Abstract
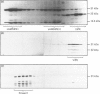
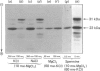
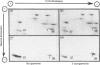
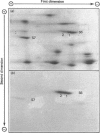
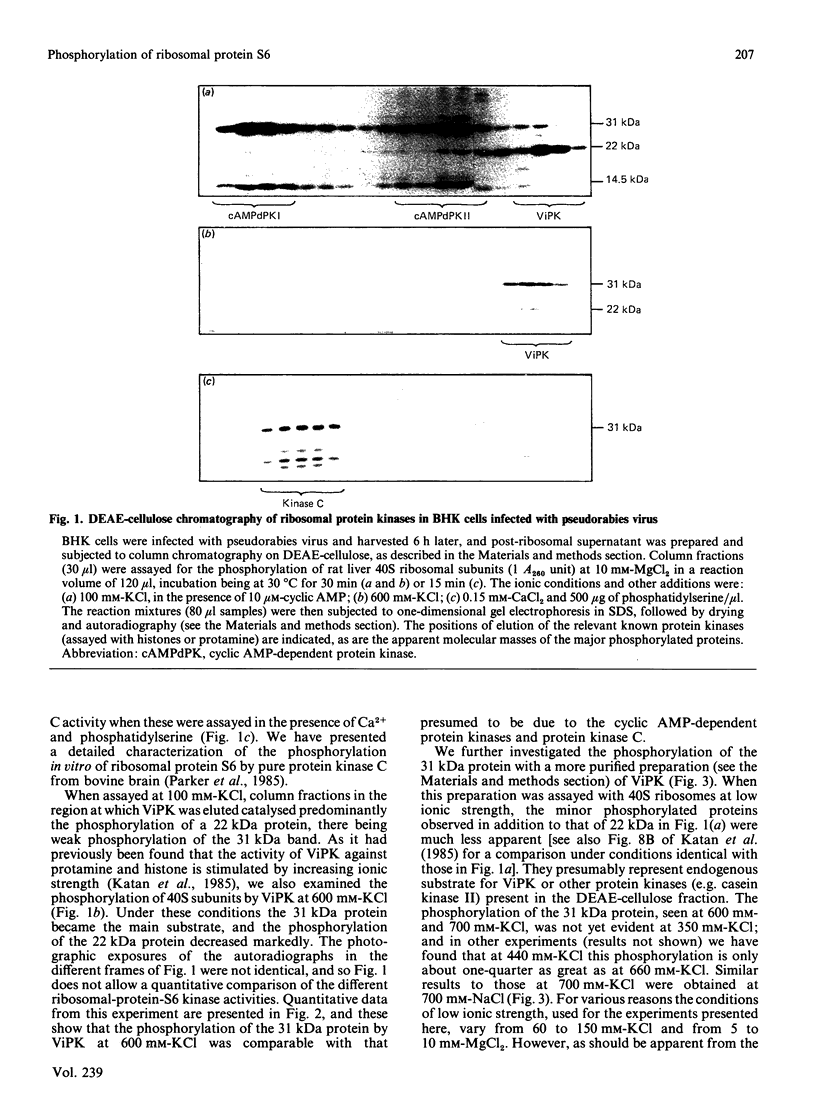
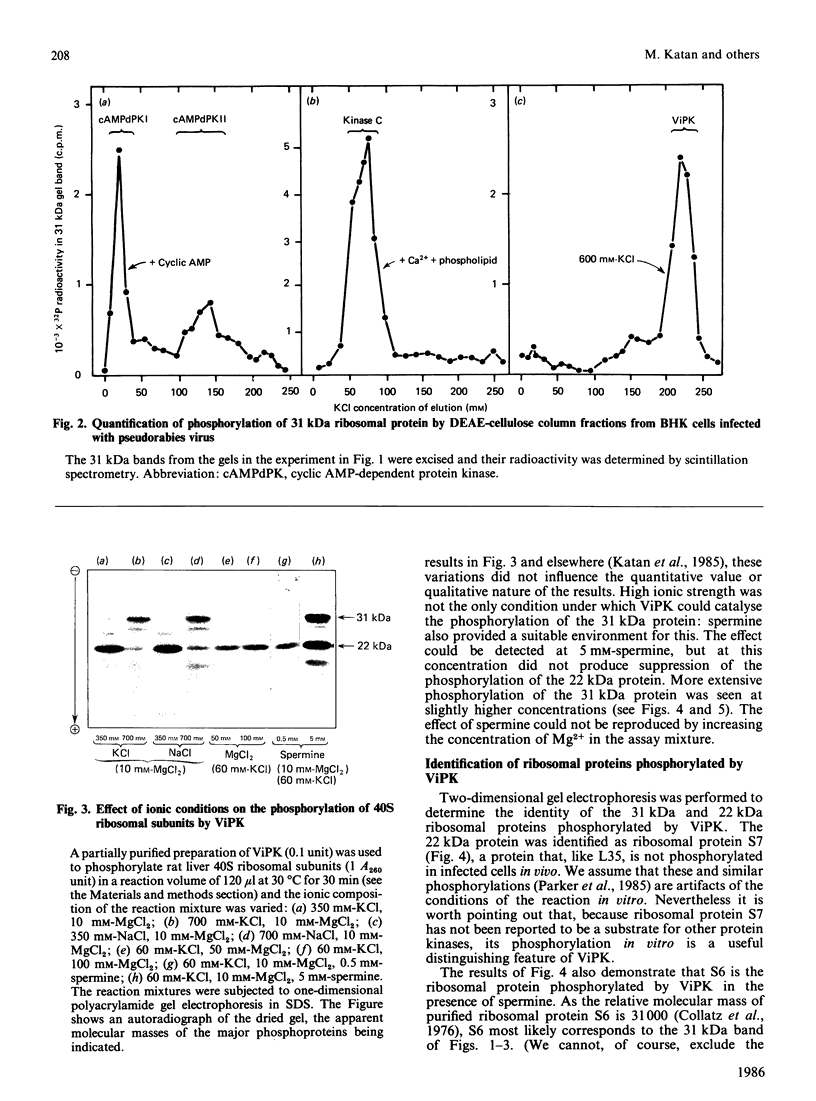
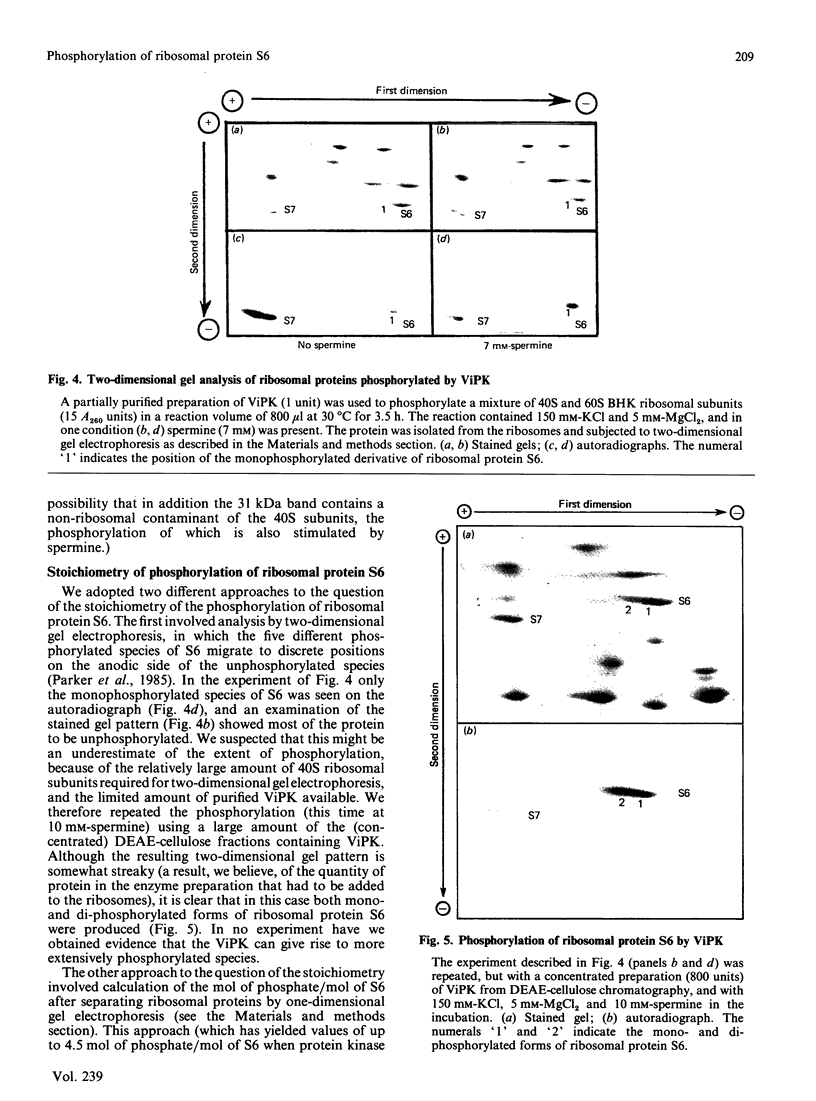
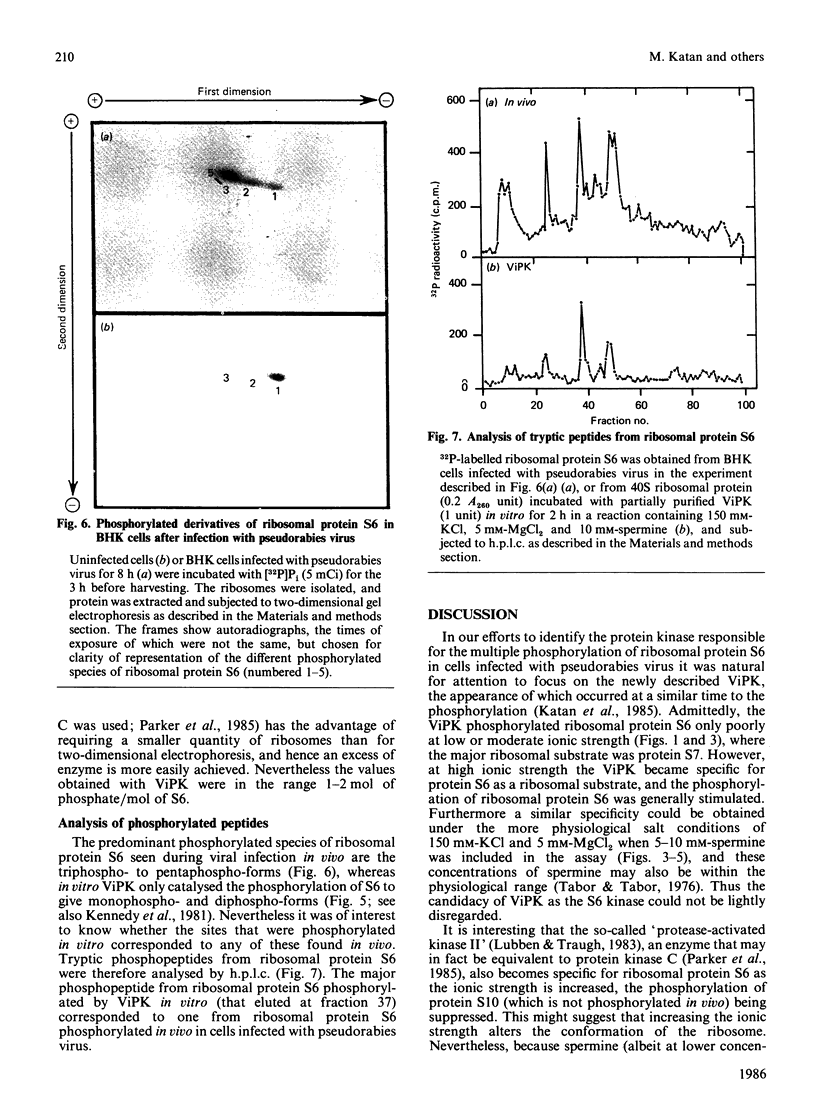
We examined the ability of protein kinase activities from BHK (baby-hamster kidney) cells infected with pseudorabies virus to catalyse the phosphorylation of ribosomal protein S6 in vitro. When the cytosol from infected cells was fractionated on DEAE-cellulose, 40S ribosomal protein kinase activity was found associated with the two isoforms of the cyclic AMP-dependent protein kinase, protein kinase C and a protein kinase (ViPK, virus-induced protein kinase) only detected in infected cells. The phosphorylation of ribosomal protein by ViPK was of particular interest because the appearance of the protein kinase and the increase in the phosphorylation of protein S6 in infected cells shared a similar time course. At moderate concentrations of KCl the major ribosomal substrate for ViPK was ribosomal protein S7, a protein not found to be phosphorylated in vivo. However, at 600 mM-KCl, or in the presence of 5-10 mM-spermine at 60-150 mM-KCl, the phosphorylation of ribosomal protein S7 was suppressed and ribosomal protein S6 became the major substrate. The maximum stoichiometry of phosphorylation obtained under the latter conditions was 1-2 mol of phosphate/mol of S6, and only mono- and di-phosphorylated forms of S6 were detected on two-dimensional gel electrophoresis. As the infection of BHK cells by pseudorabies virus results in the appearance of phosphorylated species of S6 containing up to 5 mol of phosphate/mol of S6 protein, it appears unlikely that ViPK alone can be responsible for the multiple phosphorylation seen in vivo. Nevertheless, tryptic phosphopeptide analysis did indicate that in vitro ViPK catalysed the phosphorylation of at least one of the sites on ribosomal protein S6 phosphorylated in vivo, so that a contributory role for the enzyme in the phosphorylation in vivo cannot be excluded.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. B., Estival A., Tapiovaara H., Gopalakrishna R. Altered subcellular distribution of protein kinase C (a phorbol ester receptor). Possible role in tumor promotion and the regulation of cell growth: relationship to changes in adenylate cyclase activity. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:287–306. [PubMed] [Google Scholar]
- Bittlingmaier K., Schneider D., Falke D. Influence of dibutyryl cyclic AMP on thymidine uptake by herpes simplex virus infected cells and the intracellular level of cyclic AMP. Biochim Biophys Acta. 1977 Aug 2;477(3):228–238. doi: 10.1016/0005-2787(77)90048-x. [DOI] [PubMed] [Google Scholar]
- Collatz E., Wool I. G., Lin A., Stöffler G. The isolation of eukaryotic ribosomal proteins. The purification and characterization of the 40 S ribosomal subunit proteins S2, S3, S4, S5, S6, S7, S8, S9, S13, S23/S24, S27, and S28. J Biol Chem. 1976 Aug 10;251(15):4666–4672. [PubMed] [Google Scholar]
- Gorelick F. S., Cohn J. A., Freedman S. D., Delahunt N. G., Gershoni J. M., Jamieson J. D. Calmodulin-stimulated protein kinase activity from rat pancreas. J Cell Biol. 1983 Oct;97(4):1294–1298. doi: 10.1083/jcb.97.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaerlein M., Horak I. Identification and characterization of ribosomal proteins phosphorylated in vaccinia-virus-infected HeLa cells. Eur J Biochem. 1978 Oct 16;90(3):463–469. doi: 10.1111/j.1432-1033.1978.tb12625.x. [DOI] [PubMed] [Google Scholar]
- Katan M., Stevely W. S., Leader D. P. Partial purification and characterization of a new phosphoprotein kinase from cells infected with pseudorabies virus. Eur J Biochem. 1985 Oct 1;152(1):57–65. doi: 10.1111/j.1432-1033.1985.tb09163.x. [DOI] [PubMed] [Google Scholar]
- Kennedy I. M., Stevely W. S., Leader D. P. Phosphorylation of ribosomal proteins in hamster fibroblasts infected with pseudorabies virus or herpes simplex virus. J Virol. 1981 Aug;39(2):359–366. doi: 10.1128/jvi.39.2.359-366.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Lubben T. H., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification and characterization of protease-activated kinase II. J Biol Chem. 1983 Nov 25;258(22):13992–13997. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McConkey E. H., Bielka H., Gordon J., Lastick S. M., Lin A., Ogata K., Reboud J. P., Traugh J. A., Traut R. R., Warner J. R. Proposed uniform nomenclature for mammalian ribosomal proteins. Mol Gen Genet. 1979 Jan 16;169(1):1–6. doi: 10.1007/BF00267538. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Calcium, phospholipid turnover and transmembrane signalling. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):101–112. doi: 10.1098/rstb.1983.0043. [DOI] [PubMed] [Google Scholar]
- Novak-Hofer I., Thomas G. An activated S6 kinase in extracts from serum- and epidermal growth factor-stimulated Swiss 3T3 cells. J Biol Chem. 1984 May 10;259(9):5995–6000. [PubMed] [Google Scholar]
- Novak-Hofer I., Thomas G. Epidermal growth factor-mediated activation of an S6 kinase in Swiss mouse 3T3 cells. J Biol Chem. 1985 Aug 25;260(18):10314–10319. [PubMed] [Google Scholar]
- Parker P. J., Katan M., Waterfield M. D., Leader D. P. The phosphorylation of eukaryotic ribosomal protein S6 by protein kinase C. Eur J Biochem. 1985 May 2;148(3):579–586. doi: 10.1111/j.1432-1033.1985.tb08879.x. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Katan M., Stevely W. S., Leader D. P. Characteristics of the induction of a new protein kinase in cells infected with herpesviruses. J Gen Virol. 1986 Jun;67(Pt 6):1049–1057. doi: 10.1099/0022-1317-67-6-1049. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Voorma H. O., Thomas A., Goumans H., Amesz H., van der Mast C. Isolation and purification of initiation factors of protein synthesis from rabbit reticulocyte lysate. Methods Enzymol. 1979;60:124–135. doi: 10.1016/s0076-6879(79)60012-5. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Cohen P., Caudwell B., Holland R. Differential phosphorylation of ribosomal protein S6 in isolated rat hepatocytes after incubation with insulin and glucagon. FEBS Lett. 1982 Nov 8;148(2):207–213. doi: 10.1016/0014-5793(82)80809-0. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Morgan F. J. Phosphorylation of hepatic ribosomal protein S6 on 80 and 40 S ribosomes. Primary structure of S6 in the region of the major phosphorylation sites for cAMP-dependent protein kinases. J Biol Chem. 1984 Feb 25;259(4):2084–2091. [PubMed] [Google Scholar]
- del Grande R. W., Traugh J. A. Phosphorylation of 40-S ribosomal subunits by cAMP-dependent, cGMP-dependent and protease-activated protein kinases. Eur J Biochem. 1982 Apr 1;123(2):421–428. doi: 10.1111/j.1432-1033.1982.tb19785.x. [DOI] [PubMed] [Google Scholar]