very different. Furthermore, a pembrolizumab plus lenvatinib trial of a second-line treatment for BTC also showed a limited response rate of 10%, which was similar to our study.31 In the development of ICIs, differences in carcinomas seem to be important.
Subsite-specific outcomes are often discussed for BTC. In a phase III trial of durvalumab as a first-line treatment, intrahepatic cholangiocarcinoma showed better outcomes in a subgroup analysis. In our study, however, responses were observed in GB and AV but not in extrahepatic or intrahepatic cholangiocarcinoma. Furthermore, among the 20 patients with extrahepatic or intrahepatic cholangiocarcinoma, 7 patients had received antibiotics within 1 month before the start of treatment, and biliary tract infections occurred during the treatment course in another 6 patients. In the treatment of BTC with ICIs, treatment efficacy may depend on the presence or absence of a biliary tract infection and history of antibiotic use.
Some data suggest that patients who experience IMAEs more are more likely to respond to ICIs.32 In this study, the three cases with PR had IMAEs such as hypothyroidism, hyperthyroidism, diarrhea, and increased blood corticotrophin. IMAEs may also be biomarkers for treatment of BTC with ICIs.
A limitation of this study was that it was a single-arm study with a small number of cases. However, the five centers that participated in this study handle high volumes of patients, and this contributes to the quality assurance of this study. On the other hand, a randomized trial would have been necessary to confirm the definitive effects of the ICI. In addition, the effects of antibiotic administration have been previously reported in other cancer types, and perhaps the study should have been set up to exclude such cases.
In conclusion, nivolumab plus lenvatinib was found to be safe but not sufficiently effective in the second-line treatment of BTC.
Acknowledgements
This research work was presented at ESMO Congress 2022. We are grateful to the members of the Clinical Research Support Office of the National Cancer Center Hospital for their support in preparing the manuscript and oversight of the study. We acknowledge the support of Nakanoshima Translation Center in the editing of a draft of this manuscript.
Funding
This work was supported by Ono Pharmaceutical Co., Ltd and Eisai Co., Ltd (no grant number).
Disclosure
MU reports honoraria from Taiho Pharmaceutical Co., Ltd., AstraZeneca, K.K, Yakult Honsha Co., Ltd., MSD K.K, Nihon Servier Co., Ltd., Ono Pharmaceutical Co., Ltd., Incyte Biosciences Japan GK, Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim GmbH, J-Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Novartis Pharma K.K.; and research funding from Taiho Pharmaceutical Co., Ltd., AstraZeneca, K.K, MSD K.K, Nihon Servier Co., Ltd., Ono Pharmaceutical Co., Ltd., Incyte Biosciences Japan GK, Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim GmbH, J-Pharma Co., Ltd., Eisai Co., Ltd., Novartis Pharma K.K, Astellas Pharma Inc., J-Pharma Co., Ltd., DFP (Delta Fly Pharma), Inc., Novocure GmbH, and Chiome Bioscience Inc. CM reports honoraria from Novartis Pharma K.K, Yakult Honsha Co., Ltd., Teijin Pharma, Ltd., Taiho Pharmaceutical Co., Ltd., Eisai Co., Ltd., MSD K.K, and AstraZeneca, K.K; consulting fees from Yakult Honsha Co., Ltd., MSD K.K, Nihon Servier Co., Boehringer Ingelheim GmbH, and Taiho Pharmaceutical Co., Ltd.; and research funding from Eisai Co., Yakult Honsha Co., Ltd., Ono Pharmaceutical Co., Taiho Pharmaceutical Co., J-Pharma Co., AstraZeneca, K.K, Merck Biopharma Co., Ltd., Daiichi Sankyo Co., Ltd., and Boehringer Ingelheim GmbH. MI reports honoraria from AstraZeneca, K.K, Guardant Health Japan Corp., and Incyte Biosciences Japan GK; consulting fees from AstraZeneca, K.K; and research funding from AstraZeneca, K.K, Boehringer Ingelheim GmbH, Eisai Co., J-Pharma Co., Merck Biopharma Co., Ono Pharmaceutical Co., Ltd., and Eli Lilly Japan K.K. MO reports honoraria from Taiho Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K, Bayer Yakuhin, Ltd., Ono Pharmaceutical Co., Nihon Servier Co., Ltd., Pfizer Japan Inc., and Novartis Pharma K.K.; and expert testimony from Kaneka Medix Co. FN reports honoraria from Yakult Honsha Co., Ltd., Taiho Pharmaceutical Co., Ltd., MSD K.K, Daiichi Sankyo Co., Eli Lilly Japan K.K., and Merck Biopharma Co., Ltd.; consulting fees from Yakult Honsha Co., Ltd.; and research funding from Ono Pharmaceutical Co., Ltd., Eisai Co., AstraZeneca, K.K, MSD K.K, Taiho Pharmaceutical Co., Ltd., DFP (Delta Fly Pharma), Inc., Merck Biopharma Co., Ltd., Bristol Myers Squibb, J-Pharma Co., Astellas Pharma Inc., Takeda Pharmaceutical Co., and Daiichi Sankyo Co., Ltd. JM reports honoraria from Chugai Pharmaceutical Co., and Taiho Pharmaceutical Co., Ltd.; and other financial interest from Pfizer Japan Inc. AO reports honoraria from Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Ltd., and Yakult Honsha Co., Ltd. SK reports honoraria from Ono Pharmaceutical Co., Taiho Pharmaceutical Co., Ltd., AstraZeneca, K.K, Eli Lilly Japan K.K., and Eisai Co., Ltd.; and advisory board from Zymeworks Inc. HI reports honoraria from Boston Scientific Japan, K.K, Kaneka Medix Co., Medico’s Hirata Inc., SB-KAWASUMI LABORATORIES Inc., and AstraZeneca, K.K. AK reports honoraria from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., and Eisai Co., Ltd. NO reports honoraria from Taiho Pharmaceutical Co., Ltd., AstraZeneca, K.K, Eli Lilly Japan K.K., Eisai Co., Ltd., Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., MSD K.K, and Incyte Biosciences Japan GK; advisory board from GlaxoSmithKline K.K.; and medical writing from J-Pharma Co., Ltd. MS reports honoraria from AstraZeneca, K.K, Chugai Pharmaceutical Co., Ltd., and Nihon Servier Co., Ltd. JF reports honoraria from Ono Pharmaceutical Co., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan K.K., AstraZeneca, K.K, Yakult Honsha Co., Ltd., Nihon Servier Co., Ltd., MSD K.K, Novartis Pharma K.K., Takeda Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Taiho Pharmaceutical Co., Ltd., EA Pharma Co., Ltd., TEIJIN LIMITED, Daiichi Sankyo Co., Ltd., Terumo Corporation, Incyte Biosciences Japan GK, and J-Pharma Co., Ltd.; and research funding from MSD K.K, J-Pharma Co., Ltd., DFP (Delta Fly Pharma), Inc., Taiho Pharmaceutical Co., Ltd., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., and Sanofi K.K. TO reports honoraria from AstraZeneca, K.K, Eisai Co., Ltd., Johnson & Johnson K.K., Chugai Pharmaceutical Co., Ltd., Novartis Pharma K.K, Yakult Honsha Co., Ltd., Syneos Health, Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Nihon Servier Co., Ltd., Myriad Genetics G.K., and Daiichi Sankyo Co., Ltd.; consulting fees from AstraZeneca, K.K, Ono Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Nihon Servier Co., Ltd., Novartis Pharma K.K, Eisai Co., Ltd., FUJIFILM Toyama Chemical Co., Ltd., Sumitomo Pharma Co., Ltd., and Chugai Pharmaceutical Co., Ltd., and research funding from MSD K.K, J-Pharma Co., Ltd., Eisai Co., Ltd., Novartis Pharma K.K., Bristol Myers Squibb, AstraZeneca, K.K, Chiome Bioscience Inc, Syneos Health, Incyte Biosciences Japan GK, and Sysmex corporation. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary Figure 1.
Supplementary Figure 2.
Supplementary Figure 3.
References
- 1.Randi G., Malvezzi M., Levi F., et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20(1):146–159. doi: 10.1093/annonc/mdn533. [DOI] [PubMed] [Google Scholar]
- 2.Valle J.W., Lamarca A., Goyal L., Barriuso J., Zhu A.X. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 4.Lamarca A., Palmer D.H., Wasan H.S., et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22(5):690–701. doi: 10.1016/S1470-2045(21)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyung J., Kim I., Kim K.P., et al. Treatment with liposomal irinotecan plus fluorouracil and leucovorin for patients with previously treated metastatic biliary tract cancer: the phase 2b NIFTY randomized clinical trial. JAMA Oncol. 2023;9(5):692–699. doi: 10.1001/jamaoncol.2023.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal L., Meric-Bernstam F., Hollebecque A., et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388(3):228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 8.Subbiah V., Lassen U., Elez E., et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21(9):1234–1243. doi: 10.1016/S1470-2045(20)30321-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura Y., Mizuno N., Sunakawa Y., et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019): a phase II basket study. J Clin Oncol. 2023;41(36):5569–5578. doi: 10.1200/JCO.23.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueno M., Ikeda M., Morizane C., et al. Nivolumab alone or in combination with cisplatin plus gemcitabine in Japanese patients with unresectable or recurrent biliary tract cancer: a non-randomised, multicentre, open-label, phase 1 study. Lancet Gastroenterol Hepatol. 2019;4(8):611–621. doi: 10.1016/S2468-1253(19)30086-X. [DOI] [PubMed] [Google Scholar]
- 11.Piha-Paul S.A., Oh D.Y., Ueno M., et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147(8):2190–2198. doi: 10.1002/ijc.33013. [DOI] [PubMed] [Google Scholar]
- 12.Yoo C., Javle M.M., Verdaguer Mata H., et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology. 2023;78(3):758–770. doi: 10.1097/HEP.0000000000000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Ott P.A., Hodi F.S., Buchbinder E.I. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol. 2015;5:202. doi: 10.3389/fonc.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura T., Kato Y., Ozawa Y., et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arkenau H.T., Martin-Liberal J., Calvo E., et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced or metastatic biliary tract cancer: nonrandomized, open-label, phase I trial (JVDF) Oncologist. 2018;23(12):1407-e136. doi: 10.1634/theoncologist.2018-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsui J., Yamamoto Y., Funahashi Y., et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122(3):664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto K., Kodama K., Takase K., et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340(1):97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto Y., Matsui J., Matsushima T., et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adachi Y., Kamiyama H., Ichikawa K., et al. Inhibition of FGFR reactivates IFNgamma signaling in tumor cells to enhance the combined antitumor activity of lenvatinib with anti-PD-1 antibodies. Cancer Res. 2022;82(2):292–306. doi: 10.1158/0008-5472.CAN-20-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno M., Ikeda M., Sasaki T., et al. Phase 2 study of lenvatinib monotherapy as second-line treatment in unresectable biliary tract cancer: primary analysis results. BMC Cancer. 2020;20(1):1105. doi: 10.1186/s12885-020-07365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makker V., Rasco D., Vogelzang N.J., et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20(5):711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PubMed] [Google Scholar]
- 24.Albiges L., Gurney H., Atduev V., et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2023;24(8):881–891. doi: 10.1016/S1470-2045(23)00276-0. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki E., Ikeda M., Okusaka T., et al. A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol. 2013;71(5):1141–1146. doi: 10.1007/s00280-013-2106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh D.-Y., Ruth He A., Qin S., et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8) doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 27.Kelley R.K., Ueno M., Yoo C., et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 28.Hakozaki T., Okuma Y., Omori M., Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol Lett. 2019;17(3):2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiwu H., Benjamin R.T., Thatthan S., et al. Outcomes by antibiotic use in participants with advanced biliary tract cancer treated with durvalumab or placebo plus gemcitabine and cisplatin in the phase 3 TOPAZ-1 study. J Clin Oncol. 2023;41(suppl 4) abstr550. [Google Scholar]
- 30.Makker V., Colombo N., Casado Herraez A., et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med. 2022;386(5):437–448. doi: 10.1056/NEJMoa2108330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luis V., Zarnie L., Hyun Cheol C., et al. Lenvatinib plus pembrolizumab for patients with previously treated biliary tract cancers in the multicohort phase II LEAP-005 study. J Clin Oncol. 2021;39(suppl 3) abst 321. [Google Scholar]
- 32.Ricciuti B., Genova C., De Giglio A., et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145(2):479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.