Abstract
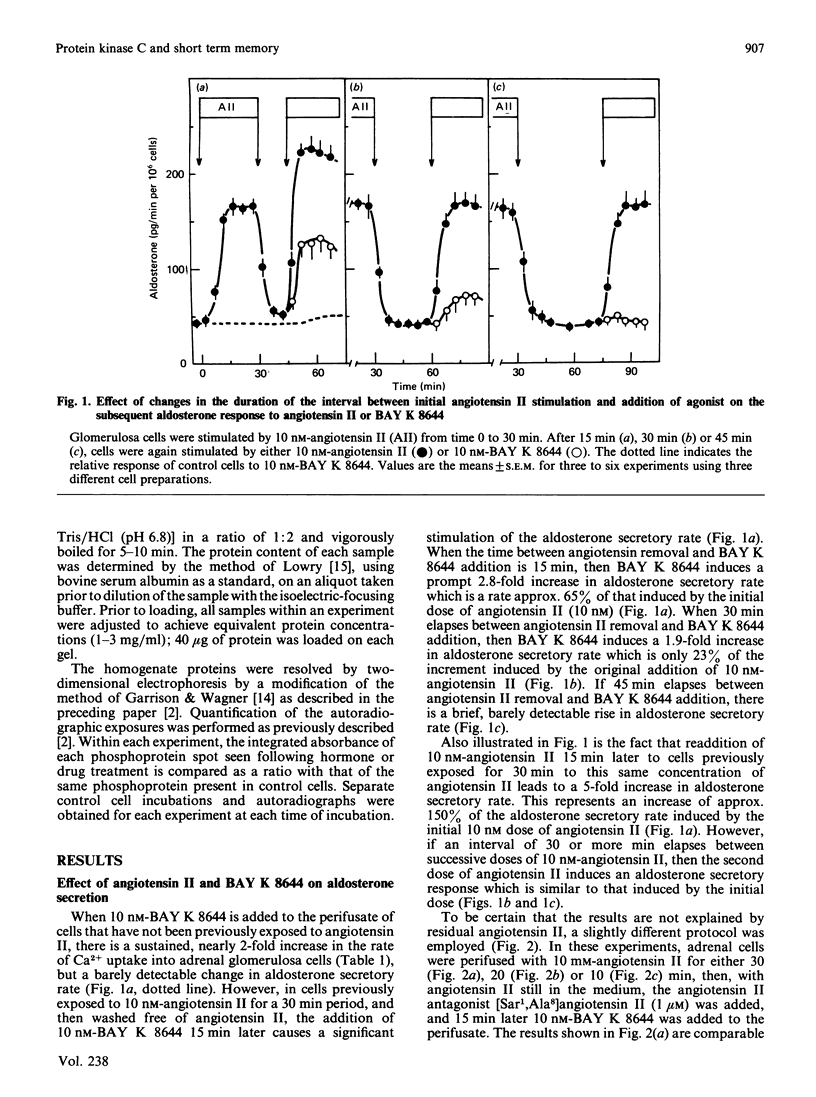
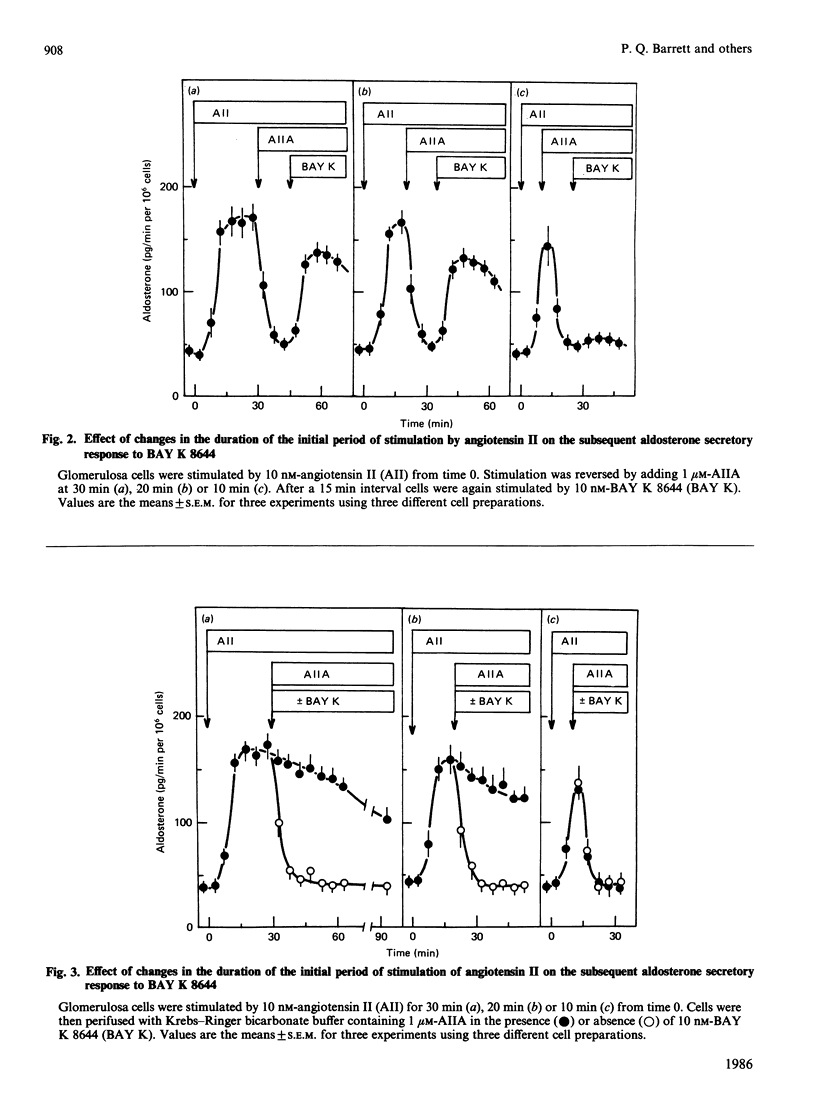
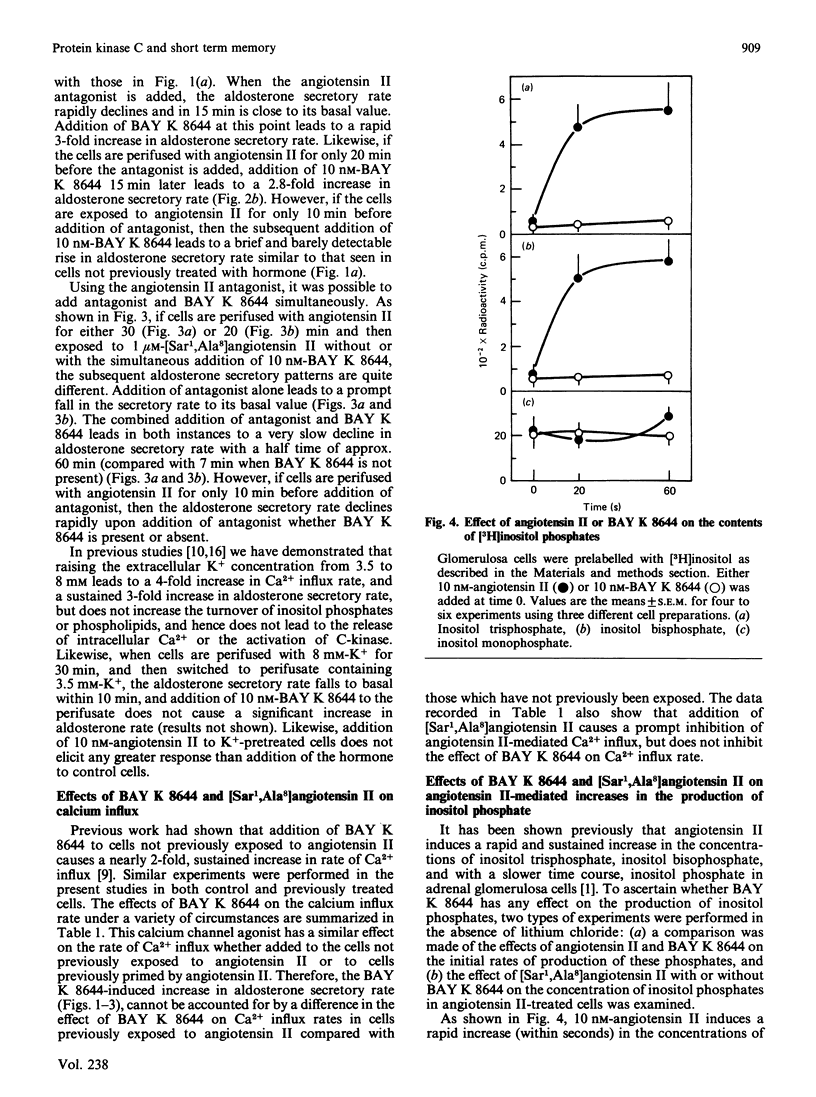
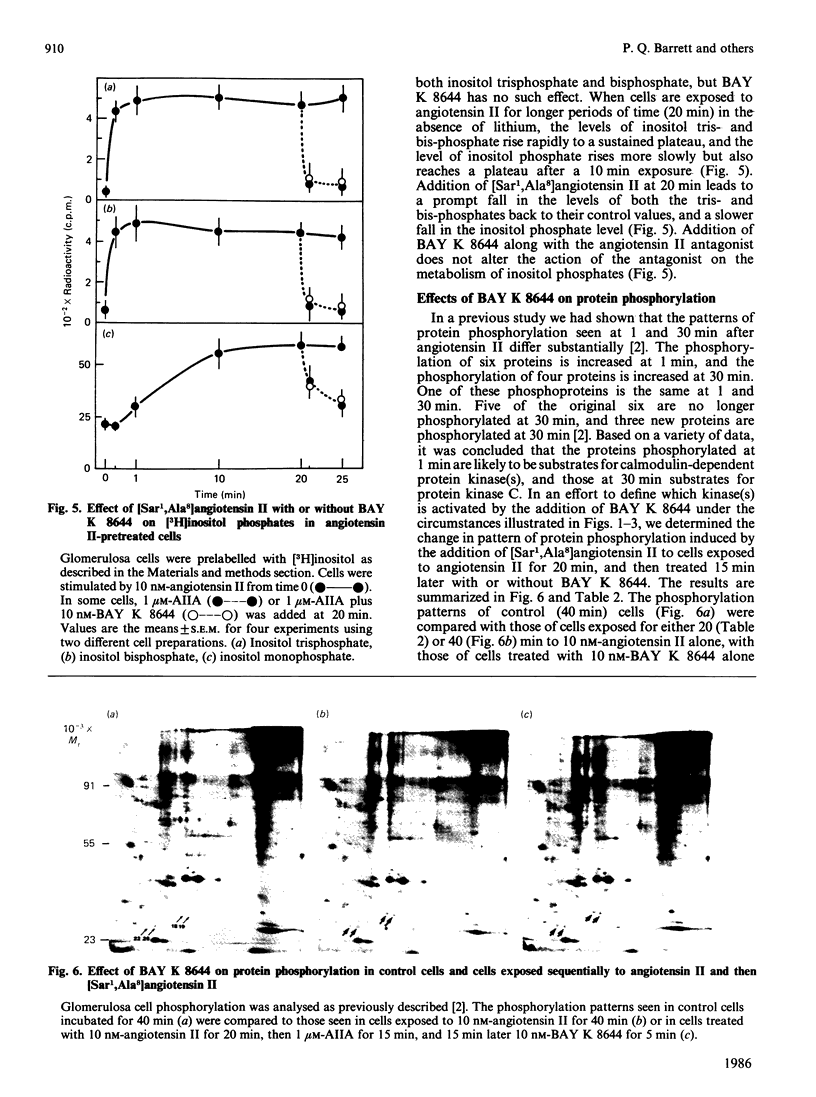
If adrenal glomerulosa cells are treated with angiotensin II for a period of 20-30 min, their subsequent response to either a rechallenge with the same concentration of angiotensin II or treatment with BAY K 8644, a calcium channel agonist, differs from the responses of control cells. Perifusion of control cells with 10 nM-angiotensin II leads to an increase in aldosterone secretory rate from 44 +/- 7 to 166 +/- 9 pg/min per 10(6) cells, but perifusion of cells pretreated for a 20 min period with angiotensin II leads to an increase in secretory rate from 51 +/- 9 to 209 +/- 18 pg/min per 10(6) cells. Likewise, treatment of control cells with 10 nM-BAY K 8644 leads to no significant increase in aldosterone secretory rate, but treatment of previously exposed cells to angiotensin II leads to an increase in rate from 51 +/- 9 to 130 +/- 11 pg/min per 10(6) cells. This memory effect is time-dependent in two ways: cells must be exposed to angiotensin II for 20 min or more before it is apparent; the longer the time between removal of angiotensin II and the rechallenge, the less effect these agents have on aldosterone secretory rate. When cells are exposed to angiotensin II for 20 min and then treated with [Sar1,Ala8]angiotensin II, a competitive antagonist of angiotensin II action, the aldosterone secretory rate falls to basal with a half time of 5-7 min. If BAY K 8644 is added simultaneously with [Sar1,Ala8]angiotensin II, the secretory rate falls with a halftime of 35-60 min. BAY K 8644 increases Ca2+ influx rate to the same extent in the presence or absence of [Sar1,Ala8]angiotensin II, and does not alter the effect of either angiotensin II or [Sar1,Ala8]angiotensin II on the production of inositol tris-, bis-, or mono-phosphate. In cells treated with 10 nM-angiotensin II for either 20, 30 or 45 min, the extent of phosphorylation of four cellular proteins is increased. If cells treated for 20 min with angiotensin II are then treated with [Sar1,Ala8]angiotensin II, and examined 15 min later (35 min), there is no longer an increase in the extent of phosphorylation of any of the four proteins. If such cells are then treated with 10 nM-BAY K 8644 and re-examined 5 min later (40 min), all four patients show an increase in the extent of phosphorylation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett P. Q., Kojima I., Kojima K., Zawalich K., Isales C. M., Rasmussen H. Temporal patterns of protein phosphorylation after angiotensin II, A23187 and/or 12-O-tetradecanoylphorbol 13-acetate in adrenal glomerulosa cells. Biochem J. 1986 Sep 15;238(3):893–903. doi: 10.1042/bj2380893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi P., Büki B., Muscsi I., Spät A. Polyphosphoinositide metabolism in adrenal glomerulosa cells. Mol Cell Endocrinol. 1985 Jun;41(1):105–112. doi: 10.1016/0303-7207(85)90147-9. [DOI] [PubMed] [Google Scholar]
- Foster R., Rasmussen H. Angiotensin-mediated calcium efflux from adrenal glomerulosa cells. Am J Physiol. 1983 Sep;245(3):E281–E287. doi: 10.1152/ajpendo.1983.245.3.E281. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Wagner J. D. Glucagon and the Ca2+-linked hormones angiotensin II, norepinephrine, and vasopressin stimulate the phosphorylation of distinct substrates in intact hepatocytes. J Biol Chem. 1982 Nov 10;257(21):13135–13143. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Kreutter D., Rasmussen H. The temporal integration of the aldosterone secretory response to angiotensin occurs via two intracellular pathways. J Biol Chem. 1984 Dec 10;259(23):14448–14457. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Effects of ANG II and K+ on Ca efflux and aldosterone production in adrenal glomerulosa cells. Am J Physiol. 1985 Jan;248(1 Pt 1):E36–E43. doi: 10.1152/ajpendo.1985.248.1.E36. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Intracellular calcium and adenosine 3',5'-cyclic monophosphate as mediators of potassium-induced aldosterone secretion. Biochem J. 1985 May 15;228(1):69–76. doi: 10.1042/bj2280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Role of calcium fluxes in the sustained phase of angiotensin II-mediated aldosterone secretion from adrenal glomerulosa cells. J Biol Chem. 1985 Aug 5;260(16):9177–9184. [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara B. C., Cranna C. E., Booth R., Stansfield D. A. The preparation and purification of isolated rat corpus-luteum cells and their use in studying the relationship between cholesterol biosynthesis and the lutropin-stimulated formation of progesterone. Biochem J. 1980 Nov 15;192(2):559–567. doi: 10.1042/bj1920559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Rasmussen H. Activation of tracheal smooth muscle contraction: synergism between Ca2+ and activators of protein kinase C. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8835–8839. doi: 10.1073/pnas.82.24.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Williams B. C., McDougall J. G., Tait J. F., Tait S. A. Calcium efflux and steroid output from superfused rat adrenal cells: effects of potassium, adrenocorticotropic hormone, 5-hydroxytryptamine, adenosine 3':5'-cyclic monophosphate and angiotensins II and III. Clin Sci (Lond) 1981 Nov;61(5):541–551. doi: 10.1042/cs0610541. [DOI] [PubMed] [Google Scholar]



