Abstract
Functional analysis of the roles of the nuclear receptor response elements (NRREs) in the transcription and replication of hepatitis B virus (HBV) in the context of its whole genome has been hampered by the extensive overlapping of the NRREs with the regions encoding viral proteins. We introduced point mutations that inactivate the NRREs individually without altering the open reading frames of viral proteins. These mutations in the context of a plasmid containing 1.2 copies of the HBV genome were transiently transfected into the human hepatoma cell line Huh7. Inactivation of the NRRE in either the preC promoter (NRREpreC) or enhancer I (NRREenhI) led to moderate reductions in synthesis of viral RNAs. Concurrent inactivation of both NRREs led to 7- to 8-fold reductions in synthesis of the preC, pregenomic, and preS RNAs and a 15-fold reduction in synthesis of the S RNA. The accumulation of viral DNA in the cytoplasmic nucleocapsids and virion particles in the culture medium was also reduced seven- to eightfold. These results suggest that these NRREs are critical for the efficient propagation of HBV in hepatocytes. In cotransfection experiments we also found that overexpression of PPARα-RXRα in the presence of their respective ligands led to a fourfold increase in pregenomic RNA synthesis and a four- to fivefold increase in viral DNA synthesis, while it had little or no effect on synthesis of the other viral RNAs. Similar effects were observed with overexpression of PPARγ-RXRα in the presence of their respective ligands. This activation was dependent on NRREpreC, because the increase in synthesis of viral RNA and DNA was not observed when this site was mutated. Likewise, no activation of synthesis of pregenomic RNA and viral DNA by PPARα-RXRα was observed in a naturally occurring NRREpreC− mutant of HBV. Our results suggest that interactions between nuclear receptors and NRREs present in the HBV genome may play critical roles in regulating its transcription and replication during HBV infection of hepatocytes.
Human hepatitis B virus (HBV) is the pathogen for viral hepatitis B. Chronic infection with HBV is associated with liver cirrhosis and primary hepatocellular carcinoma. Its 3.2-kb genome contains four overlapping open reading frames encoding the surface antigens (preS1, preS2, and S proteins), core antigens (preC and C proteins), reverse transcriptase (P protein), and transactivator (X protein). These genes are under the control of the preS, S, preC, pregenomic, and X promoters. Transcription from these promoters is regulated by two enhancer regions named enhancer I and enhancer II. Synthesis of HBV DNA takes place within the nucleocapsid in the cytoplasm of infected hepatocytes. The pregenomic RNA plays pivotal roles in the viral life cycle, serving both as the template for viral DNA synthesis and as the mRNA encoding the C and P proteins, components of the nucleocapsid (16, 17).
Nuclear receptors (NRs) are a superfamily of transcription factors which share domains of similar functionality and sequence. NRs bind to their respective response elements (NRREs) in a sequence-specific manner, leading to altered regulation of transcription from nearby promoters. Many NRs also bind ligands which affect their functional activities (54). NRs play important roles in regulating embryogenesis, cell differentiation, and a variety of cellular functions (30, 52).
The peroxisome proliferator-activated receptors (PPARs), a subfamily of NRs, consist of PPARα, PPARγ, and PPARδ. They play key roles in lipid metabolism (7). The PPARs function as part of a heterodimeric complex with another subfamily of NRs, the retinoid X receptors (RXRs) (36). The NRRE for PPAR-RXR is typically a direct repeat of two NR half-site sequences (5′-AGGTCA-3′) separated by 1 bp (DR1) (39). PPARα is expressed at high levels in the liver (28). While PPARγ is normally expressed at low levels in hepatocytes (53), its expression is induced to high levels in hepatoma cells when de novo cholesterol synthesis is inhibited (13). Although the physiological ligands for the PPARs remain unknown, many synthetic peroxisome proliferators such as Wy-14,643 and clofibric acid can function as ligands for PPARα (28). A number of saturated, polyunsaturated, and branch-chained fatty acids naturally present in cells, such as metabolites of prostaglandin J2, can function as ligands for PPARγ (14, 31). The ligand for the RXRs has been identified as 9-cis-retinoic acid, a metabolite of vitamin A (24, 33).
The hepatocyte nuclear factor 4α (HNF4α), a liver-enriched NR, is a transcription factor which activates transcription from the promoters of a variety of the genes essential for liver development and function (35, 48). Its physiological ligands are unknown, although fatty acyl-coenzyme A thioesters can funtion as ligands for it (23). HNF4α forms homodimers and binds to a DR1 element (15, 29).
NRs have been found to play important roles in the life cycles of a number of animal tumor viruses (19, 32, 37, 58, 63, 64). Tur-Kaspa et al. identified a glucocorticoid response element situated upstream of the enhancer I region of the HBV genome (55, 56). In recent years, three more NRREs have been identified in enhancer I (NRREenhI) (18, 26, 27), enhancer II (NRREenhII) (22), and the preC promoter (NRREpreC) (42, 61) of the HBV genome. Both NRREpreC and NRREenhI allow for the binding of PPAR-RXR and a number of orphan NRs such as HNF4α and chicken ovalbumin upstream promoter transcription factor 1 (COUP-TF1) (18). On the other hand, NRREenhII only allows for the binding of HNF4α (22).
The HBV NRREs are embedded in enhancer and promoter elements which overlap extensively with coding regions for viral proteins. Therefore, it is difficult to mutate individual NRREs without altering the amino acid sequences of viral proteins as well. Thus, to date, most of our knowledge regarding the biological functions of the HBV NRREs has been obtained utilizing reporter plasmids containing only a portion of the viral genome.
As part of the effort to understand the biology and pathology of HBV infection and the molecular mechanisms of chronic and fulminant hepatitis B, a few naturally occurring HBV variants with mutations in NRREs have been analyzed in the context of the whole HBV genome. However, the base pair changes present in all of these variants also cause alterations in some of the amino acids of viral proteins (2, 4, 9, 34). Thus, while the data from these studies indicated that the NRREs probably function as cis-acting regulatory elements in the life cycle of HBV, the individual contribution of each NRRE to transcription and replication could not be determined definitively. A transgenic mouse model has also been used to investigate regulation of HBV transcription and replication by NRs. Guidotti et al. (20) reported that treatment of HBV transgenic mice with synthetic compounds which are known ligands for PPARα results in a slight increase in viral RNA synthesis and a large increase in viral DNA synthesis in the livers of female transgenic mice.
In an effort to elucidate the mechanisms by which NRs activate HBV viral DNA synthesis and to explore the possible use of NRs and their ligands for treatment of HBV infection, we mutated NRREs in the HBV genome and examined viral RNA and DNA synthesis in hepatoma cells in the presence of coexpressed NRs. Previously, we reported that NRs exert differential regulation of transcription from the preC and pregenomic promoters in the context of a subgenomic fragment of HBV, with the effect varying with the NR (61). We report here that both NRREpreC and NRREenhI do, indeed, function as cis-acting, positive regulatory elements for viral transcription and replication in the context of the whole HBV genome. We also report that PPAR-RXR and their ligands efficiently upregulate synthesis of viral DNA through differential regulation of synthesis of the viral RNAs. These results suggest that NRs may play important roles in modulating the propagation of HBV in hepatocytes during different stages and courses of its infection.
MATERIALS AND METHODS
Site-directed mutagenesis and plasmid construction.
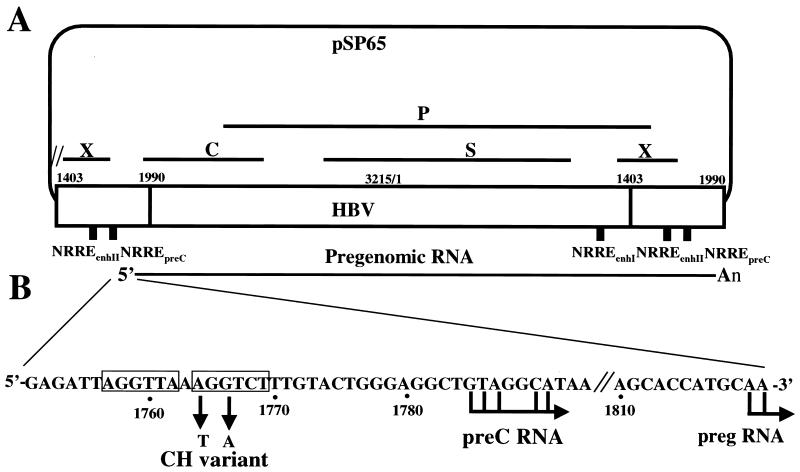
Point mutations were introduced into the NRREpreC and NRREenhI of the HBV genome by a two-step PCR-based mutagenesis method (11). All base pair changes were confirmed by DNA sequence analysis. Replication-competent wild-type and NRRE mutant plasmids were constructed by insertion into plasmid pSP65 of 1.2 copies in tandem of HBV subtype adr (nucleotides [nt] 1403 to 3215 and 1 to 1991) containing either wild-type or mutant NRREs to minimize the redundancy of HBV sequences while enabling synthesis of full-length pregenomic RNA (Fig. 1A). In NRREpreC− mutant plasmids, both copies of NRREpreC were mutated.
FIG. 1.
(A) Schematic diagram of plasmid containing 1.2 copies of the HBV genome. A 3.8-kb, terminally redundant variant of the HBV genome, indicated by the large open rectangle, was inserted into plasmid pSP65. The locations of the P, S, C, and X open reading frames are indicated by solid lines. The locations of the NRREs in enhancer I, enhancer II, and the preC promoter are indicated by small solid rectangles. Shown at the bottom is the structure of the pregenomic RNA synthesized from this plasmid. (B) Nucleotide sequence of the region of the HBV genome surrounding the NRREpreC. The half-site sequences in the NRREpreC are boxed. The sequence changes in a naturally occurring variant of HBV (CH variant) are shown below the boxed sequences. Right-pointing arrows indicate locations of transcription initiation sites used in the synthesis of preC and pregenomic RNA (preg RNA).
Oligonucleotides and EMSAs.
Electrophoretic mobility-shift assays (EMSAs) were performed as described previously (58, 64). The sources of the plasmids for synthesis of recombinant NR proteins and for transfection experiments were described previously (61). Recombinant proteins COUP-TF1, PPARα, RXRα, and HNF4α were synthesized in a coupled transcription-translation rabbit reticulocyte lysate system (Promega). The NRREpreC 25-bp synthetic oligonucleotide probe had the sequence 5′-GAGATTAGGTTAAAGGTCTTTGTAC-3′. The NRREenhI 29-bp synthetic oligonucleotide probe had the sequence 5′-ACAATATCTGAACCTTTACCCCGTTGCCC-3′. The nucleotide sequences of the mutant NRRE oligonucleotide probes were the same as the wild-type sequences except for the changes indicated (Fig. 1B and 2A). Competition EMSAs were performed as described previously (58, 64).
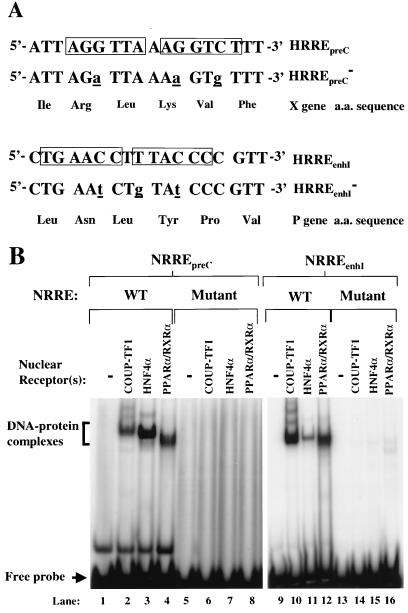
FIG. 2.
Inactivation of the NRREs in the preC promoter and enhancer I. (A) Sequences of the point mutations introduced into NRREpreC and NRREenhI such that translation of the open reading frames of the X and P genes remains unaffected. The imperfect direct repeats of the NR half-site sequence are boxed. The base pair changes introduced by mutagenesis are shown as underlined lowercase letters. (B) EMSAs used to determine the binding of NRs to wild-type (WT) and mutant NRREs. Radiolabeled double-stranded oligonucleotides containing the wild-type and mutant NRRE sequences were used as probes. DNA-protein complexes are indicated by the bracket; free probes are indicated by the arrow.
Cell line and transfections.
The human hepatoma cell line Huh7 was grown at 37°C with 5% CO2 in a 1:1 mixture of Dulbecco's modified Eagle's medium and F12 medium supplemented with 10% fetal bovine serum. Transient transfections were done according to the calcium phosphate precipitation method (44) using plasmid pUC18 as the carrier DNA. Each 60-mm tissue culture dish of cells was transfected with a total of 10 μg of plasmid DNA including 3 μg of wild-type or mutant HBV plasmid DNA. The β-galactosidase (β-Gal) expression plasmid pEQ176 (0.75 μg per 60-mm dish) (46) was included as an internal control in each transfection mixture. In the cotransfection experiments, 1 μg of a PPARα or PPARγ expression plasmid and 0.25 μg of an RXRα expression plasmid were included in each dish of cells. Cells were plated in medium supplemented with 5% charcoal-treated fetal bovine serum 24 h before transfection. After transfection with the calcium phosphate-DNA precipitates for 8 h, the cells were washed and incubated in medium containing 5% charcoal-treated serum and the indicated ligands for PPAR and RXR until they were harvested.
Isolation and analysis of viral RNAs.
Forty-eight hours after transfection, total cellular RNA was isolated using an RNeasy Mini kit (Qiagen). The relative amounts of the viral RNAs were determined by primer extension analysis as described previously (61). The primer for the preC and pregenomic RNAs was a 24-mer corresponding to HBV nt 1994 to 2017, the primer for the S RNA was a 20-mer corresponding to nt 137 to 156, and the primer for the preS RNA was a 24-mer corresponding to nt 3012 to 3035. The primer for β-Gal RNA was a 17-mer (5′-GTTTTCCCAGTCACGAC-3′).
The total polyadenylated mRNA from transfected Huh7 cells was isolated with oligo(dT) cellulose (60). Northern blot analysis of the viral mRNAs was performed as described previously (6). Briefly, denatured RNA was resolved in a 0.6 M formaldehyde–1% agarose gel, transferred to a nylon membrane, and probed with a radiolabeled HBV probe. The blot was then stripped and reprobed with a radiolabeled β-Gal probe. Probes were prepared using a random-prime labeling system (Amersham) with the 3.2-kb HBV fragment or a 2.9-kb β-Gal fragment as the template.
Isolation and analysis of viral DNA.
Forty-eight hours after transfection, the Huh7 cells were harvested. Viral nucleic acid was isolated from cytoplasmic nucleocapsids as described previously (49). The relative amounts of viral DNA were determined by Southern blot analysis (5). To isolate extracellular viral DNA, the medium in which the Huh7 cells had been growing was harvested 5 days after transfection. Virion particles were precipitated with 10% polyethylene glycol, and the viral DNA was isolated according to a method described by Summers et al. (50). The viral DNA was then subjected to Southern blot analysis. The radiolabeled HBV probe was the same as the one used in Northern blot analyses. To normalize for the efficiency of transfection, β-Gal assays were performed with lysates from the same cells.
RESULTS
Generation of NRREenhI and NRREpreC mutants of HBV defective in binding NRs.
Site-directed mutagenesis was performed to introduce nucleotide changes into NRREenhI and NRREpreC. These changes were designed such that the amino acid-coding capacities of the X and P open reading frames would not be affected (Fig. 2A). To examine the binding of NRs to these mutant NRREs, EMSAs were performed with recombinant NRs and radiolabeled synthetic oligonucleotides. As expected, we found that the mutations in these two NRREs drastically reduced binding by COUP-TF1, HNF4α, and PPARα-RXRα (Fig. 2B). Competition EMSAs were also performed to measure the affinities of these NRs for the mutant NRREs relative to the wild-type NRREs. The mutations in NRREpreC reduced its affinities for COUP-TF1, HNF4α, and PPARα-RXRα 43-, 9-, and 12-fold, respectively (data not shown). The mutations in NRREenhI reduced its affinities for COUP-TF1, HNF4α, and PPARα-RXRα 8-, 5-, and 17-fold, respectively (data not shown). Thus, we conclude that these point mutations significantly reduce binding by recombinant COUP-TF1, HNF4α, and PPARα-RXRα.
Effects of mutant NRREenhI and NRREpreC on HBV RNA and DNA synthesis.
The above NRRE mutations were introduced singly and doubly into a replication-competent plasmid containing 1.2 copies of the HBV genome (Fig. 1A) to generate plasmids NRREpreC−, NRREenhI−, and the double mutant NRREpreC−,enhI−. These mutant as well as wild-type plasmids were introduced in parallel into Huh7 cells by transient transfection. After incubation at 37°C for 48 h, the cells were harvested, and RNA and DNA were isolated. Northern blot analysis showed that these mutations in the NRREs led to reduced synthesis of all of the viral RNAs relative to that with wild-type NRREs (Fig. 3A).
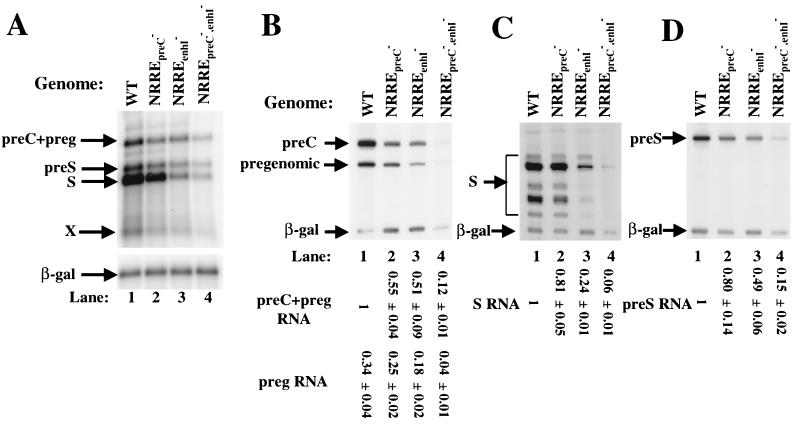
FIG. 3.
Reduced viral RNA synthesis in NRRE mutants of HBV. (A) Autoradiogram of Northern blot analysis of the viral RNAs accumulated in Huh7 cells transfected with wild-type (WT) and NRRE mutant plasmids. One-half of the poly(A)-selected RNA from a 60-mm dish of cells was loaded in each lane and analyzed as described in Materials and Methods. (B through D) Autoradiograms of 8 M urea–8% polyacrylamide gels showing the results of primer extension assays used to quantify the preC and pregenomic RNAs (B), S RNAs (C), and preS RNA (D). The positions of the viral and β-Gal RNAs are indicated by arrows. preg, pregenomic. One-sixth of the RNA from a 60-mm dish of cells was used in each reaction along with radiolabeled primers specific for the viral and β-Gal RNAs. Numbers at the bottom give the amount of viral RNA in each lane relative to that synthesized from the wild-type plasmid. These numbers were determined with a PhosphorImager, normalized to β-Gal, and represent the means ± standard errors of the data obtained from three experiments similar to the one for which results are shown. The amount of preC RNA can be calculated by subtracting the pregenomic RNA from the preC-plus-pregenomic RNA in each lane.
To examine in greater detail the effects of the NRRE mutations on synthesis of the viral RNAs, the HBV RNAs were analyzed by primer extension assays with primers specific for the preC and pregenomic, S, and preS RNAs (Fig. 3B, C, and D, respectively). The mutations in NRREenhI resulted in twofold reductions in preC, pregenomic, and preS RNA synthesis and a fourfold reduction in S RNA synthesis (lanes 3 in Fig. 3B, C, and D, respectively). The mutations in NRREpreC resulted in slight reductions in pregenomic, S, and preS RNA synthesis and a twofold reduction in preC RNA synthesis (lanes 2 in Fig. 3B, C, and D, respectively). However, when both of the NRREs were mutant, synthesis of the preC, pregenomic, and preS RNAs was reduced 7- to 8-fold and that of S RNA was reduced 15-fold (Fig. 3B through D, lanes 4). We were unable to detect the RNA species corresponding to the X RNA by primers designed for its detection (data not shown). However, Northern blot analysis showed that the mutants NRREpreC−, NRREenhI−, and NRREpreC−,enhI− reduced synthesis of X RNA approximately 2-, 4-, and 10-fold, respectively (Fig. 3A, lanes 2 to 4 versus lane 1).
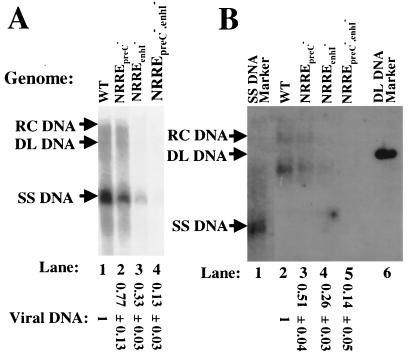
Accumulation of HBV DNA in cytoplasmic nucleocapsids was examined by Southern blot analysis. As predicted from the effects of the NRRE mutations on synthesis of the pregenomic RNA, the mutations in NRREpreC led to a slight reduction in viral DNA synthesis, while the mutations in NRREenhI resulted in a threefold reduction in viral DNA synthesis (Fig. 4A, lanes 2 and 3 versus lane 1). When both of the NRREs were mutant, viral DNA synthesis was reduced approximately eightfold (Fig. 4A, lane 4).
FIG. 4.
Reduced viral DNA synthesis in NRRE mutants of HBV. (A) Autoradiogram of Southern blot analysis of viral DNA isolated from nucleocapsids present in the cytoplasm of transfected Huh7 cells. Cells were harvested 48 h posttransfection, and nucleocapsid viral DNA was prepared. One-third of the DNA from a 60-mm dish of cells was loaded in each lane. The total viral DNA from relaxed circular (RC) to single-stranded (SS) DNA in each lane was quantitated with a PhosphorImager. Positions of viral DNAs are indicated by arrows. WT, wild type; DL DNA, duplex linear DNA. The smears between the specific indicated DNA structures are the result of HBV DNAs with incomplete strands. Numbers at the bottom are means ± standard errors of data relative to the wild type obtained in three experiments similar to the one for which results are shown. (B) Autoradiogram of Southern blot analysis of extracellular viral DNA. Culture medium was harvested for HBV virions 5 days posttransfection. One-half of the viral DNA from the medium of a 60-mm dish of cells was loaded in each lane. Lanes 1 and 6, molecular markers for SS and DL DNAs, respectively. The DNA band below the DL DNA in lanes 2 through 4 is likely DL or RC DNA with an incomplete plus strand. Data were quantified as for panel A.
To examine whether the effects of the NRRE mutations on synthesis of intracellular viral DNA in nucleocapsids were also reflected in accumulation of extracellular viral DNA in virion particles, HBV virion DNA was isolated from the tissue culture medium of the transfected Huh7 cells and analyzed likewise. The mutations in NRREpreC, NRREenhI, or both NRREs resulted in two-, four-, and sevenfold reductions of virion DNA levels in the medium, respectively (Fig. 4B). Thus, we conclude that both of these NRREs in HBV function as positive regulatory elements, with mutations in NRREenhI having greater negative effects on viral RNA and DNA synthesis than mutations in NRREpreC and with loss of the function of both NRREs having at least multiplicative effects.
Effects of PPARα-RXRα and PPARγ-RXRα on viral RNA and DNA synthesis.
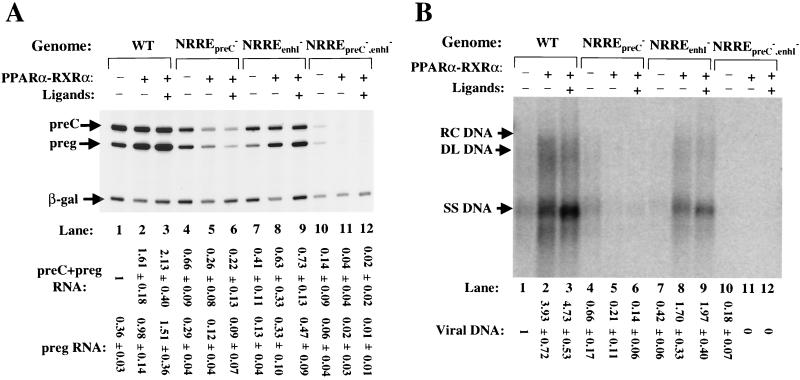
NRREpreC and NRREenhI are the only NRREs in the HBV genome to which PPARα-RXRα and PPARγ-RXRα are known to bind, with both PPARs requiring the presence of RXR to form DNA-protein complexes on these NRREs (61). Data from competition EMSAs indicated that both PPARα-RXRα and PPARγ-RXRα bind NRREpreC with a higher affinity than they do NRREenhI (61). Furthermore, although PPARα is enriched in the liver (28) while PPARγ is not (53), immunoshift assays and Western blot analysis with appropriate antisera failed to detect either PPARα or PPARγ in nuclear extracts of Huh7 cells (data not shown) (42). Thus, we studied the effects of PPAR-RXR on viral gene expression and DNA synthesis by cotransfection of Huh7 cells with PPAR and RXR expression plasmids along with wild-type or NRRE-mutant HBV-containing plasmids. After incubation at 37°C for 40 h in medium containing or lacking ligands for these receptors, the cells were harvested and analyzed as above. We found that PPARα-RXRα activated synthesis of pregenomic RNA from the wild-type HBV genome 2 1/2- or 4-fold when the ligands for PPARα and RXRα were absent or present in the medium, respectively (Fig. 5A, lanes 2 and 3 versus lane 1). PPARα-RXRα also activated synthesis of S RNA from the wild-type genome almost twofold (data not shown) but had little or no effect on synthesis of preC (Fig. 5A, lanes 1 to 3) and preS (data not shown) RNAs. As expected from increased synthesis of pregenomic RNA, overexpression of PPARα-RXRα also resulted in four- to fivefold-increased synthesis of intracellular viral DNA (Fig. 5B, lanes 1 to 3).
FIG. 5.
PPARα-RXRα activates synthesis of RNA and DNA of HBV. After incubation with the indicated DNA in a calcium phosphate precipitate for 8 h, Huh7 cells were washed and incubated in medium containing 1 mM clofibric acid (Sigma), a ligand for PPARα, and 1 μM 9-cis-retinoic acid (Sigma), a ligand for RXRα, for 40 h before they were harvested for RNA and DNA. (A) Autoradiogram showing the primer extension reactions of preC and pregenomic (preg) RNAs. One-sixth of the RNA from a 60-mm dish of cells was used in each primer extension reaction. WT, wild type. (B) Autoradiogram of Southern blot analysis of cytoplasmic viral DNA. One-third of the DNA from a 60-mm dish of cells was loaded in each lane. Quantitations were performed as described in the legends to Fig. 3 and 4. Numbers at the bottom are means ± standard errors of data obtained from three experiments similar to the one for which results are shown. RC DNA, relaxed circular DNA; DL DNA, duplex linear DNA; SS DNA, single-stranded DNA.
The effect of PPARα-RXRα on viral RNA synthesis was found to be dependent on a functional NRREpreC, because synthesis of preC and pregenomic RNAs and synthesis of S RNA were reduced three- and twofold, respectively, when this site was mutated (Fig. 5A, lanes 4 to 6; also data not shown). As a result of reduced synthesis of pregenomic RNA, the accumulation of intracellular viral DNA was also reduced three- to fourfold (Fig. 5B, lanes 4 to 6). On the other hand, overexpression of PPARα-RXRα still activated synthesis of pregenomic RNA three- to fourfold and that of S RNA twofold from the NRREenhI− plasmid (Fig. 5A, lanes 7 to 9; also data not shown) and increased the accumulation of cytoplasmic viral DNA four- to fivefold (Fig. 5B, lanes 7 to 9).
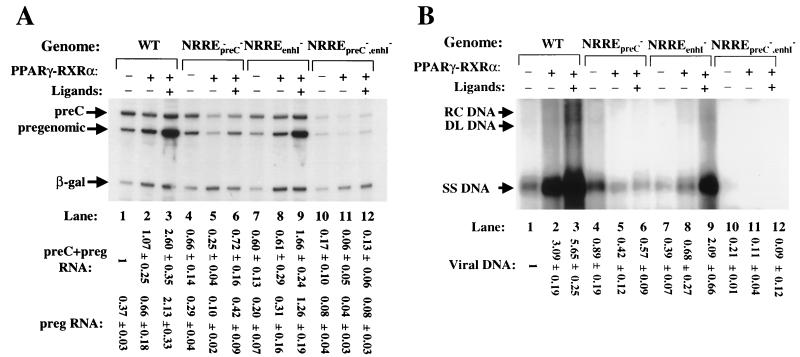
To examine the possibility that endogenous PPARα may have interfered somewhat with the outcome of these cotransfection experiments, analogous experiments were also performed with a PPARγ expression plasmid in the presence or absence of PPARγ's ligand, 15-deoxy-Δ12,14-prostaglandin J2. In this case, activation of synthesis of pregenomic RNA was found to be even more dependent on the presence of the ligand, with the increase in pregenomic RNA synthesis from the wild-type HBV template being less than twofold in charcoal-treated serum, yet greater than fivefold when the ligand was added to the medium (Fig. 6A, lane 2 versus lane 3). As expected, the accumulation of intracellular viral DNA increased concomitantly with pregenomic RNA synthesis (Fig. 6B, lanes 1 to 3). On the other hand, PPARγ-RXRα in the presence of their respective ligands increased S RNA synthesis only twofold and had little or no effect on preS RNA synthesis (data not shown). The effects of PPARγ-RXRα were also found to be largely dependent on the presence of a functional NRREpreC. Overexpression of PPARγ-RXRα led to a threefold decrease in preC and pregenomic RNA synthesis in the NRREpreC− mutant (Fig. 6A, lane 4 versus lane 5). This unexpected repression was observed only when ligands were not present in the medium (Fig. 6A, lane 5 versus lane 6). On the other hand, synthesis of pregenomic RNA still increased sixfold when NRREenhI was mutated (Fig. 6A, lanes 7 to 9), consistent with PPAR-RXR acting primarily through NRREpreC.
FIG. 6.
PPARγ-RXRα activates synthesis of viral RNA and DNA in Huh7 cells. The amounts of RNA and DNA used in each assay were the same as those stated for Fig. 5. Transfection of Huh7 cells was carried out as described for Fig. 5 except that a PPARγ-specific ligand, 15-deoxy-Δ12,14-prostaglandin J2, was used (8 μM) (Cayman Chemical). (A) Autoradiogram showing primer extension reactions used to quantify preC and pregenomic (preg) RNAs. (B) Autoradiogram of a Southern blot analysis of cytoplasmic viral DNA. Quantitations were performed as described in the legends to Fig. 3 and 4. Numbers at the bottom are means ± standard errors of data obtained from three experiments similar to the one for which results are shown. RC DNA, relaxed circular DNA; DL DNA, duplex linear DNA; SS DNA, single-stranded DNA.
In conclusion, PPARα-RXRα and PPARγ-RXRα activate synthesis of pregenomic RNA and viral DNA in transiently transfected Huh7 cells. This activation is largely dependent on their interaction with NRREpreC; NRREenhI plays only a minor role. The presence of ligands to the receptors greatly stimulates this activation of viral RNA and DNA synthesis, especially by PPARγ-RXRα.
Effects of PPARα-RXRα on viral RNA and DNA synthesis in a naturally occurring HBV variant.
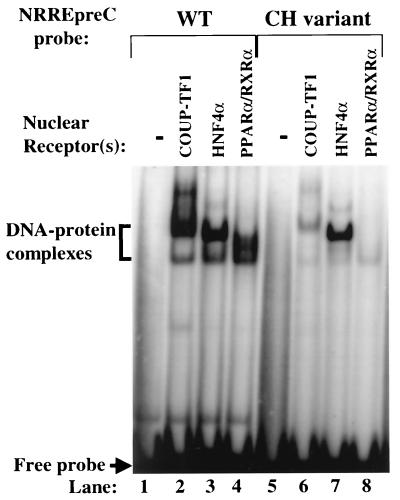
A variant of HBV is frequently found in chronic and, in certain areas of Asia, fulminant hepatitis B patients (8, 40). This variant (CH variant) contains changes in two of the conserved base pairs in the HBV NRREpreC: A to T at position 1764 and G to A at position 1766 (Fig. 1B). It has been reported previously that these two point mutations abolish binding of COUP-TF1 to NRREpreC (9, 10). To determine whether the base pair changes also affect the binding of HNF4α and PPARα-RXRα to this site, EMSAs were performed as described above, but with a 25-bp radiolabeled oligonucleotide corresponding to the NRREpreC sequence in the CH variant as a probe. The two base pair changes in this variant abolished binding to NRREpreC by COUP-TF1, as expected, and by PPARα-RXRα (Fig. 7). However, they had little effect on the binding of HNF4α (Fig. 7, lane 7 versus lane 3). Competition EMSAs with radiolabeled wild-type NRREpreC as the probe and unlabeled wild-type versus CH variant NRREpreC oligonucleotides as competitors showed that the mutations in the CH variant reduced its affinities for COUP-TF1 and PPARα-RXRα 27- and 7-fold, respectively, but had no effect on the binding of HNF4α to NRREpreC (data not shown).
FIG. 7.
Effects of base pair changes in the NRREpreC of the CH variant of HBV on its binding to NRs. Shown here are results of EMSAs performed with the indicated NRs and radiolabeled, double-stranded oligonucleotides containing the wild-type (WT) and CH variant NRREpreC sequences as probes. Bracket, DNA-protein complexes; arrow, free probe.
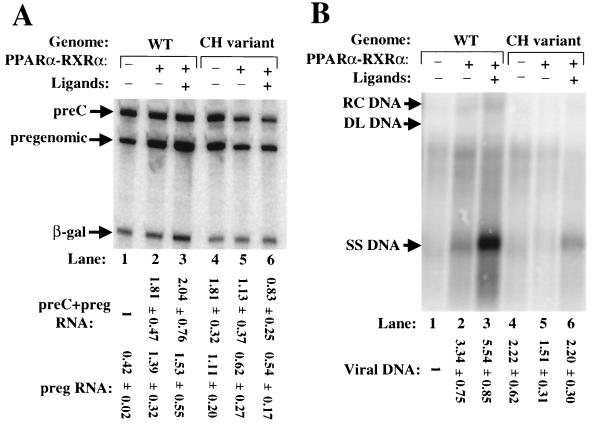
To study the effects of the mutations in the CH variant on expression of HBV, these mutations were introduced into the plasmid containing 1.2 copies of the wild-type HBV genome. Transfection experiments were performed with coexpressed PPARα-RXRα as described above. We found that mutations in the CH variant led to a two- to threefold increase in synthesis of pregenomic RNA but had very little effect on synthesis of preC RNA (Fig. 8A, lane 1 versus lane 4). Coexpressed PPARα-RXRα reduced synthesis of both preC and pregenomic RNAs from the CH variant genome approximately twofold, while it increased synthesis of pregenomic RNA from the wild-type genome three- to fourfold (Fig. 8A). Coexpressed PPARα-RXRα had little or no effect on the accumulation of intracellular viral DNA from the CH variant in the presence or absence of their ligands (Fig. 8B). Thus, we conclude that PPARα-RXRα and their ligands fail to significantly affect the expression of the HBV genome in the CH variant.
FIG. 8.
Sequence changes in the NRREpreC of the CH variant of HBV abolish activation of synthesis of viral RNA and DNA by PPARα-RXRα. (A) Autoradiogram of primer extension reactions used to quantify preC and pregenomic (preg) RNAs synthesized from the wild-type (WT) and CH variant HBV plasmids. Transient transfections of Huh7 cells and the concentrations of ligands for PPARα and RXRα added to the medium were as described for Fig. 5. (B) Autoradiogram of Southern blot analysis of cytoplasmic viral DNA present in the same cells. Numbers at the bottom are means ± standard errors of data quantified from three experiments similar to the one for which results are shown. RC DNA, relaxed circular DNA; DL DNA, duplex linear DNA; SS DNA, single-stranded DNA.
DISCUSSION
Using mutants in the NRREs that abrogate NR binding without disrupting the overlapping coding regions for viral proteins (Fig. 2A), we have examined here the biological functions of NRREs in the context of a replication-competent HBV genome. We found that both NRREpreC and NRREenhI function as cis-acting, positive regulatory elements. Mutagenesis of either of these NRREs led to decreased synthesis of both viral RNA and DNA, with the effects being greatest when both sites were mutated (Fig. 3 and 4). Overexpression of PPAR-RXR led to increased synthesis of both viral pregenomic RNA and viral DNA (Fig. 5 and 6). Addition of ligands for the PPARs and RXRα to the medium further increased synthesis of pregenomic RNA. This activation was found to be dependent only on a functional NRREpreC, although both NRREs can bind PPAR-RXR (Fig. 5 and 6). Lastly, a naturally occurring variant of HBV was shown to be defective both in the binding of PPARα-RXRα to its NRREpreC (Fig. 7) and in activation of synthesis of pregenomic RNA and viral DNA by PPARα–RXRα and their ligands (Fig. 8). Thus, we conclude that PPAR-RXR and their ligands likely play important roles in the replication of HBV, acting primarily via binding of NRREpreC.
NRREpreC and NRREenhI are positive regulatory elements essential for efficient viral gene expression and DNA synthesis.
NRREpreC was identified in the regulatory region of the preC and pregenomic promoters of HBV. EMSAs and DNase I footprinting analyses showed that NRs can bind to this site (42, 61). Since NRREpreC is embedded in the preC promoter and distal to other HBV promoters, it has been hypothesized to regulate primarily synthesis of preC and pregenomic RNAs. However, its biological functions have not been characterized previously in the context of a replication-competent HBV genome in hepatoma cell lines. We have found in this study that mutations in NRREpreC result in reduced synthesis of preC and pregenomic RNAs (Fig. 3B) and have little or no effect on synthesis of S and preS RNAs (Fig. 3C and D).
Seemingly contrary to the present findings, we previously reported that mutations in NRREpreC lead to increased synthesis of preC RNA (61). However, this study was performed with expression plasmids containing only a 588-bp subregion of the HBV genome (nt 1403 to 1990; see Fig. 1). Therefore, proper functioning of NRREpreC as a positive regulatory element may require it to be situated at its normal location within the context of the whole HBV genome so that appropriate protein-protein interactions with numerous other regulatory factors can occur within their proper contexts.
Increased synthesis of preC RNA by NRREpreC− mutants in the context of HBV subgenomic fragments was also observed in cell-free transcription assays with nuclear extracts prepared from HepG2 and HeLa cells (61). However, COUP-TF1 is much more abundant than HNF4α and PPARα in HepG2 and HeLa nuclear extracts (unpublished data) and likely outcompetes these other NRs for binding, functioning as a repressor of RNA synthesis.
The HBV enhancer I is a composite regulatory region that consists of several motifs to which liver-enriched and ubiquitous protein factors are known to bind (3, 12, 41, 43, 47). It has been shown to upregulate all viral promoters in the HBV genome (1, 25). However, most of our knowledge concerning transcriptional regulation by enhancer I has been obtained using reporter plasmids. Garcia et al. (18) reported that NRREenhI and an adjacent element, EF-C, function interdependently to confer enhancer and liver-specific activity on enhancer I. We showed here that mutations in NRREenhI reduce synthesis of preC, pregenomic, and preS RNAs twofold and reduce synthesis of S RNA fourfold (Fig. 3). These differential effects of NRREenhI on the various viral promoters may be due to the different natures or extents of its interactions with the viral promoters, presumably via protein-protein contacts. These proteins may include NRs, their coactivators and corepressors, other regulatory factors, and components of the basal transcription machinery.
We reported previously that a number of NRs, including HNF4α, COUP-TF1, and PPARα-RXRα, have higher affinities for NRREpreC than for NRREenhI (61). Yet our analysis of NRRE mutants here suggests that NRREenhI plays a global role in regulating viral transcription and replication, while NRREpreC only moderately activates synthesis of pregenomic RNA and viral DNA (Fig. 3 and 4). One possible explanation to reconcile these findings is that the function of NRREenhI is activated synergistically in vivo by other elements such as EF-C that are also present in enhancer I.
In the NRREenhI−,preC− double mutant, synthesis of preC, pregenomic, and preS RNAs was down 7- to 8-fold and synthesis of S RNA was down 15-fold (Fig. 3), at least a multiplicative effect relative to the two individually mutated NRREs. One hypothesis consistent with this finding is that the presence of NRREpreC may compensate in part for loss of NRREenhI to enable NRRE-promoter interactions essential for efficient viral RNA synthesis. Thus, viral gene expression occurs at a basal level only when both NRREs are mutated. The very low level of viral RNA synthesis observed with the double mutant also suggests that the function of NRREenhI cannot be compensated for by other elements in enhancer I.
We did not include here HBV plasmids in which NRREenhII was also mutated. Nevertheless, the very fact that the NRREpreC NRREenhI double mutant exhibited very low levels of RNA synthesis indicates that NRREenhII alone is not sufficient to sustain synthesis of viral RNA and DNA at wild-type levels. It has been reported that enhancer II plays a key role as a liver-specific activator of the preC and pregenomic promoters (57, 59, 62). We found that a variant of a previously reported subgenomic plasmid in which the entire enhancer II region (i.e., nt 1403 to 1733) is deleted synthesized 1/10 as much preC and pregenomic RNA as the wild type (X. Yu, unpublished data). On the other hand, an NRREenhII point mutant defective in binding HNF4α synthesized approximately one-half of the wild-type level of preC and pregenomic RNAs in Huh7 cells within the context of the same subgenomic plasmid (61; X. Yu, unpublished data). Therefore, while enhancer II as a whole is important for synthesis of the preC and pregenomic RNAs from that HBV subgenomic plasmid, NRREenhII probably does not contribute significantly to the function of enhancer II. A detailed analysis of the regulation of synthesis of HBV RNA and DNA by HNF4α will be reported elsewhere (X. Yu and J. E. Mertz, unpublished data).
In the studies presented here, we were careful to ensure that the mutations in the NRREs did not affect the sequences of the viral proteins encoded by these regions of the HBV genome (Fig. 2A). Nevertheless, the theoretical possibility exists that they may have affected posttranscriptional events such as the formation of secondary structures important for genome replication and/or stability of viral RNAs.
PPAR-RXR activation of synthesis of pregenomic RNA and viral DNA.
We showed here that PPAR-RXR and their ligands can play important roles in the replication of HBV, probably via direct binding to NRREpreC. For example, synthesis of pregenomic RNA increased four- to fivefold when PPARα-RXRα or PPARγ-RXRα was overexpressed by cotransfection with expression plasmids (Fig. 5A and 6A). As expected, synthesis of viral DNA increased concomitantly (Fig. 5B and 6B). On the other hand, overexpression of PPAR-RXR had minimal effects on synthesis of S, preC, and preS RNAs (Fig. 5A and 6A; also data not shown). The S protein is already in vast excess in HBV-infected hepatocytes, and the preS1 protein is only a minor component of the viral envelope and toxic to hepatocytes when overexpressed. This differential regulation of transcription from viral genes may avoid wasteful production of these two proteins and help to direct the cellular machinery to the synthesis of more C and P proteins, thus allowing for efficient virion production in hepatocytes.
After we completed our studies reported here, Tang and McLachlan published their finding that expression of PPARα-RXRα and HNF4 enables synthesis of the 3.5-kb HBV RNA and HBV DNA replication in otherwise nonpermissive mouse NIH 3T3 cells (51). These complementary experiments indicate clearly that these NRs are major players in controlling the transcription and replication of HBV. However, whereas they failed to detect any 3.5-kb HBV RNA in their nonliver cells in the absence of exogenously expressed NRs, we still observed some 3.5-kb HBV RNA with the double mutant in Huh7 cells, albeit at low levels (Fig. 3A). Likely, additional liver-enriched transcription factors and cis-acting regulatory elements within the HBV genome are responsible for the differences observed between Huh7 and NIH 3T3 cells.
The specific activation of the pregenomic promoter by PPAR-RXR was dependent on the presence of a functional NRREpreC, but not NRREenhI (Fig. 5A and 6A). PPAR-RXR increased synthesis of pregenomic RNA to the same extent from wild-type and NRREenhI− HBV plasmids (Fig. 5A and 6A). One model consistent with this observation is that NRREpreC may become a stronger activator of the pregenomic promoter in the presence of overexpressed PPAR-RXR and their ligands due to its higher affinity for PPAR-RXR and proximity to the promoter. On the other hand, synthesis of preC and pregenomic RNAs was actually slightly repressed by PPAR-RXR in the NRREpreC− mutant in the absence of ligand (Fig. 5A and 6A). Likewise, Tang and McLachlan reported that their NRREpreC mutant also preferentially reduced the level of pregenomic RNA and greatly reduced viral replication when PPARα-RXRα was overexpressed in NIH 3T3 cells (51). Remaining unclear is the reason for this reduced pregenomic RNA synthesis when PPAR-RXR is overexpressed in NRREpreC mutants.
Garcia et al. (18) have reported that overexpression of RXRα can activate a heterologous promoter containing multiple copies of NRREenhI 8- to 15-fold. On the other hand, we failed to observe activation of the HBV promoters within the context of a whole wild-type HBV genome when we overexpressed only RXRα in the presence of its ligand (data not shown). We also failed to observe significant activation or repression of viral promoters in Huh7 cells when we added only ligands for PPARs and RXRα to the culture medium without concomitant overexpression of NRs (data not shown). These findings are consistent with our failure to detect the PPARs in nuclear extracts prepared from Huh7 cells and suggest that the natural concentrations of PPARα and PPARγ in Huh7 cells are probably too low for efficient activation of replication of HBV.
Guidotti et al. (20) reported that treatment of HBV transgenic mice with the peroxisome proliferators Wy-14,643 and clofibric acid results in a <2-fold increase in HBV 3.5-kb RNA levels and in 2- to 3-fold and 7- to 14-fold increases in viral DNA accumulation in male and female mice, respectively. The much larger increase in viral DNA replication than in viral transcription in female mice treated with ligands was hypothesized to be due to the basal level of pregenomic RNA being low, and, therefore, the level of synthesis of the C protein in their livers also being low, with the moderate increase in pregenomic RNA after treatment with ligands raising the concentration of the C protein in hepatocytes above the threshold needed for efficient formation of nucleocapsids and viral DNA synthesis.
The HBV transgenic mouse model is an excellent system in which to study the HBV life cycle in the liver and the host immune response and pathogenesis caused by HBV infection. On the other hand, transfection of cultured cells is also a valuable tool in that it allows for ready analysis of phenotypic changes resulting from mutations in cis-acting regulatory elements and viral genes. As shown here, the use of hepatoma cell lines allows for assessment of the contributions of individual NRREs to viral gene expression in the context of functional HBV enhancers and other cis-acting regulatory elements. It also enables screening for NRs and ligands which may modulate viral gene expression and virion production.
The NRREpreC− phenotype of the CH variant of HBV.
The CH variant of HBV contains mutations in one of the two half-sites of the NRREpreC (Fig. 1B). When we introduced these two point mutations into the HBV genome, we found that synthesis of pregenomic RNA and viral DNA increased approximately twofold while preC RNA accumulation remained similar to that in the wild type (Fig. 8). Others have reported either reduced synthesis of preC RNA and secretion of HBV e antigen but no effect on synthesis of pregenomic RNA (9, 21, 45), activation of pregenomic RNA synthesis (38), no effect on viral DNA synthesis (21), or increased viral DNA synthesis (9, 38, 45). These discrepancies may be attributed to the use of different subtypes of HBV (ayw, adw2, and adr), plasmid constructs (monomer, dimer, and 1.2-mer), and hepatoma cell lines (HepG2 and Huh7).
We also studied the responses of the CH variant genome to overexpression of PPARα-RXRα in the presence of their ligands. The CH variant was found to exhibit a phenotype similar to, but less severe than, that of our artificial NRREpreC mutant, with pregenomic RNA synthesis being reduced approximately twofold and viral DNA synthesis being largely unaffected (Fig. 8). The less severe phenotype may be attributed to the following facts: (i) the CH variant still retains binding by HNF4α in its NRREpreC (Fig. 7), while our NRREpreC mutations abolish HNF4α binding as well (Fig. 2B); (ii) the mutations in the CH variant create a new HNF1 binding site in NRREpreC (34); and (iii) the mutations in the CH variant also led to changes in two of the amino acids in the X protein (34).
We have shown here dependence on a functional NRREpreC in PPARα-RXRα-mediated activation of viral RNA and DNA synthesis. Thus, the low level of responsiveness of the CH variant to PPARα-RXRα is likely due, at least in part, to inactivation of NRREpreC. Presumably, this nonresponsiveness to PPARs confers some advantage on the CH variant for maintaining its presence in hepatocytes. For example, many physiological and environmental changes and exposure to peroxisome proliferators and other compounds which can function as ligands for PPARs may upregulate the synthesis and activity of PPARs in hepatocytes. In wild-type HBV infection, this will result in elevated synthesis of viral RNA, protein, and DNA. The increased synthesis of viral C protein and its display on the cell surface may render the hepatocytes more vulnerable to the host immune system. With mutated NRREpreC, the CH variant can avoid this upregulation of its replication and thus retain the chronicity of its infection.
In summary, we conclude that NRREpreC and NRREenhI are important positive regulatory elements for HBV gene expression and replication. These NRREs differentially regulate transcription from various viral promoters to facilitate efficient nucleocapsid formation and virion production. PPAR-RXR and their ligands increase synthesis of both the pregenomic RNA and viral DNA. Analysis of artificial NRRE mutants and a naturally occurring NRRE mutant suggests that this activation is largely dependent on the function of NRREpreC.
ACKNOWLEDGMENTS
We thank Shannon Reagan for technical assistance. We also thank Dan Loeb, Jeff Habig, Paul Lambert, Richard Kraus, and Michael Farrell for helpful discussions and comments on the manuscript.
This work was supported by Public Health Service research grants CA22443 and CA07175 from the National Cancer Institute.
REFERENCES
- 1.Antonucci T K, Rutter W J. Hepatitis B virus (HBV) promoters are regulated by the HBV enhancer in a tissue-specific manner. J Virol. 1989;63:579–583. doi: 10.1128/jvi.63.2.579-583.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumert T F, Marrone A, Vergalla J, Liang T J. Naturally occurring mutations define a novel function of the hepatitis B virus core promoter in core protein expression. J Virol. 1998;72:6785–6795. doi: 10.1128/jvi.72.8.6785-6795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Levy R, Faktor O, Berger I, Shaul Y. Cellular factors that interact with the hepatitis B virus enhancer. Mol Cell Biol. 1989;9:1804–1809. doi: 10.1128/mcb.9.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock C-T, Malek N P, Tillmann H L, Manns M P, Trautwein C. The enhancer I core region contributes to the replication level of hepatitis B virus in vivo and in vitro. J Virol. 2000;74:2193–2202. doi: 10.1128/jvi.74.5.2193-2202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown T. Southern blotting. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1993. p. 2.9.1. [Google Scholar]
- 6.Brown T, Mackey K. Analysis of RNA by Northern and slot blot hybridization. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1997. p. 4.9.1. [Google Scholar]
- 7.Brun R P, Tontonoz P, Forman B M, Ellis R, Chen J, Evans R M, Spiegelman B M. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996;10:974–984. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- 8.Buckwold V, Ou J. Hepatitis B virus C-gene expression and function: the lessons learned from viral mutants. Curr Top Virol. 1999;1:71–81. [Google Scholar]
- 9.Buckwold V E, Xu Z, Chen M, Yen T S B, Ou J-H. Effects of a naturally occurring mutation in the hepatitis B virus basal core promoter on precore gene expression and viral replication. J Virol. 1996;70:5845–5851. doi: 10.1128/jvi.70.9.5845-5851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckwold V E, Xu Z, Yen T S B, Ou J-H. Effects of a frequent double-nucleotide basal core promoter mutation and its putative single-nucleotide precursor mutations on hepatitis B virus gene expression and replication. J Gen Virol. 1997;78:2055–2065. doi: 10.1099/0022-1317-78-8-2055. [DOI] [PubMed] [Google Scholar]
- 11.Butz K, Hoppe-Seyler F. Transcriptional control of human papillomavirus (HPV) oncogene expression: composition of the HPV type 18 upstream regulatory region. J Virol. 1993;67:6476–6486. doi: 10.1128/jvi.67.11.6476-6486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dikstein R, Faktor O, Ben-Levy R, Shaul Y. Functional organization of the hepatitis B virus enhancer. Mol Cell Biol. 1990;10:3682–3689. doi: 10.1128/mcb.10.7.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fajas L, Schoonjans K, Gelman L, Kim J B, Najib J, Martin G, Fruchart J C, Briggs M, Spiegelman B M, Auwerx J. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol. 1999;19:5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. 15-Deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR γ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 15.Fraser J D, Martinez V, Straney R, Briggs M R. DNA binding and transcription activation specificity of hepatocyte nuclear factor 4. Nucleic Acids Res. 1998;26:2702–2707. doi: 10.1093/nar/26.11.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganem D. Hepadnaviridae and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2703–2739. [Google Scholar]
- 17.Ganem D, Varmus H E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 18.Garcia A D, Ostapchuk P, Hearing P. Functional interaction of nuclear factors EF-C, HNF-4, and RXRα with hepatitis B virus enhancer I. J Virol. 1993;67:3940–3950. doi: 10.1128/jvi.67.7.3940-3950.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloss B, Bernard H U, Seedorf K, Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987;6:3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidotti L G, Eggers C M, Raney A K, Chi S Y, Peters J M, Gonzalez F J, McLachlan A. In vivo regulation of hepatitis B virus replication by peroxisome proliferators. J Virol. 1999;73:10377–10386. doi: 10.1128/jvi.73.12.10377-10386.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunther S, Piwon N, Will H. Wild-type levels of pregenomic RNA and replication but reduced pre-C RNA and e-antigen synthesis of hepatitis B virus with C(1653)→T, A(1762)→T and G(1764)→A mutations in the core promoter. J Gen Virol. 1998;79:375–380. doi: 10.1099/0022-1317-79-2-375. [DOI] [PubMed] [Google Scholar]
- 22.Guo W, Chen M, Yen T S B, Ou J-H. Hepatocyte-specific expression of the hepatitis B virus core promoter depends on both positive and negative regulation. Mol Cell Biol. 1993;13:443–448. doi: 10.1128/mcb.13.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 24.Heyman R A, Mangelsdorf D J, Dyck J A, Stein R B, Eichele G, Evans R M, Thaller C. 9-cis-Retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 25.Hu K Q, Siddiqui A. Regulation of the hepatitis B virus gene expression by the enhancer element I. Virology. 1991;181:721–726. doi: 10.1016/0042-6822(91)90906-r. [DOI] [PubMed] [Google Scholar]
- 26.Huan B, Kosovsky M J, Siddiqui A. Retinoid X receptor α transactivates the hepatitis B virus enhancer 1 element by forming a heterodimeric complex with the peroxisome proliferator-activated receptor. J Virol. 1995;69:547–551. doi: 10.1128/jvi.69.1.547-551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huan B, Siddiqui A. Retinoid X receptor RXRα binds to and trans-activates the hepatitis B virus enhancer. Proc Natl Acad Sci USA. 1992;89:9059–9063. doi: 10.1073/pnas.89.19.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 29.Jiang G, Nepomuceno L, Hopkins K, Sladek F M. Exclusive homodimerization of the orphan receptor hepatocyte nuclear factor 4 defines a new subclass of nuclear receptors. Mol Cell Biol. 1995;15:5131–5143. doi: 10.1128/mcb.15.9.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 31.Kliewer S A, Lenhard J M, Willson T M, Patel I, Morris D C, Lehmann J M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 32.Lee M-O, Hobbs P D, Zhang X-K, Dawson M I, Pfahl M. A synthetic retinoid antagonist inhibits the human immunodeficiency virus type 1 promoter. Proc Natl Acad Sci USA. 1994;91:5632–5636. doi: 10.1073/pnas.91.12.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin A A, Sturzenbecker L J, Kazmer S, Bosakowski T, Huselton C, Allenby G, Speck J, Kratzeisen C, Rosenberger M, Lovey A, Grippo J F. 9-cis-Retinoic acid stereoisomer binds and activates the nuclear receptor RXRα. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Buckwold V E, Hon M-W, Ou J-H. Mechanism of suppression of hepatitis B virus precore RNA transcription by a frequent double mutation. J Virol. 1999;73:1239–1244. doi: 10.1128/jvi.73.2.1239-1244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Ning G, Duncan S A. Mammalian hepatocyte differentiation requires the transcription factor HNF-4α. Genes Dev. 2000;14:464–474. [PMC free article] [PubMed] [Google Scholar]
- 36.Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 37.Moens U, Subramaniam N, Johansen B, Johansen T, Traavik T. A steroid hormone response unit in the late leader of the noncoding control region of the human polyomavirus BK confers enhanced host cell permissivity. J Virol. 1994;68:2398–2408. doi: 10.1128/jvi.68.4.2398-2408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriyama K, Okamoto H, Tsuda F, Mayumi M. Reduced precore transcription and enhanced core-pregenome transcription of hepatitis B virus DNA after replacement of the precore-core promoter with sequences associated with e antigen-seronegative persistent infections. Virology. 1996;226:269–280. doi: 10.1006/viro.1996.0655. [DOI] [PubMed] [Google Scholar]
- 39.Nakshatri H, Bhat-Nakshatri P. Multiple parameters determine the specificity of transcriptional response by nuclear receptors HNF-4, ARP-1, PPAR, RAR and RXR through common response elements. Nucleic Acids Res. 1998;26:2491–2499. doi: 10.1093/nar/26.10.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto H, Tsuda F, Akahane Y, Sugai Y, Yoshiba M, Moriyama K, Tanaka T, Miyakawa Y, Mayumi M. Hepatitis B virus with mutations in the core promoter for an e antigen- negative phenotype in carriers with antibody to e antigen. J Virol. 1994;68:8102–8110. doi: 10.1128/jvi.68.12.8102-8110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel N U, Jameel S, Isom H, Siddiqui A. Interactions between nuclear factors and the hepatitis B virus enhancer. J Virol. 1989;63:5293–5301. doi: 10.1128/jvi.63.12.5293-5301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raney A K, Johnson J L, Palmer C N A, McLachlan A. Members of the nuclear receptor superfamily regulate transcription from the hepatitis B virus nucleocapsid promoter. J Virol. 1997;71:1058–1071. doi: 10.1128/jvi.71.2.1058-1071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reith W, Ucla C, Barras E, Gaud A, Durand B, Herrero-Sanchez C, Kobr M, Mach B. RFX1, a transactivator of hepatitis B virus enhancer I, belongs to a novel family of homodimeric and heterodimeric DNA-binding proteins. Mol Cell Biol. 1994;14:1230–1244. doi: 10.1128/mcb.14.2.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Russell D W. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 2001. pp. 16.14–16.20. [Google Scholar]
- 45.Scaglioni P P, Melegari M, Wands J R. Biologic properties of hepatitis B viral genomes with mutations in the precore promoter and precore open reading frame. Virology. 1997;233:374–381. doi: 10.1006/viro.1997.8594. [DOI] [PubMed] [Google Scholar]
- 46.Schleiss M R, Degnin C R, Geballe A P. Translational control of human cytomegalovirus gp48 expression. J Virol. 1991;65:6782–6789. doi: 10.1128/jvi.65.12.6782-6789.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaul Y, Rutter W J, Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985;4:427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spath G F, Weiss M C. Hepatocyte nuclear factor 4 expression overcomes repression of the hepatic phenotype in dedifferentiated hepatoma cells. Mol Cell Biol. 1997;17:1913–1922. doi: 10.1128/mcb.17.4.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Staprans S, Loeb D D, Ganem D. Mutations affecting hepadnavirus plus-strand DNA synthesis dissociate primer cleavage from translocation and reveal the origin of linear viral DNA. J Virol. 1991;65:1255–1262. doi: 10.1128/jvi.65.3.1255-1262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Summers J, Smith P M, Huang M, Yu M. Morphogenetic and regulatory effects of mutations in the envelope proteins of an avian hepadnavirus. J Virol. 1991;65:1310–1317. doi: 10.1128/jvi.65.3.1310-1317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang H, McLachlan A. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc Natl Acad Sci USA. 2001;98:1841–1846. doi: 10.1073/pnas.041479698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thummel C S. From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell. 1995;83:871–877. doi: 10.1016/0092-8674(95)90203-1. [DOI] [PubMed] [Google Scholar]
- 53.Tontonoz P, Hu E, Graves R A, Budavari A I, Spiegelman B M. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 1994;8:1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 54.Tsai M-J, O'Malley B W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 55.Tur-Kaspa R, Burk R D, Shaul Y, Shafritz D A. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci USA. 1986;83:1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tur-Kaspa R, Shaul Y, Moore D D, Burk R D, Okret S, Poellinger L, Shafritz D A. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988;167:630–633. [PubMed] [Google Scholar]
- 57.Wang Y, Chen P, Wu X, Sun A L, Wang H, Zhu Y A, Li Z P. A new enhancer element, ENII, identified in the X gene of hepatitis B virus. J Virol. 1990;64:3977–3981. doi: 10.1128/jvi.64.8.3977-3981.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley S R, Kraus R J, Zuo F, Murray E E, Loritz K, Mertz J E. SV40 early-to-late switch involves titration of cellular transcriptional repressors. Genes Dev. 1993;7:2206–2219. doi: 10.1101/gad.7.11.2206. [DOI] [PubMed] [Google Scholar]
- 59.Yee J K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989;246:658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]
- 60.Yu X, Mertz J E. Promoters for synthesis of the pre-C and pregenomic mRNAs of human hepatitis B virus are genetically distinct and differentially regulated. J Virol. 1996;70:8719–8726. doi: 10.1128/jvi.70.12.8719-8726.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu X, Mertz J E. Differential regulation of the pre-C and pregenomic promoters of human hepatitis B virus by members of the nuclear receptor superfamily. J Virol. 1997;71:9366–9374. doi: 10.1128/jvi.71.12.9366-9374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuh C H, Ting L P. The genome of hepatitis B virus contains a second enhancer: cooperation of two elements within this enhancer is required for its function. J Virol. 1990;64:4281–4287. doi: 10.1128/jvi.64.9.4281-4287.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo F, Kraus R J, Gulick T, Moore D D, Mertz J E. Direct modulation of simian virus 40 late gene expression by thyroid hormone and its receptor. J Virol. 1997;71:427–436. doi: 10.1128/jvi.71.1.427-436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuo F, Mertz J E. Simian virus 40 late gene expression is regulated by members of the steroid/thyroid hormone receptor superfamily. Proc Natl Acad Sci USA. 1995;92:8586–8590. doi: 10.1073/pnas.92.19.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]