Abstract
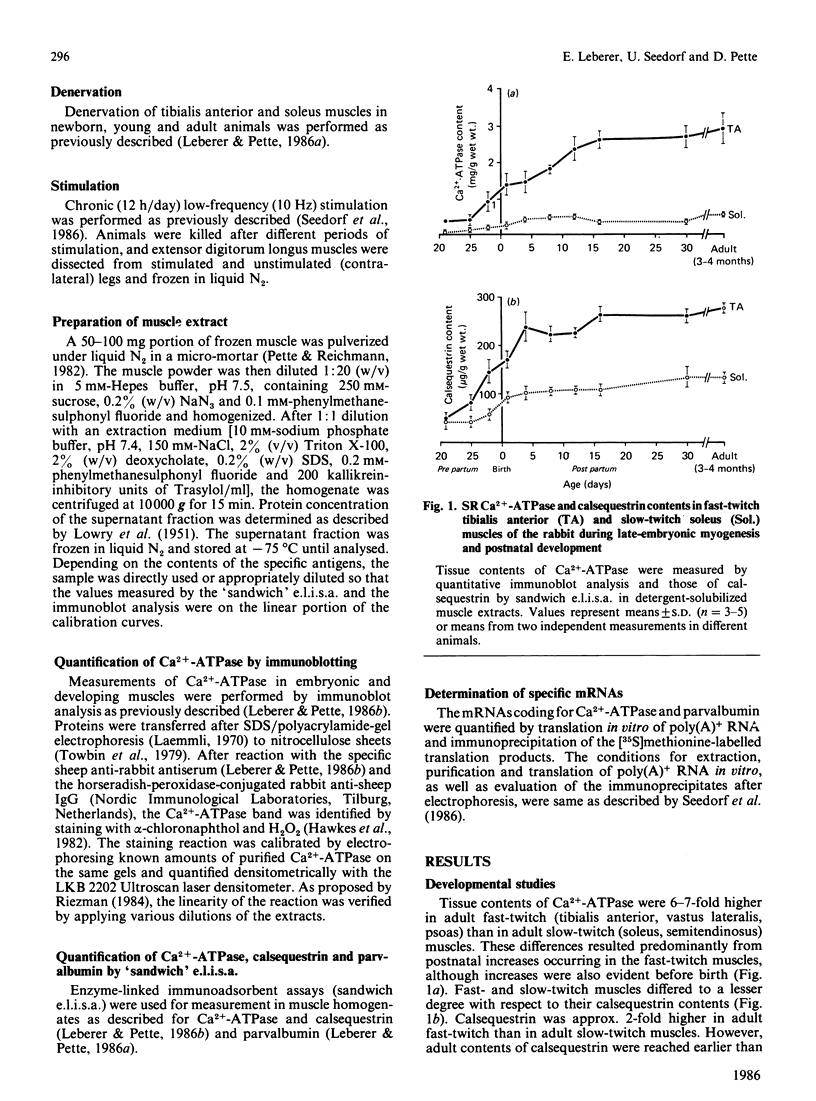
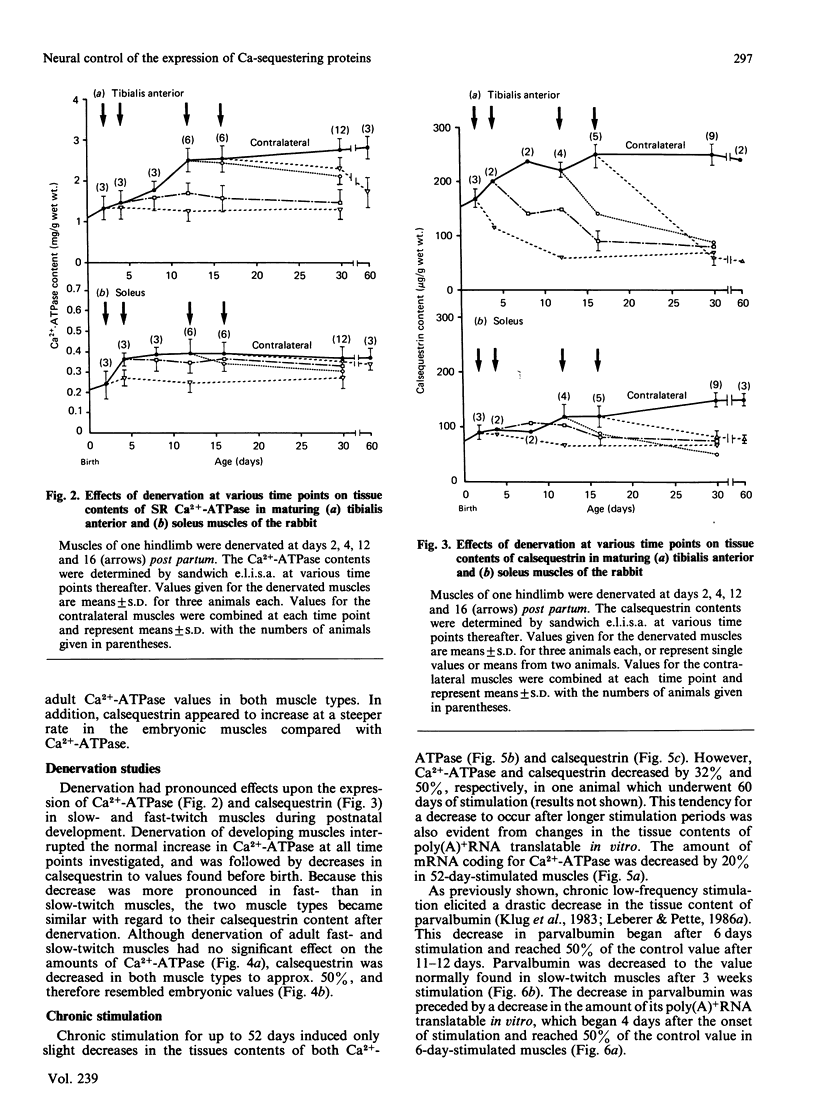
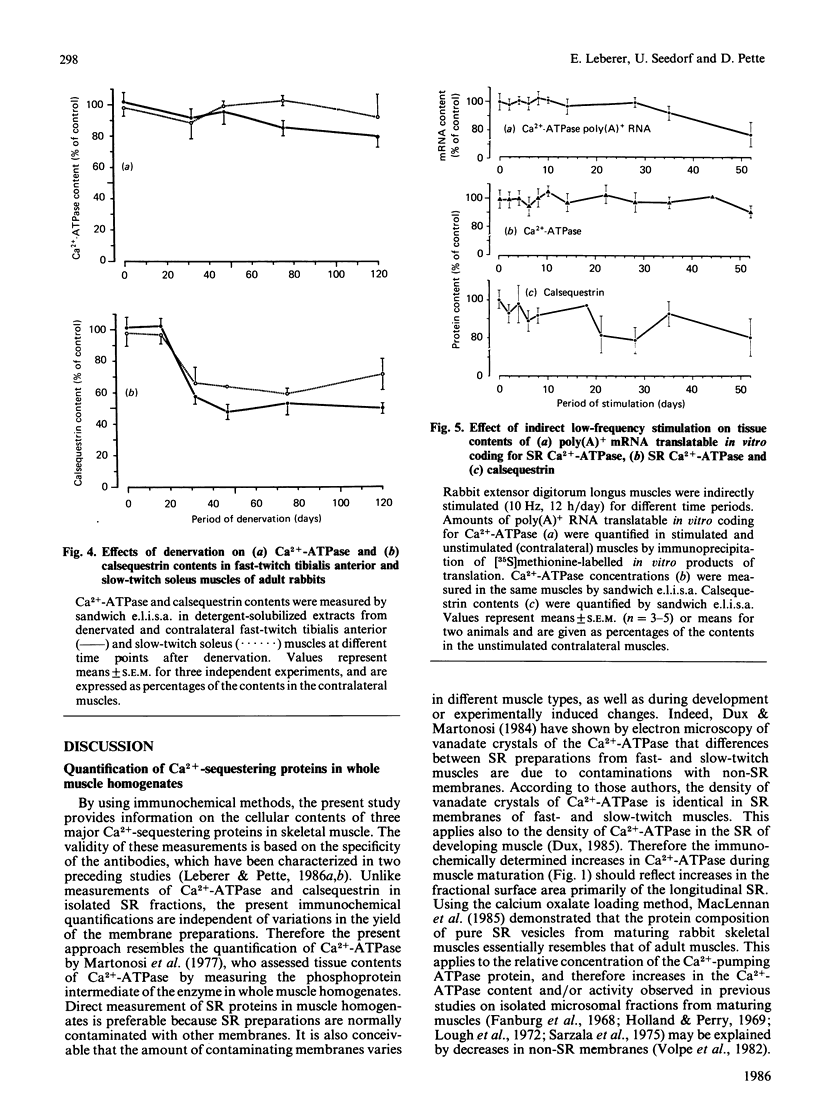
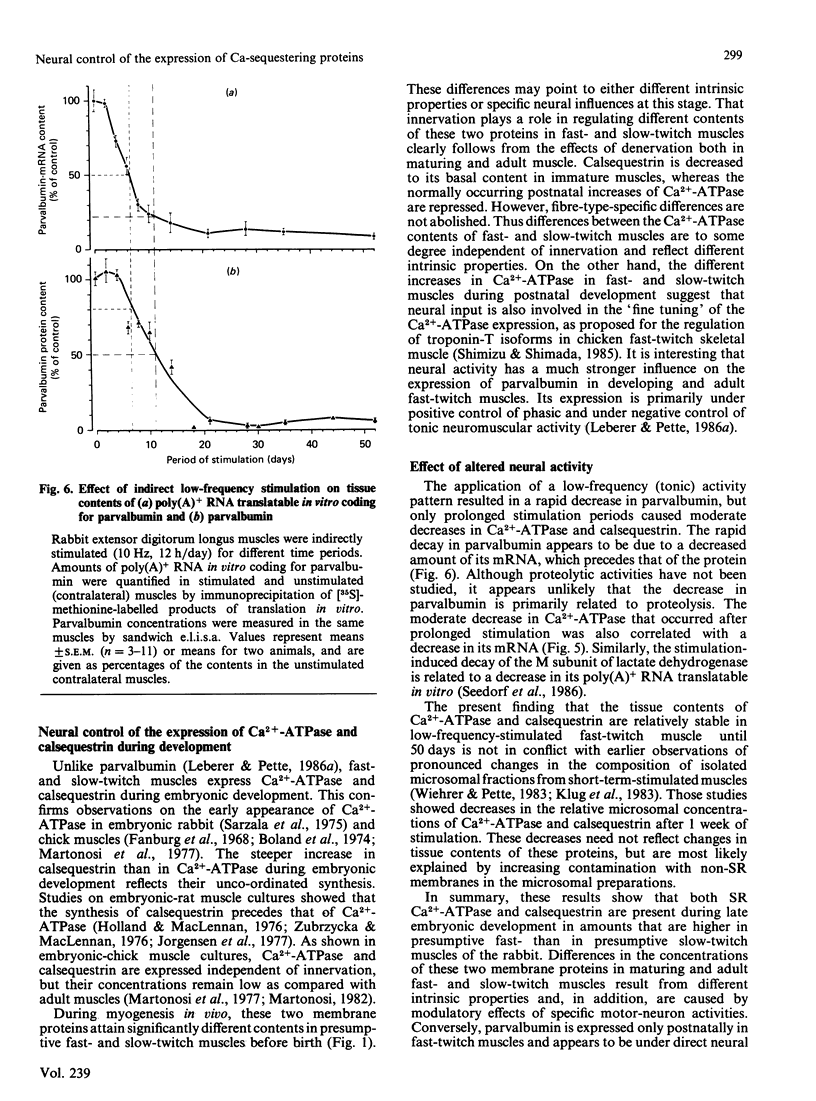
Tissue contents of the sarcoplasmic-reticulum Ca2+-ATPase (Ca2+ +Mg2+-dependent ATPase), of calsequestrin and of parvalbumin were immunochemically quantified in homogenates of fast- and slow-twitch muscles of embryonic, maturing and adult rabbits. Unlike parvalbumin, Ca2+-ATPase and calsequestrin were expressed in embryonic muscles. Presumptive fast-twitch muscles displayed higher contents of these two proteins than did presumptive slow-twitch muscles. Calsequestrin steeply increased before birth and reached adult values in the two muscle types 4 days after birth. The main increase in Ca2+-ATPase occurred during the first 2 weeks after birth. Denervation of postnatal fast- and slow-twitch muscles decreased calsequestrin to amounts typical of embryonic muscle and suppressed further increases of Ca2+-ATPase. Denervation caused slight decreases in Ca2+-ATPase in adult fast-twitch, but not in slow-twitch, muscles, whereas calsequestrin was greatly decreased in both. Chronic low-frequency stimulation induced a rapid decrease in parvalbumin in fast-twitch muscle, which was preceded by a drastic decrease in the amount of its polyadenylated RNA translatable in vitro. Tissue amounts of Ca2+-ATPase and calsequestrin were essentially unaltered up to periods of 52 days stimulation. These results indicate that in fast- and slow-twitch muscles different basal amounts of Ca2+-ATPase and calsequestrin are expressed independent of innervation, but that neuromuscular activity has a modulatory effect. Conversely, the expression of parvalbumin is greatly enhanced by phasic, and drastically decreased by tonic, motor-neuron activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boland R., Martonosi A., Tillack T. W. Developmental changes in the composition and function of sarcoplasmic reticulum. J Biol Chem. 1974 Jan 25;249(2):612–623. [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982 Jun 10;297(5866):504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- Dux L., Martonosi A. Membrane crystals of Ca2+-ATPase in sarcoplasmic reticulum of fast and slow skeletal and cardiac muscles. Eur J Biochem. 1984 May 15;141(1):43–49. doi: 10.1111/j.1432-1033.1984.tb08154.x. [DOI] [PubMed] [Google Scholar]
- Dux L. Membrane crystals of Ca2+-ATPase in sarcoplasmic reticulum of developing muscle. FEBS Lett. 1985 Apr 8;183(1):177–181. doi: 10.1016/0014-5793(85)80980-7. [DOI] [PubMed] [Google Scholar]
- Fanburg B. L., Drachman D. B., Moll D., Roth S. I. Calcium transport in isolated sarcoplasmic reticulum during muscle maturation. Nature. 1968 Jun 8;218(5145):962–964. doi: 10.1038/218962a0. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Heilmann C., Pette D. Molecular transformations in sarcoplasmic reticulum of fast-twitch muscle by electro-stimulation. Eur J Biochem. 1979 Feb 1;93(3):437–446. doi: 10.1111/j.1432-1033.1979.tb12841.x. [DOI] [PubMed] [Google Scholar]
- Heizmann C. W. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984 Sep 15;40(9):910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- Holland D. L., Perry S. V. The adenosine triphosphatase and calcium ion-transporting activities of the sarcoplasmic reticulum of developing musce. Biochem J. 1969 Sep;114(2):161–170. doi: 10.1042/bj1140161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P. C., MacLennan D. H. Assembly of the sarcoplasmic reticulum. Biosynthesis of the adenosine triphosphatase in rat skeletal muscle cell culture. J Biol Chem. 1976 Apr 10;251(7):2030–2036. [PubMed] [Google Scholar]
- Jorgensen A. O., Kalnins V. I., Zubrzycka E., MacLennan D. H. Assembly of the sarcoplasmic reticulum. Localization by immunofluorescence of sarcoplasmic reticulum proteins in differentiating rat skeletal muscle cell cultures. J Cell Biol. 1977 Jul;74(1):287–298. doi: 10.1083/jcb.74.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A. O., Kalnins V., MacLennan D. H. Localization of sarcoplasmic reticulum proteins in rat skeletal muscle by immunofluorescence. J Cell Biol. 1979 Feb;80(2):372–384. doi: 10.1083/jcb.80.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Wiehrer W., Reichmann H., Leberer E., Pette D. Relationships between early alterations in parvalbumins, sarcoplasmic reticulum and metabolic enzymes in chronically stimulated fast twitch muscle. Pflugers Arch. 1983 Dec;399(4):280–284. doi: 10.1007/BF00652753. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leberer E., Pette D. Immunochemical quantification of sarcoplasmic reticulum Ca-ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles of defined fiber composition. Eur J Biochem. 1986 May 2;156(3):489–496. doi: 10.1111/j.1432-1033.1986.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Leberer E., Pette D. Neural regulation of parvalbumin expression in mammalian skeletal muscle. Biochem J. 1986 Apr 1;235(1):67–73. doi: 10.1042/bj2350067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough J. W., Entman M. L., Bossen E. H., Hansen J. L. Calcium accumulation by isolated sarcoplasmic reticulum of skeletal muscle during development in tissue culture. J Cell Physiol. 1972 Dec;80(3):431–436. doi: 10.1002/jcp.1040800313. [DOI] [PubMed] [Google Scholar]
- Martonosi A., Roufa D., Boland R., Reyes E., Tillack T. W. Development of sarcoplasmic reticulum in cultured chicken muscle. J Biol Chem. 1977 Jan 10;252(1):318–332. [PubMed] [Google Scholar]
- Martonosi A. The development of sarcoplasmic reticulum membranes. Annu Rev Physiol. 1982;44:337–355. doi: 10.1146/annurev.ph.44.030182.002005. [DOI] [PubMed] [Google Scholar]
- Mommaerts W. F., Buller A. J., Seraydarian K. The modification of some biochemical properties of muscle by cross-innervation. Proc Natl Acad Sci U S A. 1969 Sep;64(1):128–133. doi: 10.1073/pnas.64.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müntener M., Berchtold M. W., Heizmann C. W. Parvalbumin in cross-reinnervated and denervated muscles. Muscle Nerve. 1985 Feb;8(2):132–137. doi: 10.1002/mus.880080209. [DOI] [PubMed] [Google Scholar]
- Pette D., Reichmann H. A method for quantitative extraction of enzymes and metabolites from tissue samples in the milligram range. J Histochem Cytochem. 1982 Apr;30(4):401–402. doi: 10.1177/30.4.7061832. [DOI] [PubMed] [Google Scholar]
- Sarzala M. G., Pilarska M., Zubrzycka E., Michalak M. Changes in the structure, composition and function of sarcoplasmic-reticulum membrane during development. Eur J Biochem. 1975 Sep 1;57(1):25–34. doi: 10.1111/j.1432-1033.1975.tb02273.x. [DOI] [PubMed] [Google Scholar]
- Seedorf U., Leberer E., Kirschbaum B. J., Pette D. Neural control of gene expression in skeletal muscle. Effects of chronic stimulation on lactate dehydrogenase isoenzymes and citrate synthase. Biochem J. 1986 Oct 1;239(1):115–120. doi: 10.1042/bj2390115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N., Shimada Y. Immunochemical analysis of troponin T isoforms in adult, embryonic, regenerating, and denervated chicken fast skeletal muscles. Dev Biol. 1985 Oct;111(2):324–334. doi: 10.1016/0012-1606(85)90487-7. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Luff A. R., Gergely J. Effect of cross-reinnervation on physiological parameters and on properties of myosin and sarcoplasmic reticulum of fast and slow muscles of the rabbit. J Gen Physiol. 1975 Dec;66(6):811–821. doi: 10.1085/jgp.66.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe P., Damiani E., Salviati G., Margreth A. Transitions in membrane composition during postnatal development of rabbit fast muscle. J Muscle Res Cell Motil. 1982 Jun;3(2):213–230. doi: 10.1007/BF00711943. [DOI] [PubMed] [Google Scholar]
- Wiehrer W., Pette D. The ratio between intrinsic 115 kDa and 30 kDa peptides as a marker of fibre type-specific sarcoplasmic reticulum in mammalian muscles. FEBS Lett. 1983 Jul 25;158(2):317–320. doi: 10.1016/0014-5793(83)80604-8. [DOI] [PubMed] [Google Scholar]
- Zubrzycka E., MacLennan D. H. Assembly of the sarcoplasmic reticulum. Biosynthesis of calsequestrin in rat skeletal muscle cell cultures. J Biol Chem. 1976 Dec 25;251(24):7733–7738. [PubMed] [Google Scholar]


