Abstract
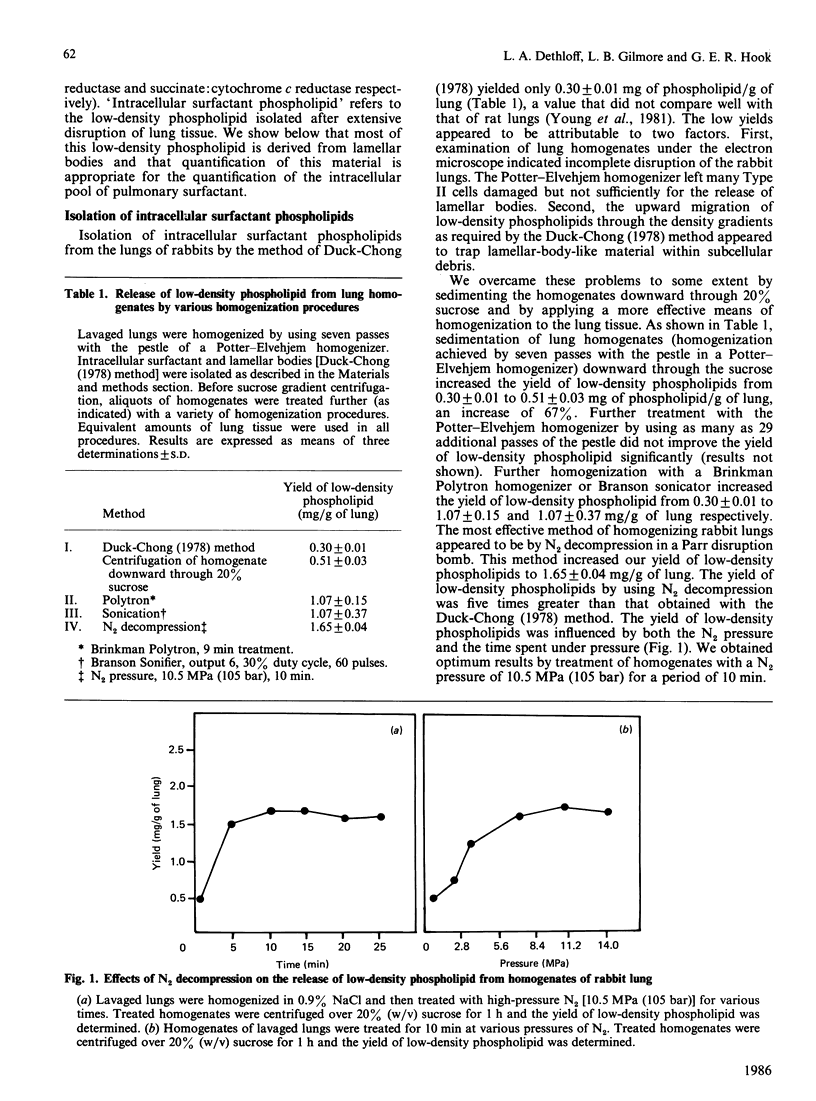
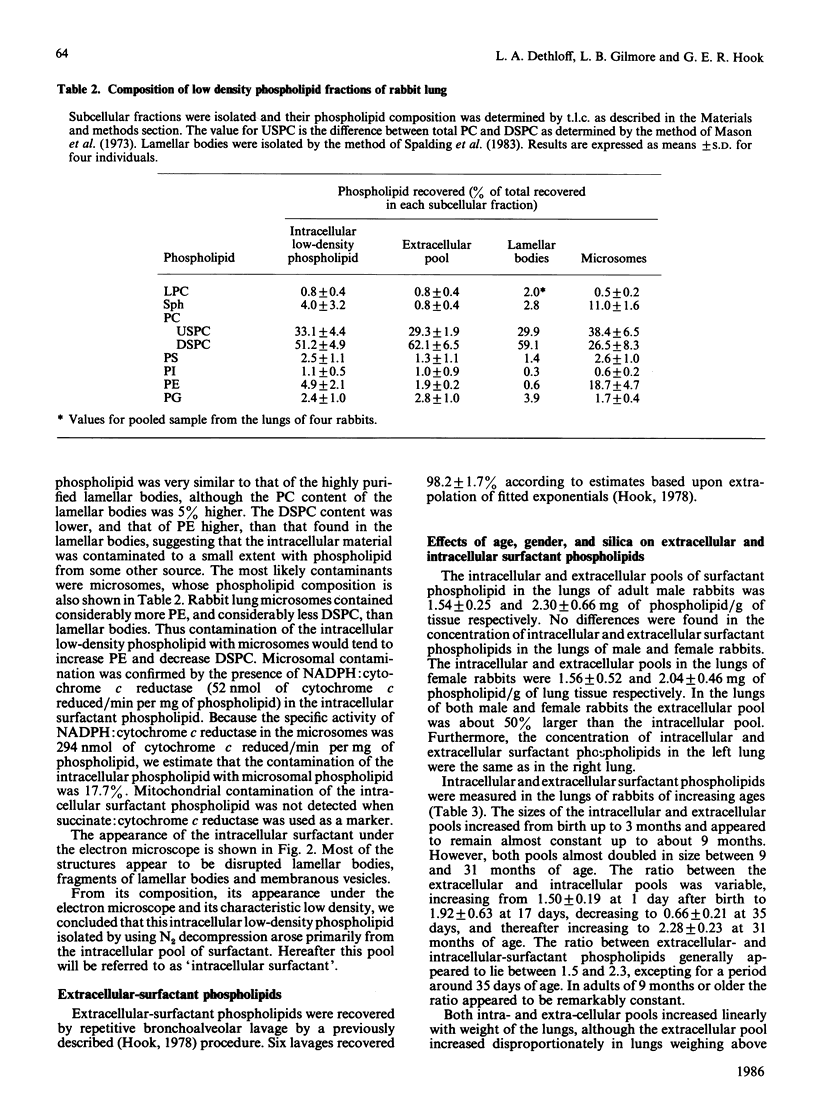
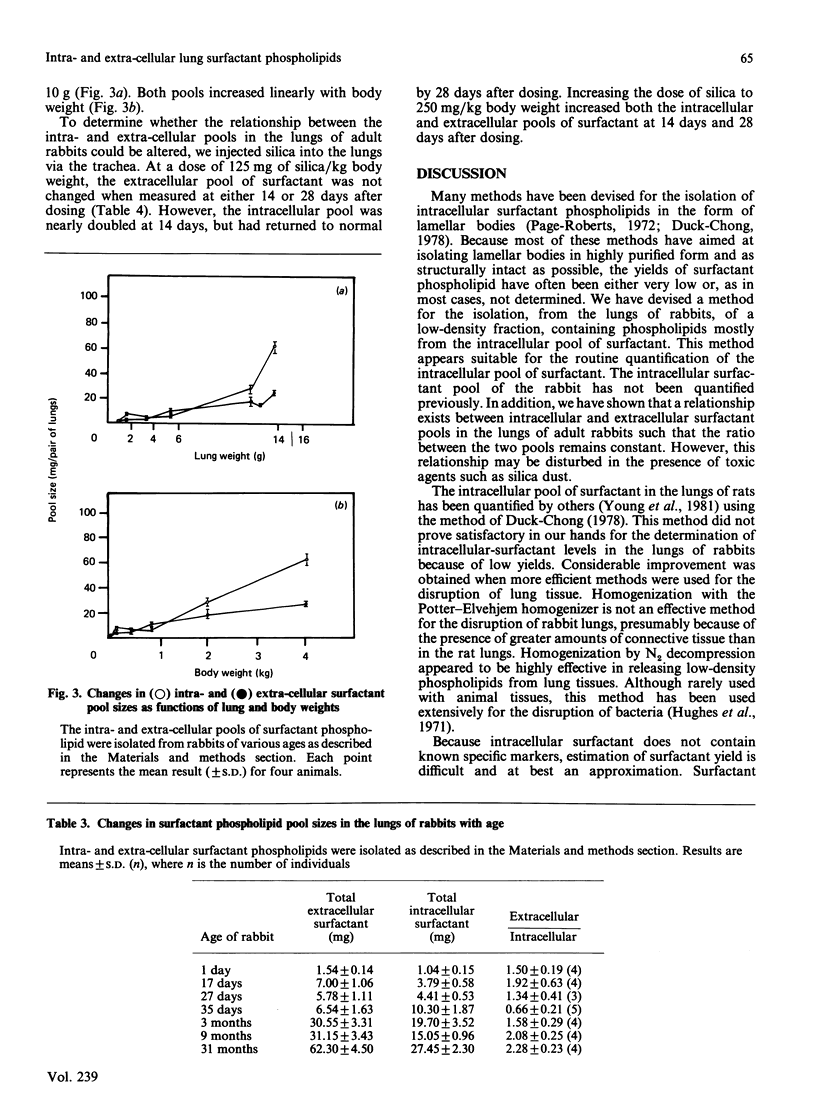
Extensive homogenization of lung tissue by nitrogen decompression in a Parr disruption bomb increased by 5-fold the yields of low-density phospholipid (d = 1.06) achieved by other methods. This intracellular phospholipid preparation was high in phosphatidylcholines (84.3%), particularly disaturated phosphatidylcholine (51.2%). On the basis of its low density, composition, and morphological appearance, we concluded that this phospholipid was derived from the intracellular compartment of pulmonary surfactant. We examined the relationship between intra- and extra-cellular surfactant pools according to age, gender and silica-induced pulmonary injury. In normal animals the intracellular pool of surfactant phospholipids increased from 1.54 +/- 0.14 mg at 1 day after birth to 62.30 +/- 4.50 mg per pair of lungs after 31 months, and over the same time period the extracellular pool increased from 1.04 +/- 0.15 mg to 27.45 +/- 2.30 mg per pair of lungs. The ratio between the extracellular and intracellular pools of surfactant increased from 1.50 +/- 0.19 at 1 day after birth to 2.28 +/- 0.23 after 31 months of age. The ratio between the two pools was not influenced by gender, but was changed by the intratracheal injection of silica into the lungs. Intratracheal injection of silica dust increased the levels of surfactant in both compartments, but not to the same extent, indicating that the ratio between the pools could be changed by toxic materials. These data suggest the existence of a size relationship between the intra- and the extra-cellular pools of surfactant, a relationship which implies a common regulatory mechanism that can be disturbed during pulmonary injury.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams M. E. Isolation and quantitative estimation of pulmonary surface-active lipoprotein. J Appl Physiol. 1966 Mar;21(2):718–720. doi: 10.1152/jappl.1966.21.2.718. [DOI] [PubMed] [Google Scholar]
- Albert R. K., Lakshminarayan S., Hildebrandt J., Kirk W., Butler J. Increased surface tension favors pulmonary edema formation in anesthetized dogs' lungs. J Clin Invest. 1979 May;63(5):1015–1018. doi: 10.1172/JCI109369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTS J. A., BROWN E. S., JOHNSON R. P. Pulmonary surface tension and the mucus lining of the lungs: some theoretical considerations. J Appl Physiol. 1958 Mar;12(2):262–268. doi: 10.1152/jappl.1958.12.2.262. [DOI] [PubMed] [Google Scholar]
- Dethloff L. A., Gilmore L. B., Brody A. R., Hook G. E. Induction of intra- and extra-cellular phospholipids in the lungs of rats exposed to silica. Biochem J. 1986 Jan 1;233(1):111–118. doi: 10.1042/bj2330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duck-Chong C. G. The isolation of lamellar bodies and their membranous content from rat lung, lamb tracheal fluid and human amniotic fluid. Life Sci. 1978 Jun 12;22(22):2025–2030. doi: 10.1016/0024-3205(78)90549-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gabor S., Zugravu E., Kováts A., Böhm B., Andrasoni D. Effects of quartz on lung surfactant. Environ Res. 1978 Jul;16(1-3):443–448. doi: 10.1016/0013-9351(78)90177-9. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Fletcher K., Wyatt I. Changes in the composition of lung lipids and the "turnover" of dipalmitoyl lecithin in experimental alveolar lipo-proteinosis induced by inhaled quartz. Br J Exp Pathol. 1974 Aug;55(4):384–395. [PMC free article] [PubMed] [Google Scholar]
- Hook G. E., Bend J. R., Hoel D., Fouts J. R., Gram T. E. Preparation of lung microsomes and a comparison of the distribution of enzymes between subcellular fractions of rabbit lung and liver. J Pharmacol Exp Ther. 1972 Sep;182(3):474–490. [PubMed] [Google Scholar]
- Hook G. E. Extracellular hydrolases of the lung. Biochemistry. 1978 Feb 7;17(3):520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Jobe A., Ikegami M., Conaway D. The significance of reutilization of surfactant phosphatidylcholine. J Biol Chem. 1983 Apr 10;258(7):4159–4165. [PubMed] [Google Scholar]
- Jacobs H., Jobe A., Ikegami M., Jones S. Surfactant phosphatidylcholine source, fluxes, and turnover times in 3-day-old, 10-day-old, and adult rabbits. J Biol Chem. 1982 Feb 25;257(4):1805–1810. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoon M. W., Wright J. R., Baritussio A., Williams M. C., Goerke J., Benson B. J., Hamilton R. L., Clements J. A. Subfractionation of lung surfactant. Implications for metabolism and surface activity. Biochim Biophys Acta. 1983 Jan 7;750(1):18–31. doi: 10.1016/0005-2760(83)90200-x. [DOI] [PubMed] [Google Scholar]
- Mason R. J. Disaturated lecithin concentration of rabbit tissues. Am Rev Respir Dis. 1973 Apr;107(4):678–679. doi: 10.1164/arrd.1973.107.4.678. [DOI] [PubMed] [Google Scholar]
- Page-Roberts B. A. Preparation and partial characterization of a lamellar body fraction from rat lung. Biochim Biophys Acta. 1972 Feb 21;260(2):334–338. doi: 10.1016/0005-2760(72)90046-x. [DOI] [PubMed] [Google Scholar]
- Pawlowski R., Frosolono M. F., Charms B. L., Przybylski R. Intra- and extracellular compartmentalization of the surface-active fraction in dog lung. J Lipid Res. 1971 Sep;12(5):538–544. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S. F., Hashim S. A., Cernansky G., Barrett C. R., Jr, Bell A. L., Jr, Liau D. F. Quantification of surfactant phospholipids in the dog lung. J Lipid Res. 1980 Nov;21(8):1004–1014. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Brain J. D. Pathways of clearance in mouse lungs exposed to iron oxide aerosols. Anat Rec. 1975 Mar;181(3):581–625. doi: 10.1002/ar.1091810304. [DOI] [PubMed] [Google Scholar]
- Spalding J. W., Ortner M. J., Tombropoulos E. G., Gilmore L. B., Hook G. E. Isolation and characterization of rabbit lung lamellar bodies. Exp Lung Res. 1983 Apr;4(3):171–190. doi: 10.3109/01902148309046059. [DOI] [PubMed] [Google Scholar]
- Tsao F. H., Zachman R. D. Phosphatidylcholine-lysophosphatidylcholine cycle pathway enzymes in rabbit lung. I. Subcellular localization and properties. Pediatr Res. 1977 Jul;11(7):849–857. doi: 10.1203/00006450-197707000-00015. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS C. H., Jr, KAMIN H. Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J Biol Chem. 1962 Feb;237:587–595. [PubMed] [Google Scholar]
- Williams M. C., Benson B. J. Immunocytochemical localization and identification of the major surfactant protein in adult rat lung. J Histochem Cytochem. 1981 Feb;29(2):291–305. doi: 10.1177/29.2.7019304. [DOI] [PubMed] [Google Scholar]
- Williams M. C. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977 Feb;72(2):260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. L., Kremers S. A., Apple J. S., Crapo J. D., Brumley G. W. Rat lung surfactant kinetics biochemical and morphometric correlation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):248–253. doi: 10.1152/jappl.1981.51.2.248. [DOI] [PubMed] [Google Scholar]



