Abstract
BACKGROUND:
This trial aimed to assess the efficacy, acceptability, and safety of a first-trimester screen-and-prevent strategy for preterm preeclampsia in Asia.
METHODS:
Between August 1, 2019, and February 28, 2022, this multicenter stepped wedge cluster randomized trial included maternity/diagnostic units from 10 regions in Asia. The trial started with a period where all recruiting centers provided routine antenatal care without study-related intervention. At regular 6-week intervals, one cluster was randomized to transit from nonintervention phase to intervention phase. In the intervention phase, women underwent first-trimester screening for preterm preeclampsia using a Bayes theorem-based triple-test. High-risk women, with adjusted risk for preterm preeclampsia ≥1 in 100, received low-dose aspirin from <16 weeks until 36 weeks.
RESULTS:
Overall, 88.04% (42 897 of 48 725) of women agreed to undergo first-trimester screening for preterm preeclampsia. Among those identified as high-risk in the intervention phase, 82.39% (2919 of 3543) received aspirin prophylaxis. There was no significant difference in the incidence of preterm preeclampsia between the intervention and non-intervention phases (adjusted odds ratio [aOR], 1.59 [95% CI, 0.91–2.77]). However, among high-risk women in the intervention phase, aspirin prophylaxis was significantly associated with a 41% reduction in the incidence of preterm preeclampsia (aOR, 0.59 [95% CI, 0.37–0.92]). In addition, it correlated with 54%, 55%, and 64% reduction in the incidence of preeclampsia with delivery at <34 weeks (aOR, 0.46 [95% CI, 0.23–0.93]), spontaneous preterm birth <34 weeks (aOR, 0.45 [95% CI, 0.22–0.92]), and perinatal death (aOR, 0.34 [95% CI, 0.12–0.91]), respectively. There was no significant between-group difference in the incidence of aspirin-related severe adverse events.
CONCLUSIONS:
The implementation of the screen-and-prevent strategy for preterm preeclampsia is not associated with a significant reduction in the incidence of preterm preeclampsia. However, low-dose aspirin effectively reduces the incidence of preterm preeclampsia by 41% among high-risk women. The screen-and-prevent strategy for preterm preeclampsia is highly accepted by a diverse group of women from various ethnic backgrounds beyond the original population where the strategy was developed. These findings underpin the importance of the widespread implementation of the screen-and-prevent strategy for preterm preeclampsia on a global scale.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03941886.
Keywords: Asia, aspirin, Fetal Medicine Foundation, first-trimester, preeclampsia, screen-and-prevent, screening, stepped wedge cluster randomized trial
Clinical Perspective.
What Is New?
Aspirin prophylaxis effectively reduces the incidence of preterm preeclampsia by 41% among high-risk women.
Low-dose aspirin is considered a safe intervention during pregnancy.
The screen-and-prevent strategy for preterm preeclampsia is highly accepted by a diverse group of women from various ethnic backgrounds beyond the original population for which the strategy was developed.
What Are the Clinical Implications?
The screen-and-prevent strategy for preterm preeclampsia can be implemented on a global scale.
Preeclampsia is a serious pregnancy-specific multisystem hypertensive disorder that affects 2% to 5% of all pregnant women worldwide.1,2 In Asia, it has been reported that the incidence of preeclampsia is approximately 2%.3 It is the second leading cause of maternal mortality (14%), accounting for approximately 63 000 maternal deaths annually worldwide.4 Preeclampsia can be classified into early, preterm, late, and term preeclampsia based on the gestational age at delivery: <34, <37, ≥34, and ≥37 weeks of gestation, respectively. These classifications are essential because of the varying adverse pregnancy outcomes associated with each category.5–9 Evidence has consistently shown that early or preterm preeclampsia is associated with an increased risk of severe pregnancy complications, including fetal growth restriction; low birth weight; preterm birth; placental abruption; hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; and stillbirth, compared to late or term preeclampsia.9–16 Given the severity of the disorder, it is important to provide effective early screening and prevention for preterm preeclampsia.
The ASPRE trial (Combined Multimarker Screening and Randomised Patient Treatment With Aspirin for Evidence-Based Preeclampsia Prevention), which predominantly involved White populations, reported a 62% (95% CI, 26%–80%) reduction in the incidence of preterm preeclampsia among high-risk women receiving daily aspirin prophylaxis at 150 mg, compared with those who received placebo, after first-trimester screening using the Fetal Medicine Foundation (FMF) combined test.17 On the basis of the results of the ASPRE trial, this screen-and-prevent strategy for preterm preeclampsia has been endorsed and recommended by several key professional organizations for clinical practice.5,18–22 We already prospectively validated the first-trimester FMF triple test in an independent cohort of 10 935 singleton pregnancies from China, Hong Kong SAR, India, Japan, Taiwan, Thailand, and Singapore.23 This test combines maternal characteristics and history, mean arterial pressure (MAP), uterine artery pulsatility index (UtA-PI), and PlGF (placental growth factor). We have demonstrated that the FMF triple test could achieve a detection rate of 64.0% for preterm preeclampsia at a 10% false-positive rate.23 These results are comparable with previously published findings for East Asians from the FMF.24,25 Before the widespread adoption of the European-derived screen-and-prevent strategy for preterm preeclampsia, the next step is to conduct an implementation trial in an independent cohort. This effort is crucial to demonstrate that this strategy can be widely implemented across the world. Therefore, the aim of the present trial was to evaluate the efficacy, acceptability, and safety of the aforementioned first-trimester screen-and-prevent strategy for preterm preeclampsia in Asia.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
This was a multicenter stepped wedge cluster randomized trial conducted between August 1, 2019, and February 28, 2022. Screening centers were organized into clusters, each containing approximately an equal number of pregnant women. These clusters included maternity/diagnostic units from Hong Kong SAR, Mainland China, Taiwan, Singapore, Thailand, the Philippines, Indonesia, Malaysia, Vietnam, and Japan (Table S1). The formation of clusters was based on the homogeneity of the trial populations. A staggered schedule was followed to implement the intervention over many time periods (Figures S1 and S2). The stepped wedge design started with a period where routine antenatal care was provided with no study-related intervention at all recruiting centers. Subsequently, at regular 6-weekly intervals, 1 cluster was randomized to transit from a nonintervention phase to an intervention phase. During the intervention phase, first-trimester screening for preterm preeclampsia using the Bayes theorem-based triple test was performed.3 For women with an adjusted risk for preterm preeclampsia ≥1 in 100, low-dose aspirin treatment was initiated from <16 weeks until 36 weeks, or until delivery or the onset of preeclampsia before 36 weeks.5
Approval for the trial was obtained from the Joint Chinese University of Hong Kong–New Territories East Cluster clinical research ethics committee (ref. No. 2018.391) in Hong Kong and the ethics committee of each participating hospital in other regions. The trial is registered with https://www.clinicaltrials.gov (Unique identifier: NCT03941886).
Participants
Women ≥18 years of age with a viable singleton pregnancy at 11–13+6 weeks of gestation who consented to participate were screened for preterm preeclampsia using maternal characteristics and history combined with maternal MAP, UtA-PI, and PlGF. Exclusion criteria included multiple pregnancies, major fetal defects identified at 11–13+6 weeks of assessment, nonviable fetus (missed spontaneous miscarriage or stillbirth), or those under other clinical interventions. All eligible women received written information describing the trial, and those who agreed to participate gave written informed consent.
Randomization and Masking
The recruiting centers formed the units of cluster randomization. An independent statistician conducted randomization using anonymous cluster codes. All clusters were randomly assigned different starting times for the intervention phase based on computer-generated random numbers. Each cluster’s random start date for the intervention phase was concealed until 3 weeks before the commencement. No masking was used because of the nature of the intervention.
Biomarker Quality Control
During the trial period, anonymized first-trimester screening maternal and biomarker data (MAP, UtA-PI, and PlGF) were returned to the quality control team (L.N.-H., D.S.S., and L.C.P.) for quality assessment every 3 months. At each assessment, target plots and cumulative sum control charts were generated for each recruiting center. Biomarker multiples of the median (MoM) distributions were considered acceptable provided that the median was within the 0.95 to 1.05 range.26
First-Trimester Screening Test for Preterm Preeclampsia
Gestational age was determined from the measurement of the fetal crown rump length.27 Maternal characteristics, medical and obstetric history, and any history of drug use or substance abuse were recorded,28 and maternal weight and height were measured. Measurements of MAP, UtA-PI, and PlGF followed well-established standardized protocols.29–33 MAP was measured by validated automated devices (BP3AQ1 Microlife, Taipei, Taiwan).34 Transabdominal color Doppler ultrasound imaging was used to identify the uterine arteries, and pulsed-wave Doppler was used to measure the left and right UtA-PI, with the average value recorded.35 All operators performing Doppler studies had received the appropriate Certificate of Competence from the FMF (https://fetalmedicine.org/). PlGF concentrations were measured by 1 of 3 automated immunoanalyzers: AutoDELFIA or DELFIA Xpress system (PlGF 1-2-3 kits; Revvity Inc, Waltham, MA), B.R.A.H.M.S KRYPTOR analyzer (Thermo Fisher Scientific, Hennigsdorf, Germany), or Cobas e411 system (Roche Diagnostics, Rotkreuz, Switzerland). Risks for preterm preeclampsia were estimated using the FMF competing risk model by combining maternal factors with the values of MAP, UtA-PI, and PlGF.22 The preterm preeclampsia risk calculator has been integrated into several software applications, including Viewpoint, GE HealthCare Technologies Inc (Chicago, IL), Lifecycle, Revvity Inc (Waltham, MA), and Astraia Obstetric Module, Astraia GmbH (Munich, Germany).
The measured values of MAP, UtA-PI, and PlGF were converted into MoM values with adjustment for maternal characteristics, past and current obstetrics history, and immunoanalyzers using published formulae.36 The central tendency of PlGF and MAP MoMs distribution was assessed using the initial 400 women screened at each center because our previous study indicated that the FMF PlGF and MAP MoM formulae did not result in a distribution with a median of 1 MoM, as required by the risk estimation model.26 To address this, at each site, the distribution of PlGF and MAP MoMs was recentered to have a median of 1 MoM by applying a biomarker site-specific correction factor.
In the Bayes theorem first-trimester triple test, the risk of development of preeclampsia increased with advancing maternal age; increasing weight; Black and South Asian racial origin; medical history of chronic hypertension, diabetes, and systemic lupus erythematosus or antiphospholipid syndrome; conception by in vitro fertilization; family history of preeclampsia; maternal history of preeclampsia; higher MAP MoM; higher UtA-PI MoM; and lower PlGF MoM.22
Intervention
During each cluster intervention period, women with an adjusted risk ≥1 in 100 were invited to attend a follow-up visit for further counseling about the high-risk status, benefits, and side effects of aspirin prophylaxis. Those without known bleeding disorders, active peptic ulcer disease, or hypersensitivity to aspirin were offered low-dose aspirin at <16 weeks until 36 weeks, or until delivery or the onset of preeclampsia before 36 weeks.
All aspirin tablets used in the trial are locally registered products. The regimen of low-dose aspirin depended on maternal weight.5 High-risk women with a maternal weight <40 kg were offered aspirin at a daily dose of 100 mg, whereas those with a maternal weight of ≥40 kg were offered aspirin at doses of 150 mg or 160 mg or 162 mg based on the available preparations at each recruiting center (Table S1). Participants were provided with a drug diary to record daily aspirin self-administration and any side effects. Compliance was reported as the tablet count percentage difference of the total number of tablets consumed against the total number of tablets prescribed. Adherence was considered good, moderate, and poor if the reported intake of tablets was ≥85%, between 50% and <85%, and ≤50%, respectively.17 For those who declined aspirin prophylaxis, follow-up visits continued until delivery. In cases of hypersensitivity to nonsteroidal anti-inflammatory drugs, the potential risk of cross-sensitivity would be explained, and participants would choose whether to use aspirin prophylaxis.
Compliance and adverse events were assessed during follow-up visits at 19 to 24, 30 to 34, and 35 to 37 weeks of gestation and during telephone interviews at 16 weeks of gestation, 28 weeks of gestation, and 30 days after the last dose of aspirin was taken. Low-risk women were followed up according to standard local protocols. We reached out to both the participants and their obstetricians to confirm the use of aspirin and collect data on pregnancy outcomes for those who received pregnancy care and delivered elsewhere.
Data Management
All data acquired for first-trimester preeclampsia screening were stored in a secure, password-encrypted database. Pregnancy outcome data for both high-risk and low-risk participants were recorded on paper-based case report forms. These data included gestational age at delivery, onset of labor, mode of delivery, indications for iatrogenic delivery, sex and birth weight of neonate, Apgar scores, neonatal intensive care unit admission status, hypertensive complications of pregnancy, and aspirin intake during pregnancy. Single data entry was used locally. All screening and outcome data were then sent to trial coordinators (L.N.-H., L.C.P.) for validation. In cases where information was ambiguous, it was returned to the recruiting center for confirmation or revision before being recorded in our secure password-encrypted database.
Primary Outcome
The primary outcome measure was delivery with preeclampsia before 37 weeks of gestation.
Secondary Outcomes
Secondary outcomes were composite adverse outcomes of pregnancy including preeclampsia, gestational hypertension, low birth weight <5th percentile, stillbirth, placental abruption with delivery <34, <37, and ≥37 weeks of gestation; neonatal mortality; composite neonatal morbidity including grade II or above intraventricular hemorrhage, neonatal sepsis confirmed by cultures, neonatal anemia requiring transfusion, respiratory distress syndrome requiring surfactant and ventilation, and necrotizing enterocolitis requiring surgical intervention; composite neonatal therapy including neonatal high dependency or neonatal intensive care unit admission and ventilation—need of positive pressure or intubation; small for gestational age (SGA) with different cutoffs of low birth weight percentile: <3rd, <5th and <10th; stillbirth; spontaneous preterm birth (sPTB) at <34 and <37 weeks of gestation; acceptability for preeclampsia screening; acceptability for aspirin treatment; and gestational age at delivery.
Preeclampsia and gestational hypertension were defined according to the guidelines of the International Society for the Study of Hypertension in Pregnancy.18 Preeclampsia was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg on ≥2 occasions measured 4 hours apart in previously normotensive women, accompanied by ≥1 of the following new-onset conditions at or after 20 weeks of gestation: proteinuria, evidence of other maternal organ dysfunction such as acute kidney injury, liver involvement, elevated liver enzymes, neurological complications, hematological complications, or uteroplacental dysfunction.18 SGA neonate was defined as birth weight <10th, <5th, or <3rd percentile for gestational age, adjusted for maternal weight and height, past obstetric history and newborn sex.37 sPTB was defined as delivery at <37 weeks of gestation with ≥1 of the following factors: spontaneous onset of labor with intact membranes, preterm premature rupture of membranes (PPROM), and cervical insufficiency.38
Sample Size
The sample size calculation was based on the primary outcome measure, which is the difference in proportions of delivery with preterm preeclampsia between nonintervention and intervention groups. In total, we recruited 7 clusters for the trial, and the trial duration was set at 78 weeks (Figures S1 and S2). Following the method of Hussey and Hughes,38a it was determined that a sample size of 340 participants per cluster per time interval would allow us to detect a 2.7% difference in proportions by intervention.17 This calculation assumed a 10% screen positive rate and an intraclass correlation coefficient of 0.001 (based on pilot data) over 28 time steps, with 80% statistical power and a 2-sided type 1 error rate of 5%. Accounting for an anticipated 10% loss to follow-up, it was estimated that 378 participants (25 per working day) were required for each cluster during a 3-week time interval. Consequently, the total sample size for the entire trial was projected to be 68 250.
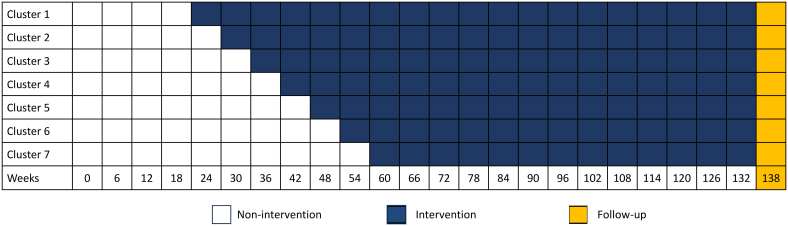
However, the global COVID-19 pandemic significantly affected recruitment rate. The trial design was adjusted on the assumption that the recruitment rate was halved. By extending the intervention phase by 60 weeks (equivalent to 20 time steps), the aim was to maintain a trial power of ≈65%, while keeping all other assumptions unchanged. Ultimately, a total of 42 897 women participated in the trial. The adjusted step wedge design is illustrated in Figure 1.
Figure 1.
Adjusted step-wedge caused by COVID-19.
Statistical Analysis
Both intention-to-treat analysis and per-protocol analysis were conducted. The intention-to-treat analysis was the primary approach and included all trial participants who took ≥1 dose of aspirin during pregnancy. Per-protocol analysis was conducted on the primary outcome for participants who demonstrated ≥90% compliance with low-dose aspirin prophylaxis. No imputation of missing data values was performed. Histograms were used to identify any potential outliers in trial outcomes.
All baseline characteristics were descriptively compared between the nonintervention and intervention phases. Continuous data were presented as either mean and SD or median and interquartile range. Categorical data are expressed as numbers and percentages.
The accuracy of the FMF triple test in the nonintervention cases was assessed using area under the receiver operating characteristic curve, calibration, and determining the detection rate at fixed false-positive rates of 5%, 10%, 15%, and 20%. Calibration during the nonintervention period was also assessed visually by comparing the observed incidence with the predicted risk for preterm preeclampsia by the FMF triple test. To compare the proportion of preterm preeclampsia between nonintervention and intervention phases, covariate unadjusted odds ratio and adjusted odds ratio (aOR) were determined. The odds ratio measuring the association between the intervention and the outcome was obtained by generalized linear mixed-effect model analysis (binomial distribution assumed using logit link function), controlling for covariates of cluster and study time period only, whereas the aOR was obtained by generalized linear mixed-effect model analysis controlling for covariates of cluster, study time periods, and individual-level characteristics. The cluster and time period effect were respectively treated as a random-effect term and a fixed-effect term in the models. The intraclass correlation coefficient was determined by dividing the between-cluster variance by total variance using the latent variable method.39 The variance terms were estimated by fitting an unconditional generalized linear mixed-effects model with only a random intercept for clusters, and the latent variable method approximates the observed binary response by representing an unobserved thresholded continuous variable. The 95% CI and P values were calculated based on the Wald statistic.
For the secondary outcomes including adverse outcomes of pregnancy, neonatal death, composite neonatal morbidity, composite neonatal therapy, SGA, and sPTB, both crude odds ratio and aOR were estimated using the same generalized linear mixed-effects model analysis. Acceptability of preeclampsia screening and aspirin prophylaxis were assessed by the proportions of all eligible women who agreed to undergo preeclampsia screening and high-risk women who agreed to commence aspirin prophylaxis, respectively. The acceptability of preeclampsia screening and aspirin prophylaxis was plotted using cluster bar charts. Safety of low-dose aspirin was described by the proportions of participants who experienced aspirin-related adverse side effects (eg, allergic reaction, nausea, vomiting, upper and lower gastrointestinal symptoms, or pyrexia) during the study period.
A sensitivity analysis was conducted to compare primary results between the intention-to-treat analysis and the analysis excluding women who took aspirin for other medical indications. To identify potential subgroups showing a more favorable intervention effect, post hoc subgroup analyses of the primary and the secondary outcomes were conducted by stratifying participants according to the presence or absence of risk factors: obesity (body mass index >30 kg/m2), diabetes, chronic hypertension, family history of preeclampsia, history of smoking, advanced maternal age ≥35 years, assisted conception (in vitro fertilization and ovulation induction), nulliparity, and history of preeclampsia. To examine the robustness of time-varying treatment effect of aspirin prophylaxis on the preeclampsia outcomes, a post hoc analysis of mixed-effect Cox proportional-hazards model was applied accounting for time-dependent covariates of treatment initiation time and end time, individual-level baseline covariates, fixed-period effect, and random-cluster effect. Tied survival times in the Cox model were handled by Efron approximation. Because the subgroup analyses and post hoc analysis were conducted for exploratory purposes, adjustments for the inflation of type 1 error because of the multiple comparisons were not made.
A P value of <0.05 was considered statistically significant. All analyses were carried out using SAS statistical software version 9.4.
RESULTS
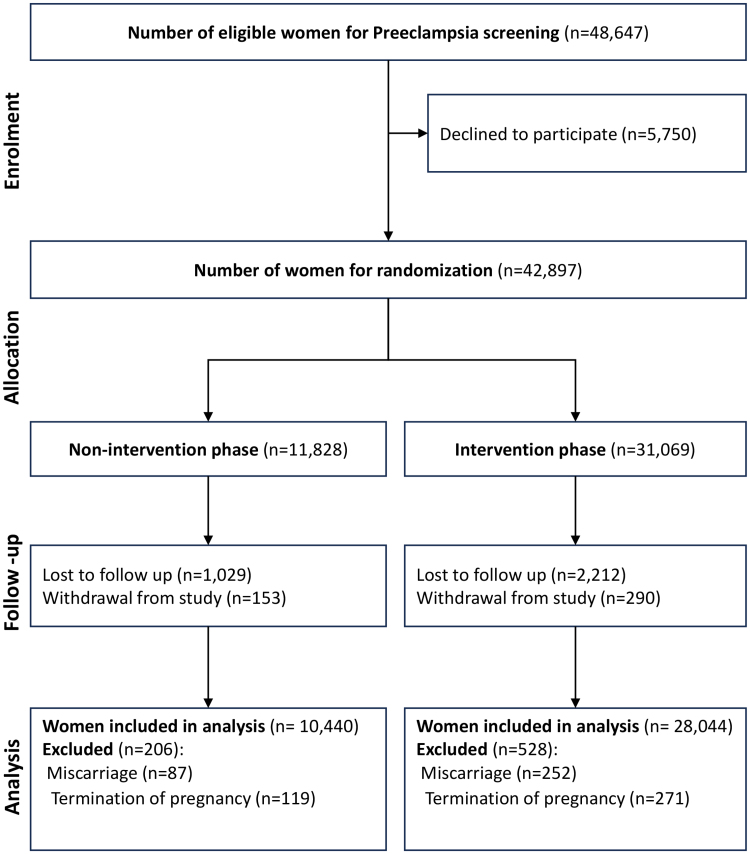
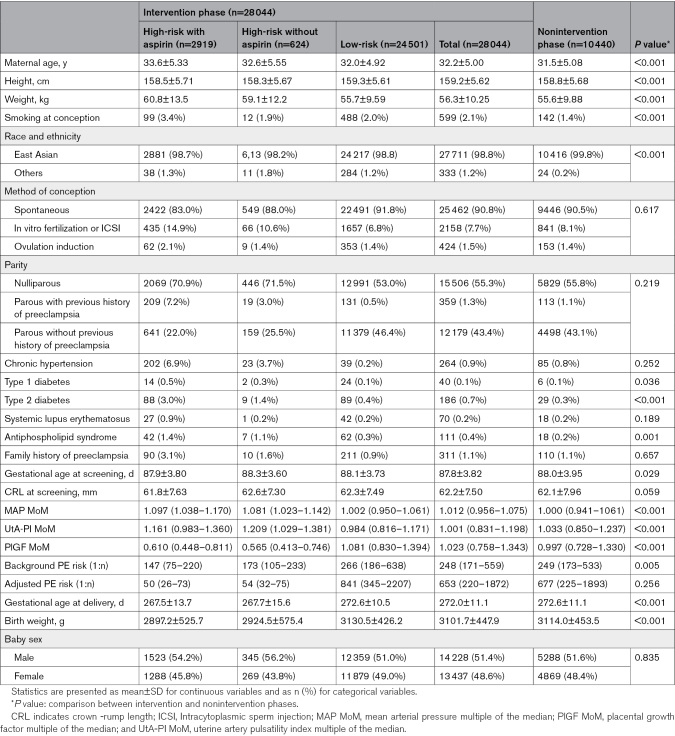
Between August 1, 2019, and February 28, 2022, a total of 48 647 women with singleton pregnancies were offered the first-trimester FMF triple test for preterm preeclampsia screening. Among these women, 5750 (11.82%) declined the screening. Among the remaining 42 897 women, 11 828 (27.57%) participated in the nonintervention phase, whereas 31 069 (72.43%) women participated in the intervention phase. During the follow-up period, an additional 4413 (10.29%) cases were excluded because of miscarriage (n=339), termination of pregnancy (n=390), loss to follow-up (n=3241), and withdrawal from the trial (n=443). As a result, 10 440 (27.13%) women in the nonintervention phase and 28 044 (72.87%) women in the intervention phase were included for analysis. In the intervention phase, 3543 (12.63%) women were identified as high-risk for developing preterm preeclampsia (Figure 2). Table 1 provides an overview of the baseline demographic and clinical characteristics of participants in both the nonintervention and intervention phases. The number of individuals recruited by week and cluster is presented in Table S2.
Figure 2.
Flowchart of population.
Table 1.
Maternal and Pregnancy Characteristics
Predictive Performance of First-Trimester Screening for Preterm Preeclampsia
In the nonintervention cohort, the first-trimester FMF triple test with MAP, UtA-PI, and PlGF achieved an area under the receiver operating characteristic curve of 0.890 (95% CI, 0.851–0.928) and had detection rates of 62.0%, 70.9%, 77.2%, and 78.5% at 5%, 10%, 15%, and 20% fixed false-positive rates, respectively, for the prediction of preterm preeclampsia (Figure S3). On calibration of the model, the intercept was 0.0002, and the calibration slope was 0.846 (95% CI, 0.842–0.850), which was close to 1.0 and suggests a good agreement between the predicted risks and observed incidence of preterm preeclampsia (Figure S4).
Acceptability
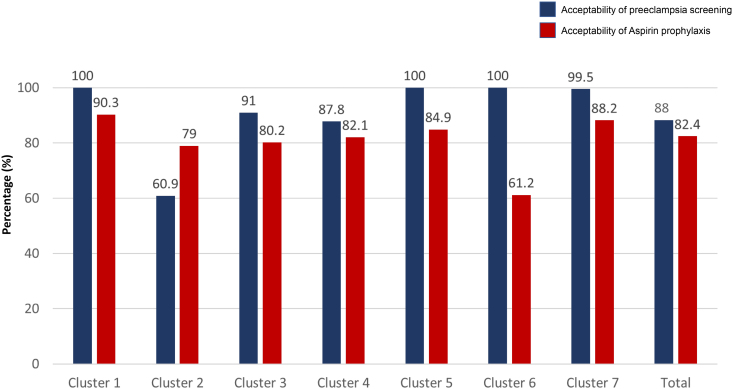
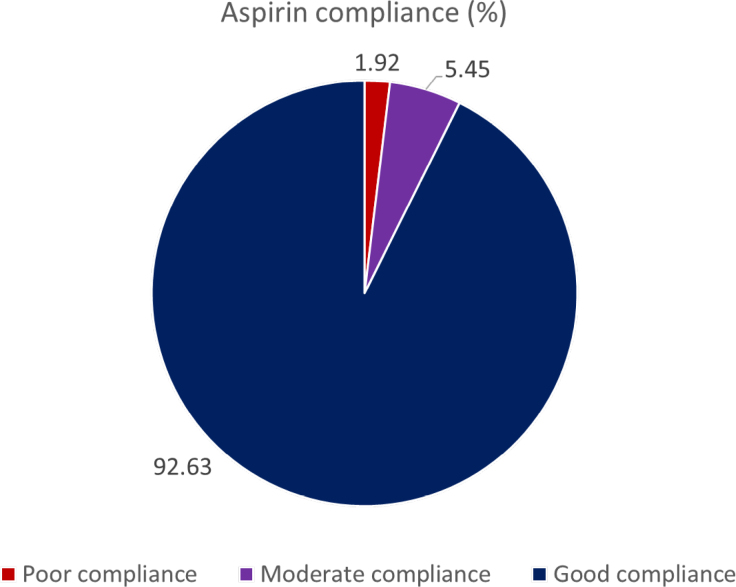
Overall, 88.04% (42 897 of 48 725) of women accepted undergoing first-trimester screening for preterm preeclampsia. Among those identified as high-risk for developing preterm preeclampsia in the intervention phase, 82.39% (2919 of 3543) received aspirin prophylaxis (Figure 3). The main reason for declining aspirin prophylaxis was that some women were already receiving care in another hospital (28.21%; 176 of 624). In addition, some women chose not to attend the counseling session for aspirin prophylaxis because of COVID-19 concerns (16.83%; 105 of 624). Other significant reasons for not taking aspirin included concerns about side effects (15.54%; 97 of 624) and a desire for more evidence about the benefits of aspirin prophylaxis (11.54%; 72 of 624; Table S5). Among the high-risk women who took aspirin during the intervention phase, 92.63% (2704 of 2919) demonstrated good adherence. A moderate level of adherence was observed in 5.45% (159 of 2919) of participants, whereas 1.92% (56 of 2919) of participants exhibited poor adherence (Figure 4).
Figure 3.
Acceptability of preeclampsia screening and aspirin prophylaxis for high-risk women in the intervention phase.
Figure 4.
Percentage of different groups of aspirin compliance.
Efficacy
Primary Outcomes
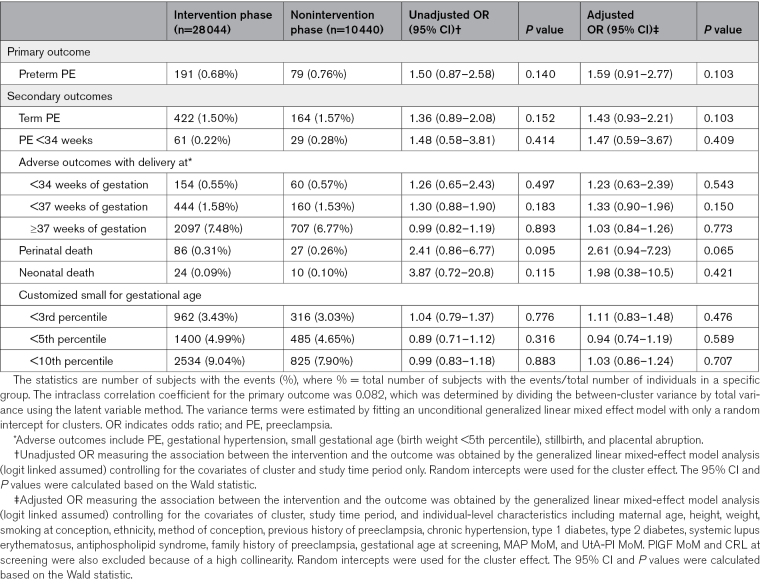
In the intervention phase of the trial, preterm preeclampsia was observed in 191 of 28 044 participants (0.68%), whereas in the nonintervention phase, it occurred in 79 of 10 408 participants (0.76%). However, there was no difference in the incidence of preterm preeclampsia between the intervention and nonintervention phases (aOR, 1.59 [95% CI, 0.91–2.77]; P=0.103; Table 2).
Table 2.
Comparison of Primary and Secondary Outcomes Between Intervention and Nonintervention Phases
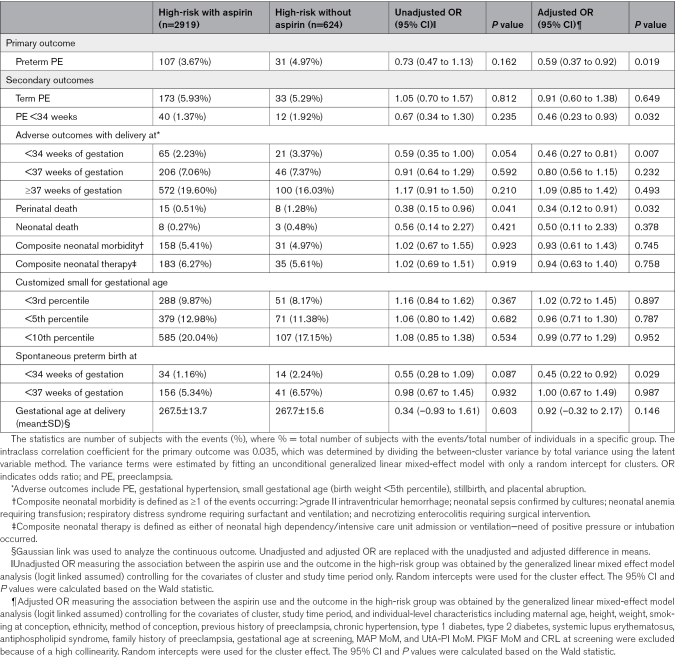
Among high-risk women in the intervention phase, aspirin prophylaxis was significantly associated with a 41% reduction in the incidence of preterm preeclampsia compared with no aspirin treatment (aOR, 0.59 [95% CI, 0.37–0.92]; P=0.019; Table 3). Furthermore, a reduction of 48% was observed when aspirin compliance was ≥90% (aOR, 0.52 [95% CI, 0.33–0.82]; P=0.005; Table S4). A significant reduced risk in preeclampsia was also observed (adjusted hazard ratio, 0.23 [95% CI, 0.15–0.34]; P<0.001) when time-varying treatment effect of aspirin prophylaxis was accounted for.
Table 3.
Comparison of Primary and Secondary Outcomes Between High-Risk Subjects With and Without Using Aspirin in Intervention Phase
Secondary Outcomes
The aspirin effect on secondary outcomes, quantified as aOR with 95% CI, is presented in Table 2 and Table S3. There was no significant difference observed between the intervention and nonintervention phases in the incidence of any secondary outcomes.
Among the high-risk women in the intervention phase, aspirin prophylaxis was associated with a 54%, 54%, 55%, and 76% reduction in the incidence of preeclampsia with delivery at <34 weeks (aOR, 0.46 [95% CI, 0.23–0.93]; P=0.032), maternal composite adverse outcomes with delivery at <34 weeks of gestation (aOR, 0.46 [95% CI, 0.27–0.81]; P=0.007), sPTB at <34 weeks of gestation (aOR, 0.45 [95% CI, 0.22–0.92]; P=0.029), and perinatal death (aOR, 0.34 [95% CI, 0.12–0.91]; P=0.032), respectively (Table 3). When considering women with good aspirin compliance of ≥90%, the effect size of aspirin increased, showing associations with preeclampsia with delivery at <34 weeks (aOR, 0.40 [95% CI, 0.19–0.83]; P=0.013), maternal composite adverse outcomes with delivery <34 weeks of gestation (aOR, 0.39 [95% CI, 0.22–0.70]; P=0.002), sPTB at <34 weeks of gestation (aOR, 0.43 [95% CI, 0.21–0.89]; P=0.023), perinatal death (aOR, 0.32 [95% CI, 0.12–0.90]; P=0.031), and a delayed gestational age at delivery of 1.32 days (adjusted difference in means, 1.32 [95% CI, 0.09–2.54]; P=0.035; Table S4).
Safety
Among the high-risk women taking aspirin, the most commonly reported side effect was dyspepsia or heartburn, which affected 1.13% of participants (33 of 2923). Vaginal bleeding was reported by 0.89% of participants (26 of 2923; Table S6).
On severe adverse events, in the group of high-risk women taking aspirin, 3 participants (0.10%) had composite fetal chromosomal abnormalities, and 21 participants (0.72%) had fetal structural defects. Among the high-risk women who did not take aspirin, no fetal chromosomal abnormalities were reported, and 4 of 621 (0.64%) had fetal structural defects. In addition, the incidences of estimated blood loss of ≥1000 mL during delivery in high-risk women with and without aspirin were 2.37% (69 of 2919) and 1.44% (9 of 624), respectively. The between-group difference in the incidence of these events was not statistically significant (Table S7).
DISCUSSION
This trial, which was conducted in a large Asian population, did not show any significant reduction in the incidence of preterm preeclampsia between the nonintervention and intervention periods. This lack of significance can be attributed to the underrecruitment because of the negative impact of the COVID-19 pandemic. In addition, although the nonintervention phase was implemented in the prepandemic or early stages of the COVID-19 pandemic, the intervention phase was implemented during the critical period of the COVID-19 outbreak when the number of affected cases in the community was extremely high. There is strong evidence suggesting that both asymptomatic and symptomatic COVID-19 infection significantly increases the odds of developing preeclampsia by 1.5-fold and 2-fold, respectively.40 Furthermore, severe COVID-19 has been associated with the development of a preeclampsia-like syndrome,41 which potentially leads to an increased incidence of preterm preeclampsia in the intervention phase. However, we observed that, in the intervention phase, aspirin prophylaxis was associated with a significant reduction of 41% to 48% in the incidence of preterm preeclampsia, compared with high-risk women who did not receive aspirin. The extent of reduction depended on the level of aspirin compliance. This result is comparable to the ASPRE trial (aOR, 0.38 [95% CI, 0.20–0.74]), which was conducted in a mixed European population.17,42 Recent meta-analyses also support the effectiveness of aspirin prophylaxis when initiated before 16 weeks of gestation at a daily dose of ≥100 mg.43–46
Our trial provides further evidence supporting the use of aspirin for high-risk women can effectively prevent the occurrence of maternal composite adverse outcomes, including preeclampsia, gestational hypertension, SGA, stillbirth, and placental abruption requiring delivery <34 weeks of gestation, sPTB <34 weeks of gestation, and perinatal death.46–48 These outcomes fall under the umbrella of great obstetrical syndromes.49,50 It is important to emphasize that great obstetrical syndromes requiring delivery at <34 weeks is strongly associated with a significant increase in the risks of maternal mortality (odds ratio, 9.7 [95% CI, 1.3–71.3]), respiratory morbidity (aOR, 21.0 [95% CI, 15.6–28.3]), cardiovascular morbidity (aOR, 21.6 [95% CI, 14.0–33.4]), acute renal failure (aOR, 32.1 [95% CI, 15.8–64.9])51 and perinatal death or severe neonatal outcomes (aOR, 16.4 [95% CI, 14.5–18.6]).52
In addition, we have previously demonstrated the utility of first-trimester combined tests for predicting various conditions individually, including preeclampsia, SGA, sPTB, and gestational diabetes.3,53–55 However, further studies are required to establish an effective first-trimester combined screening test for the composite outcome great obstetrical syndromes requiring delivery at <34 weeks. It is also crucial to explore alternative therapeutic approaches to maximize the prevention of preterm preeclampsia. There remains a subset of high-risk women who do not respond to aspirin and develop preeclampsia. By adopting a precision medicine approach, we may be able to offer personalized, targeted prophylaxis options for these women. The high acceptance rates of the screening test and aspirin prophylaxis for preterm preeclampsia among Asian women indicates that the screen-and-prevent strategy for preterm preeclampsia can be implemented in different settings across the world. The use of low-dose aspirin was not associated with an increased risk of side effects or severe adverse events, which is in line with the existing literature.17,56
This trial has several strengths. First, this is the first stepped wedge randomized trial evaluating the clinical applicability of the screen-and-prevent strategy for preterm preeclampsia in a completely different population compared to the European cohort where it was originally developed.5 Second, the multicenter stepped wedge design enables assessment of the real-life performance of screening and prevention for preterm preeclampsia, taking into consideration various social-economic factors that can affect the implementation and effectiveness of the strategy, such as resource settings, local policies, and the influence of COVID-19 pandemic measures. In addition, biomarkers were measured using standardized protocols and subjected to regular quality control processes. These biomarkers were then converted into MoMs, and the prediction model was appropriately applied,26 ensuring consistency across different study sites.
However, it is crucial to acknowledge limitations. The COVID-19 pandemic presented significant challenges and had a substantial detrimental impact on the trial. At the beginning of the pandemic, we extensively discussed whether to continue the trial. Understanding the potential clinical benefits of administering aspirin prophylaxis to high-risk women, all site investigators agreed to continue the trial for as long as possible during the pandemic. Because of pandemic-related constraints, the intended sample size of 68 000 pregnant women was not achieved, resulting in a 20-week trial extension and reduced statistical power because of a decrease of sample size to 42 897. The pandemic-related preventive measures, such as social distancing, travel restrictions, and fear of face-to-face contact, adversely affected recruitment rates and led to a higher rate of loss to follow-up (7.8%) among the high-risk women. It is also important to note that a small percentage of the low-risk women (0.11%; 27 of 24 529) chose to use low-dose aspirin based on recommendations from private doctors. However, this has had no impact on the findings. Another limitation is that aspirin compliance was evaluated using a self-reported method, which can lead to recall bias, although it is the most commonly used method.57 Platelet activation and function assays,58 as well as metabolomic analysis,59 exist that can provide a more objective and accurate assessment of direct aspirin adherence. However, high costs, limited reproducibility, and the need for laboratory expertise limit their use in clinical studies.58,60 A significant limitation arose because of the selection of recruiting centers. These centers were primarily located in relatively larger and more developed cities in Asia, capable of offering first-trimester clinical visits. Despite the inherent selection bias, ensuring the feasibility of the trial remained a priority. In addition, we acknowledge the potential presence of time-varying treatment effect.61,62 According to existing evidence,59 aspirin prophylaxis would take effect when it is initiated between 11 and 15 weeks and completed at 36 weeks. The treatment period is therefore approximately 20 weeks. In this trial, >90% of high-risk women in the aspirin group have received at least 20 weeks of aspirin prophylaxis, and we believe that using the typical immediate treatment effect does not influence our results. Apart from that, we acknowledge that the intraclass correlation coefficient of the primary analysis is higher than our assumed value in sample size based on pilot data (0.082 versus 0.001), resulting in an underestimation of study power.
In conclusion, our results demonstrate that: (1) the implementation of the screen-and-prevent strategy for preterm preeclampsia is not associated with a significant reduction in the incidence of preterm preeclampsia, but in the intervention phase, low-dose aspirin prophylaxis effectively reduces the incidence of preterm preeclampsia by 41% among the high-risk women; (2) the screen-and-prevent strategy for preterm preeclampsia is highly accepted by a large number of women with different ethnic backgrounds from the original population where the strategy was developed; and (3) low-dose aspirin is considered a safe intervention during pregnancy. These findings underpin the importance of the widespread implementation of the screen-and-prevent strategy for preterm preeclampsia on a global scale.
ARTICLE INFORMATION
This work was presented as an abstract at the 21st World Congress in Fetal Medicine, Lisbon, Portugal, June 23–27, 2024.
Acknowledgments
The authors thank all the participants and their attending obstetricians, nurses, midwives, research assistants, sonographers, and the medical professionals who helped in the recruitment and follow-up of participants.
L.C.P. conceptualized the trial. L.C.P. and M.K.C.C. contributed to the trial design. L.C.P. supervised trial implementation. L.C.P., L.N.-H., and D.S.S. supervised data collection. M.K.C.C. was the lead statistician and led the development of the statistical analysis plan. L.C.P., M.K.C.C., and L.N.-H. directly accessed and verified the underlying data reported in the article and analyzed the data. L.C.P. and L.N.-H. led interpretation of the data. L.N.-H. and M.K.C.C. drafted the tables and figures. L.N.-H. and L.C.P. wrote the first draft of the article. L.N.-H., L.T.D., A.S.T.T., D.-A.N., R.K.P., A.S., M.Z., Y.H., B.L., A.K., P.Y., A.G., M.K., S.L., T.-Y.C., N.C., T.N., H.L., S.W.S., W.C.L., Z.A.M., A.A., N.M.W.L., N.H.Y.L., S.L.L., I.Y.M.W., X.L., D.S.S., M.K.C.C., and L.C.P. reviewed the article and approved the final draft. All authors had full access to all the data in the trial and had final responsibility for the decision to submit for publication.
Sources of Funding
Reagents and equipment for the measurement of serum PlGF were provided at no cost by Thermo Fisher Scientific, Hennigsdorf, Germany; Revvity Inc (formerly PerkinElmer Life and Analytical Sciences), Waltham, MA; and Roche Diagnostics, Rotkreuz, Switzerland. The PE risk calculators, including Viewpoint, Astraia, and Lifecycle, were provided at no cost for the duration of the trial by GE HealthCare Technologies Inc, Chicago, IL; Astraia GmbH, Munich, Germany; and Revvity Inc, respectively. This trial was also supported by a start-up grant from the faculty of medicine, Chinese University of Hong Kong. The study sponsors were not involved in the study design, data collection, analysis, interpretation, report writing, or decision to submit the article for publication.
Disclosures
L.C.P. has received speaker fees and consultancy payments from Roche Diagnostics and Ferring Pharmaceuticals. In addition, she has received in-kind contributions from Roche Diagnostics, Revvity Inc (formerly PerkinElmer Life and Analytical Sciences), Thermo Fisher Scientific, Ningbo Aucheer Biological Technology Co, Ltd, and GE HealthCare. D.S.S. has received in-kind contributions from Revvity Inc, Thermo Fisher Scientific, Roche Diagnostics, Diabetomics, and Ningbo Aucheer Biological Technology Co, Ltd. R.K.P. is chief executive officer of Ritz Medical Co Ltd, a genetic testing company, and holds shares in and receives executive compensation from it. The other authors report no conflicts.
Supplemental Materials
Figures S1–S4
Tables S1–S7
APPENDIX
FORECAST Collaborators
Iok Seng Wong, Yunyu Chen, Jiao Liu, Jing Lin, Ada W. T. Tse, Lo Wong, Fangzi Liu (Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China), Runmei Ma (Kunming Angel Women and Children’s Hospital, Teaching Hospital of Kunming University of Science and Technology, Kunming, China), Jiang Yan Min (Department of Obstetrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China), Mahesh A. Choolani (Yong Loo Lin School of Medicine, National University of Singapore, Singapore), Tuangsit Wataganara (Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand), Osamu Shimokawa (Clinical Laboratory, Ritz Medical Co, Ltd, Osaka, Japan)
Supplementary Material
Nonstandard Abbreviations and Acronyms
- aOR
- adjusted odds ratio
- ASPRE
- Combined Multimarker Screening and Randomised Patient Treatment With Aspirin for Evidence-Based Preeclampsia Prevention
- FMF
- Fetal Medicine Foundation
- MAP
- mean arterial pressure
- MoM
- multiples of the median
- PlGF
- placental growth factor
- SGA
- small for gestational age
- sPTB
- spontaneous preterm birth
- UtA-PI
- uterine artery pulsatility index
L. Nguyen-Hoang and L.T. Dinh contributed equally.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.124.069907.
For Sources of Funding and Disclosures, see page 1234.
Circulation is available at www.ahajournals.org/journal/circ .
Contributor Information
Linh Thuy Dinh, Email: drdinhlinhobgyn@gmail.com.
Angela S.T. Tai, Email: angelatai1211@gmail.com.
Duy-Anh Nguyen, Email: anhnguyen.hogh@gmail.com.
Ritsuko K. Pooh, Email: ritsuko.pooh.brain@fetal-medicine-pooh.jp.
Arihiro Shiozaki, Email: as1000039709@gmail.com.
Mingming Zheng, Email: drmingmingzheng@163.com.
Yali Hu, Email: huyali57@163.com.
Bin Li, Email: ynuleebin@126.com.
Piengbulan Yapan, Email: pong_lovelymoon@hotmail.com.
Arundhati Gosavi, Email: obggat@nus.edu.sg.
Suchaya Luewan, Email: suchaya.l@cmu.ac.th.
Tung-Yao Chang, Email: tychang@fetalmedicine.tw.
Noppadol Chaiyasit, Email: nchaiyas@gmail.com.
Tongta Nanthakomon, Email: ntongta@gmail.com.
Huishu Liu, Email: huishuliu@hotmail.com.
Steven W. Shaw, Email: dr.shaw@me.com.
Wing Cheong Leung, Email: hiuyuhillaryleung@cuhk.edu.hk.
Zaleha Abdullah Mahdy, Email: zaleha@ppukm.ukm.edu.my.
Angela Aguilar, Email: angelasaguilarmd@gmail.com.
Hillary H.Y. Leung, Email: hiuyuhillaryleung@cuhk.edu.hk.
Nikki M.W. Lee, Email: nikkimaywinglee@cuhk.edu.hk.
Iok Seng Wong, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China.
Yunyu Chen, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China.
Jiao Liu, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China.
Jing Lin, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China.
Ada W. T. Tse, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China
Lo Wong, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China.
Fangzi Liu, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Chinese University of Hong Kong, Hong Kong SAR, China.
Runmei Ma, Kunming Angel Women and Children’s Hospital, Teaching Hospital of Kunming University of Science and Technology, Kunming, China.
Jiang Yan Min, Department of Obstetrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China.
Mahesh A. Choolani, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Tuangsit Wataganara, Faculty of Medicine, Siriraj Hospital, Bangkok, Thailand.
Osamu Shimokawa, Clinical Laboratory, Ritz Medical Co, Ltd, Osaka, Japan.
Collaborators: Iok Seng Wong, Yunyu Chen, Jiao Liu, Jing Lin, Ada W. T. Tse, Lo Wong, Fangzi Liu, Runmei Ma, Jiang Yan Min, Mahesh A. Choolani, Tuangsit Wataganara, and Osamu Shimokawa
REFERENCES
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631–644. doi: 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- 2.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011. doi: 10.1016/S0140-6736(15)00070-7 [DOI] [PubMed] [Google Scholar]
- 3.Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, Shaw SW, Seshadri S, Choolani M, Wataganara T, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221:650.e1–650.e16. doi: 10.1016/j.ajog.2019.09.041 [DOI] [PubMed] [Google Scholar]
- 4.Say L, Chou D, Gemmill A, Tunçalp O, Moller A-B, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 5.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145:1–33. doi: 10.1002/ijgo.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayrink J, Costa ML, Cecatti JG. Preeclampsia in 2018: revisiting concepts, physiopathology, and prediction. ScientificWorldJournal. 2018;2018:6268276. doi: 10.1155/2018/6268276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller EC, Boehme AK, Chung NT, Wang SS, Lacey JV, Lakshminarayan K, Zhong C, Woo D, Bello NA, Wapner R, et al. Aspirin reduces long-term stroke risk in women with prior hypertensive disorders of pregnancy. Neurology. 2019;92:e305–e316. doi: 10.1212/WNL.0000000000006815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papageorghiou AT. Predicting and preventing pre-eclampsia-where to next? Ultrasound Obstet Gynecol. 2008;31:367–370. doi: 10.1002/uog.5320 [DOI] [PubMed] [Google Scholar]
- 9.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060 [DOI] [PubMed] [Google Scholar]
- 10.Roberts JM, Rich-Edwards JW, McElrath TF, Garmire L, Myatt L; Global Pregnancy Collaboration. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension. 2021;77:1430–1441. doi: 10.1161/HYPERTENSIONAHA.120.14781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orabona R, Donzelli CM, Falchetti M, Santoro A, Valcamonico A, Frusca T. Placental histological patterns and uterine artery Doppler velocimetry in pregnancies complicated by early or late pre-eclampsia. Ultrasound Obstet Gynecol. 2016;47:580–585. doi: 10.1002/uog.15799 [DOI] [PubMed] [Google Scholar]
- 12.von Dadelszen P, Payne B, Li J, Ansermino JM, Broughton Pipkin F, Côté A-M, Douglas MJ, Gruslin A, Hutcheon JA, Joseph KS, et al. ; PIERS Study Group. Prediction of adverse maternal outcomes in pre-eclampsia: development and validation of the fullPIERS model. Lancet. 2011;377:219–227. doi: 10.1016/S0140-6736(10)61351-7 [DOI] [PubMed] [Google Scholar]
- 13.Kim YM, Chaemsaithong P, Romero R, Shaman M, Kim CJ, Kim J-S, Qureshi F, Jacques SM, Ahmed AI, Chaiworapongsa T, et al. The frequency of acute atherosis in normal pregnancy and preterm labor, preeclampsia, small-for-gestational age, fetal death and midtrimester spontaneous abortion. J Matern Fetal Neonatal Med. 2015;28:2001–2009. doi: 10.3109/14767058.2014.976198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lekva T, Sugulle M, Moe K, Redman C, Dechend R, Staff AC. Multiplex analysis of circulating maternal cardiovascular biomarkers comparing preeclampsia subtypes. Hypertension. 2020;75:1513–1522. doi: 10.1161/HYPERTENSIONAHA.119.14580 [DOI] [PubMed] [Google Scholar]
- 15.Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641–652. doi: 10.1515/jpm.2011.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, Yeo L, Romero R. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol. 2014;10:531–540. doi: 10.1038/nrneph.2014.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377:613–622. doi: 10.1056/NEJMoa1704559 [DOI] [PubMed] [Google Scholar]
- 18.Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC, et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022;27:148–169. doi: 10.1016/j.preghy.2021.09.008 [DOI] [PubMed] [Google Scholar]
- 19.Sotiriadis A, Hernandez-Andrade E, da Silva Costa F, Ghi T, Glanc P, Khalil A, Martins WP, Odibo AO, Papageorghiou AT, Salomon LJ, et al. ; ISUOG CSC Pre-eclampsia Task Force. ISUOG practice guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet Gynecol. 2019;53:7–22. doi: 10.1002/uog.20105 [DOI] [PubMed] [Google Scholar]
- 20.Queensland Clinical Guidelines. Hypertension and pregnancy. Guideline No. MN21.13-V9-R26. Queensland Health. February 2021. Accessed December 23, 2023. https://www.health.qld.gov.au/__data/assets/pdf_file/0034/139948/g-hdp.pdf [Google Scholar]
- 21.Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int J Gynaecol Obstet. 2019;145:1–33. doi: 10.1002/ijgo.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright D, Wright A, Nicolaides KH. The competing risk approach for prediction of preeclampsia. Am J Obstet Gynecol. 2020;223:12–23.e7. doi: 10.1016/j.ajog.2019.11.1247 [DOI] [PubMed] [Google Scholar]
- 23.Chaemsaithong P, Pooh RK, Zheng M, Ma R, Chaiyasit N, Tokunaka M, Shaw SW, Seshadri S, Choolani M, Wataganara T, et al. Prospective evaluation of screening performance of first-trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221:650.e1–650.e16. doi: 10.1016/j.ajog.2019.09.041 [DOI] [PubMed] [Google Scholar]
- 24.O’Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11-13 weeks gestation. Am J Obstet Gynecol. 2016;214:103.e1–103.e12. doi: 10.1016/j.ajog.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 25.Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, Singh M, Greco E, Wright A, Maclagan K, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstetr Gynecol. 2018;51:743–750. doi: 10.1002/uog.19039 [DOI] [PubMed] [Google Scholar]
- 26.Chaemsaithong P, Sahota D, Pooh RK, Zheng M, Ma R, Chaiyasit N, Koide K, Shaw SW, Seshadri S, Choolani M, et al. First-trimester pre-eclampsia biomarker profiles in Asian population: multicenter cohort study. Ultrasound Obstet Gynecol. 2020;56:206–214. doi: 10.1002/uog.21905 [DOI] [PubMed] [Google Scholar]
- 27.Sahota DS, Leung TY, Leung TN, Chan OK, Lau TK. Fetal crown-rump length and estimation of gestational age in an ethnic Chinese population. Ultrasound Obstet Gynecol. 2009;33:157–160. doi: 10.1002/uog.6252 [DOI] [PubMed] [Google Scholar]
- 28.Wright D, Syngelaki A, Akolekar R, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am J Obstet Gynecol. 2015;213:62.e1–62.e10. doi: 10.1016/j.ajog.2015.02.018 [DOI] [PubMed] [Google Scholar]
- 29.Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11-13 weeks’ gestation. Fetal Diagn Ther. 2012;31:42–48. doi: 10.1159/000335366 [DOI] [PubMed] [Google Scholar]
- 30.Chaemsaithong P, Ting YH, Cheng KYY, Poon CYL, Leung TY, Sahota DS. Uterine artery pulsatility index in the first trimester: assessment of intersonographer and intersampling site measurement differences. J Matern Fetal Neonatal Med. 2018;31:2276–2283. doi: 10.1080/14767058.2017.1341481 [DOI] [PubMed] [Google Scholar]
- 31.Khalil A, Nicolaides KH. How to record uterine artery Doppler in the first trimester. Ultrasound Obstet Gynecol. 2013;42:478–479. doi: 10.1002/uog.12366 [DOI] [PubMed] [Google Scholar]
- 32.Drouin O, Johnson JA, Chaemsaithong P, Metcalfe A, Huber J, Schwarzenberger J, Winters E, Stavness L, Tse AWT, Lu J, et al. Transverse technique: complementary approach to measurement of first-trimester uterine artery Doppler. Ultrasound Obstet Gynecol. 2018;52:639–647. doi: 10.1002/uog.18917 [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. World Health Organization; 2010. Accessed December 23, 2023. https://www.who.int/publications/i/item/9789241599221. [PubMed]
- 34.Roberts L, Chaemsaithong P, Sahota DS, Nicolaides KH, Poon LCY. Protocol for measurement of mean arterial pressure at 10-40 weeks’ gestation. Pregnancy Hypertens. 2017;10:155–160. doi: 10.1016/j.preghy.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 35.Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;30:742–749. doi: 10.1002/uog.5157 [DOI] [PubMed] [Google Scholar]
- 36.Tan MY, Syngelaki A, Poon LC, Rolnik DL, O'Gorman N, Delgado JL, Akolekar R, Konstantinidou L, Tsavdaridou M, Galeva S, et al. Screening for pre-eclampsia by maternal factors and biomarkers at 11-13 weeks’ gestation. Ultrasound Obstet Gynecol. 2018;52:186–195. doi: 10.1002/uog.19112 [DOI] [PubMed] [Google Scholar]
- 37.Sahota DS, Kagan KO, Lau TK, Leung TY, Nicolaides KH. Customized birth weight: coefficients and validation of models in a UK population. Ultrasound Obstet Gynecol. 2008;32:884–889. doi: 10.1002/uog.5372 [DOI] [PubMed] [Google Scholar]
- 38.Di Renzo GC, Roura LC, Facchinetti F, Antsaklis A, Breborowicz G, Gratacos E, Husslein P, Lamont R, Mikhailov A, Montenegro N, et al. Guidelines for the management of spontaneous preterm labor: identification of spontaneous preterm labor, diagnosis of preterm premature rupture of membranes, and preventive tools for preterm birth. J Matern Fetal Neonatal Med. 2011;24:659–667. doi: 10.3109/14767058.2011.553694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials [published online July 7, 2006]. Contemp Clin Trials. 2007 Feb;28(2):182-91. doi: 10.1016/j.cct.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 39.Goldstein H, Browne W, Rasbash J. Partitioning Variation in Multilevel Models. Understanding Statistics, 1 (4), 223–231. https://doi.org/10.1207/S15328031US0104_02 [Google Scholar]
- 40.Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68–89.e3. doi: 10.1016/j.ajog.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serrano B, Bonacina E, Garcia-Ruiz I, Mendoza M, Garcia-Manau P, Garcia-Aguilar P, Gil J, Armengol-Alsina M, Fernández-Hidalgo N, Sulleiro E, et al. Confirmation of preeclampsia-like syndrome induced by severe COVID-19: an observational study. Am J Obstetr Gynecol MFM. 2023;5:100760. doi: 10.1016/j.ajogmf.2022.100760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright D, Poon LC, Rolnik DL, Syngelaki A, Delgado JL, Vojtassakova D, de Alvarado M, Kapeti E, Rehal A, Pazos A, et al. Aspirin for evidence-based preeclampsia prevention trial: influence of compliance on beneficial effect of aspirin in prevention of preterm preeclampsia. Am J Obstet Gynecol. 2017;217:685.e1–685.e5. doi: 10.1016/j.ajog.2017.08.110 [DOI] [PubMed] [Google Scholar]
- 43.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216:110–120.e6. doi: 10.1016/j.ajog.2016.09.076 [DOI] [PubMed] [Google Scholar]
- 44.Cui Y, Zhu B, Zheng F. Low-dose aspirin at </=16 weeks of gestation for preventing preeclampsia and its maternal and neonatal adverse outcomes: a systematic review and meta-analysis. Exp Ther Med. 2018;15:4361–4369. doi: 10.3892/etm.2018.5972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2018;218:287–293.e1. doi: 10.1016/j.ajog.2017.11.561 [DOI] [PubMed] [Google Scholar]
- 46.Henderson JT, Vesco KK, Senger CA, Thomas RG, Redmond N. Aspirin use to prevent preeclampsia and related morbidity and mortality: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:1192–1206. doi: 10.1001/jama.2021.8551 [DOI] [PubMed] [Google Scholar]
- 47.Turner JM, Robertson NT, Hartel G, Kumar S. Impact of low-dose aspirin on adverse perinatal outcome: meta-analysis and meta-regression. Ultrasound Obstet Gynecol. 2020;55:157–169. doi: 10.1002/uog.20859 [DOI] [PubMed] [Google Scholar]
- 48.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019:CD004659. doi: 10.1002/14651858.CD004659.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171 [DOI] [PubMed] [Google Scholar]
- 50.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–635. doi: 10.1080/14767050902866804 [DOI] [PubMed] [Google Scholar]
- 51.Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124:771–781. doi: 10.1097/AOG.0000000000000472 [DOI] [PubMed] [Google Scholar]
- 52.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019 [DOI] [PubMed] [Google Scholar]
- 53.Nguyen-Hoang L, Papastefanou I, Sahota DS, Pooh RK, Zheng M, Chaiyasit N, Tokunaka M, Shaw SW, Seshadri S, Choolani M, et al. Collaborators. Evaluation of screening performance of first-trimester competing-risks prediction model for small-for-gestational age in Asian population. Ultrasound Obstet Gynecol. 2024;63:331–341. doi: 10.1002/uog.27447 [DOI] [PubMed] [Google Scholar]
- 54.Chiu CPH, Feng Q, Chaemsaithong P, Sahota DS, Lau YY, Yeung YK, Yim LW, Chung JPW, Poon LC. Prediction of spontaneous preterm birth and preterm prelabor rupture of membranes using maternal factors, obstetric history and biomarkers of placental function at 11-13 weeks. Ultrasound Obstet Gynecol. 2022;60:192–199. doi: 10.1002/uog.24917 [DOI] [PubMed] [Google Scholar]
- 55.Shen L, Sahota DS, Chaemsaithong P, Tse WT, Chung MY, Ip JKH, Leung TY, Poon LCY. First trimester screening for gestational diabetes mellitus with maternal factors and biomarkers. Fetal Diagn Ther. 2022;49:256–264. doi: 10.1159/000525384 [DOI] [PubMed] [Google Scholar]
- 56.Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2014;160:695–703. doi: 10.7326/M13-2844 [DOI] [PubMed] [Google Scholar]
- 57.Oladejo M, Bewley S. Adherence in pregnancy: a systematic review of the literature. Fetal Maternal Med Rev. 2012;23:201–229. doi: 10.1017/s0965539512000113 [Google Scholar]
- 58.Shanmugalingam R, Wang X, Motum P, Fulcher I, Lee G, Kumar R, Hennessy A, Makris A. Clinical influence of nonadherence with prophylactic aspirin in preventing preeclampsia in high-risk pregnancies: a multicenter, prospective, observational cohort study. Hypertension. 2020;75:1125–1132. doi: 10.1161/HYPERTENSIONAHA.119.14107 [DOI] [PubMed] [Google Scholar]
- 59.Li X, Milosavljevic A, Elsea SH, Wang CC, Scaglia F, Syngelaki A, Nicolaides KH, Poon LC. Effective aspirin treatment of women at risk for preeclampsia delays the metabolic clock of gestation. Hypertension. 2021;78:1398–1410. doi: 10.1161/HYPERTENSIONAHA.121.17448 [DOI] [PubMed] [Google Scholar]
- 60.Navaratnam K, Alfirevic A, Alfirevic Z. Low dose aspirin and pregnancy: how important is aspirin resistance? BJOG. 2016;123:1481–1487. doi: 10.1111/1471-0528.13914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kenny A, Voldal EC, Xia F, Heagerty PJ, Hughes JP. Analysis of stepped wedge cluster randomized trials in the presence of a time-varying treatment effect. Stat Med. 2022;41:4311–4339. doi: 10.1002/sim.9511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maleyeff L, Li F, Haneuse S, Wang R. Assessing exposure-time treatment effect heterogeneity in stepped-wedge cluster randomized trials. Biometrics. 2023;79:2551–2564. doi: 10.1111/biom.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.