Abstract
Neutral memories can be modulated via intentional memory control paradigms such as directed forgetting. In addition, previous studies have shown that neutral visual memories can be modulated indirectly, via remember and forget instructions towards competing verbal memories. Here we show that direct modulation of neutral verbal memory strength is impaired by negative visual context, and that negative visual context is resistant to indirect memory modulation. Participants were directly instructed to intentionally remember or forget newly encoded neutral verbal information. Importantly, this verbal information was interleaved with embedded negative visual context. Results showed that negative visual context eliminated the well-documented effect of direct instructions to intentionally remember verbal content. Furthermore, negative visual memory was highly persistent, overcoming its sensitivity to indirect modulation shown in previous studies. Finally, these memory effects persisted to the following day. These results demonstrate the dominance of negative visual context over neutral content, highlighting the challenges associated with memory modulation in psychopathologies involving maladaptive processing of negative visual memories.
Introduction
In daily life, we often experience events we wish to forget. Humans can actively forget unwanted memories through intentional memory control, such as directed forgetting (DF), in which individuals are instructed to remember or forget newly encoded information [1, 2]. Studies have shown that the instruction to forget, compared to the instruction to remember, prompts prefrontal inhibitory control over mnemonic hippocampal activity, resulting in decreased memory strength [1, 2]. Thus far, DF paradigms demonstrated reliable direct memory modulation effects for neutral stimuli [3–6]. Additional studies have shown that neutral memories can be modified indirectly during online processing [7–10], and post-encoding via mechanisms of memory competition [11].
Nevertheless, modulation of negative memories is a more challenging endeavor. Negative emotional memories show high resistance to deliberate suppression [12–14], and require extensive neuro-cognitive engagement [15–19]. Following their consolidation, adverse memories become even less susceptible to direct modulation [14, 20, 21]. Consolidated emotional memories become distributed across multiple neural networks [21], possibly persisting through involuntary recurrent reactivation, and in some cases result in pathological outcomes such as intrusive memories [22, 23]. In the same vein, effective regulation of negative memories can support resilience and mental health [24–26], and is key in the treatment of psychopathologies such as depression, fear disorders, and post-traumatic stress disorder (PTSD) [27–29].
Previously, we found that neutral visual context is susceptible to indirect memory modulation [11]. Here, we show that negative visual context attenuates direct modulation of neutral verbal memory strength, and that this negative visual context is resistant to indirect memory modulation. Participants studied two lists of neutral words, embedded within negative pictorial context. To test direct modulation of neutral memory, participants received a cue to either forget or remember the words from the list they had just studied. To evaluate indirect modulation of the emotional memory, participants did not receive any instructions regarding the negative pictures (Fig 1A) (see Materials and methods). After completing the study phase, we measured verbal and visual memory through free recall and two-alternative forced choice recognition tests, respectively (Fig 1B). To monitor memory persistence effects, participants returned to the lab on the following day to perform another verbal and visual memory test.
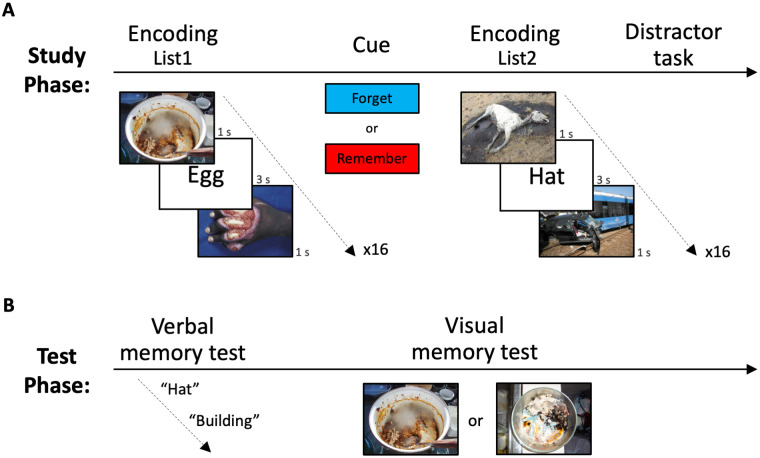
Fig 1. Study design.
(A) Participants were instructed to study two lists of neutral words embedded within negative pictures. After studying List1, participants were presented with a cue to either forget or remember the studied words. (B) On the day of encoding and on the following day, we measured participant’s memory strength for the words (direct memory modulation), and the pictures (indirect memory modulation). The pictures illustrated are from the DIsgust-RelaTed-Images (DIRTI), and the Nencki Affective Picture System (NAPS) databases [35, 37].
Materials and methods
Participants
Forty-three healthy volunteers (22 females, Mage = 23.84 years, SD = 2.45, Range = 18–30) were recruited through online and printed advertisements. All participants reported at least 6 hours of sleep the night before the experimental sessions, and reported no history of neurological, physical, or mental disorders. One participant was excluded due to difficulty in understanding task instructions and two participants did not attend the second day of the study, one due to the emotional content of the experiment and the other for medical reasons. Sample size was determined based on an averaged reported effect size (η2p = 0.127, power = 0.95) found in previous list-method directed forgetting studies [30–32] and in accordance with previous experiments involving a similar paradigm (n = 40, see main experiment in [11]). Study recruitment was conducted between 2/6/2021-4/09/2022. Participants provided written informed consent prior to testing and were compensated $20 or course credit. The Tel Aviv University Institutional Review Board approved the study, and all methods were performed in accordance with the relevant guidelines and regulations.
Procedure
Participants performed a directed forgetting paradigm, with emotionally negative pictures interleaved between the to be remembered/forgotten words [11]. Participants observed two lists of neutral words (16 per list), embedded within negative pictorial context (32 per list) [33–37], with each trial presenting a triplet of picture-word-picture (Fig 1A). To avoid semantic overlap between presented words and pictures, the studied words were of objects with minimum relation to the pictures that depicted negative scenes. Each word was presented for 3 sec and each picture for 1 sec. Inter-stimulus-interval (ISI) between trials was 3 sec. Both words and pictures were randomized across and within lists [11].
Participants were told that they are about to see a list of words interleaved with pictures, and were instructed to study the words for a future memory test. Importantly, these instructions were given only regarding the words (direct memory modulation), and not the pictures (indirect memory modulation). After studying the words of List1, participants received a cue to either forget (n = 20) or remember (n = 22) this list of words. All participants were instructed to remember the words of List2. To reduce recency effects and deliberate words memorization, participants solved arithmetic problems for 1 minute as a distractor before the test phase [38, 39] (Fig 1A).
Participants’ verbal and visual memory strength was measured immediately following the distractor task (Fig 1B). First, participants were asked to write as many words as they can recall from both studied lists (List1 and List2), including from the list they were instructed to forget (List1). There was no time limit for this test. Next, participants performed a visual memory test on all the pictures they have seen during the study phase. In each trial participants saw two pictures on the screen, one which previously appeared in the study phase, and a foil picture with the same semantic content and similar visual features. Participants had to choose the picture they have seen before and were asked to answer as quickly and as accurately as possible.
To monitor memory persistence effects, participants returned to the lab on the following day to perform another verbal and visual memory test on the same stimuli encoded the previous day. Participants performed the second day memory tests in the same laboratory room, with the same research assistant and approximately at the same time of the day. Following the memory test participants rated the valence of the study pictures [40] to assure that the pictures were perceived as negative. Participants were exposed to all studied negative pictures (N = 64) together with new 32 neutral valence pictures, operationalized as pictures with scores between 4 and 6 on a 1–9 Self-Assessment Manikin scale [11]. Participants’ ratings confirmed that the pictures were experienced as negative. Participants rated the study pictures as negative (M = 3.16, SE = 0.09) (rating lower than 4 on the Manikin scale), relative to neutral pictures, in both experimental conditions (F(1,38) = 378.389, p<0.001, η2p = 0.909).
Data analysis
Only exact recall of the studied words, or words with phonetically equivalent spelling mistakes (< 1.1%) were scored by the coder as correct answers in the verbal memory test. For each list of words and pictures we calculated the percent of correct responses. We then winsorized data points falling above two standard-deviations from the mean in each experimental condition and list to the highest score inside the range of -2<Z<2 [41]. In the picture recognition test, we excluded trials with a response time lower than 300 ms (< 0.001%) [11]. We also calculated, for each list, the average valence rating of the negative contextual pictures.
A two-way mixed ANOVA was performed for word or picture memory strength, with instruction (remember/forget) as a between-subjects factor and list (List 1, List 2) as a within-subject factor. We applied nonparametric permutation analysis for comparisons that did not withstand the equal variance assumption. Null results were additionally confirmed with a complimentary Bayesian analysis with instruction (remember/forget) as a between-subjects factor and list (List 1, List 2) as a within-subject factor. Bayesian analysis was performed using the statistical program JASP and its default priors (https://jasp-stats.org/).
Results
Memory performance day 1
Verbal memory
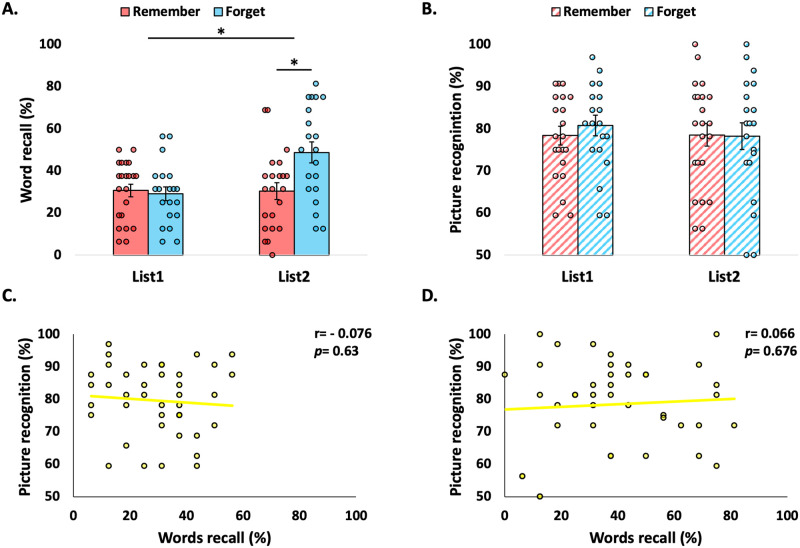
Verbal memory strength, under the influence of negative visual context, showed an instruction x list interaction (F(1,40) = 7.513, p = 0.009, η2p = 0.158). Negative context eliminated the well-documented directed forgetting cost effect [42, 43] (Fig 2A, left panel), i.e., the beneficial effect of the instruction to remember the words compared to the instruction to forget the words, resulting in similar List1 verbal memory strength under both conditions (t(40) = -0.366, p = 0.717, d = -0.113; further supported by a complimentary Bayesian analysis, BF01 = 3.125, error % = 0.014). This was contrary to neutral visual context, which did not affect DF memory modulation in our previous study [11]. However, under negative context, verbal memory strength on List2 was greater following the instruction to forget the words, compared to the instruction to remember the words (t(40) = 2.89, p = 0.006, d = 0.896), also known as the directed forgetting benefit effect [44, 45] (Fig 2A, right panel).
Fig 2. The dominance of negative visual context over neutral verbal memory.
(A) Under negative context, the instruction to remember List1 words (light red) did not enhance verbal memory, compared to the instruction to forget the words (light blue). List2 verbal memory strength was greater following the instruction to forget the words (light blue), relative to the instruction to remember the words (light red). (B) Negative visual memory showed resistance to indirect modulation, with comparable visual memory strength in List1 versus List2 when participants were instructed to remember or to forget the words. (C) There was no correlation between verbal and visual memory strength for List1. (D) Similarly, there was no correlation between verbal and visual memory strength for List2. Error bars represent ±1 Standard error of the mean (S.E.M.) *p<0.05.
Negative visual memory
Next, we asked whether instructions to remember/forget the words indirectly modulated negative visual memory strength. Results showed that negative visual memory was resistant to the established indirect modulation effect shown with neutral memories [11], with comparable visual memory strength in List1 relative to List2 when instructions were to remember or forget the words (F(1,40) = 0.486, p = 0.49, η2p = 0.012; further supported by a complimentary Bayesian analysis, BF01 = 29.861, error % = 1.691) (Fig 2B). Additionally, in contrast to a previous study with neutral visual context [11], when using negative visual context there was no significant correlation between verbal and visual memory strength neither for List1 (r = -0.076, 95% CI[0.225,-0.36], p = 0.63, BF01 = 4.651) (Fig 2C) nor for List2 (r = 0.066, 95% CI[0.351,-0.234], p = 0.676, BF01 = 4.78) (Fig 2D).
Memory performance day 2
Verbal memory
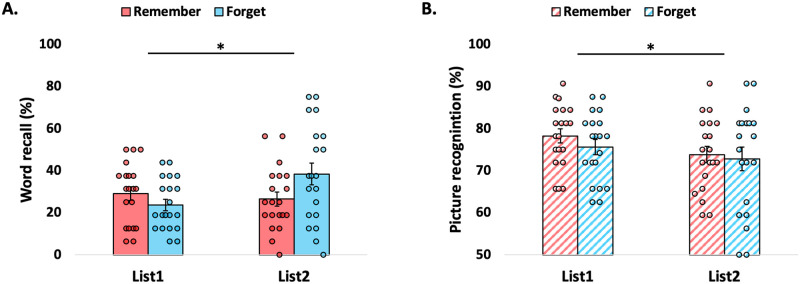
These results remained stable in the subsequent day of testing, with verbal memory strength showing an instruction x list interaction (F(1,38) = 4.96, p = 0.032, η2p = 0.115). Verbal memory strength remained similar between the remember and forget conditions for List1 (t(38) = -1.257, p = 0.216, d = -0.397; further supported by a complimentary Bayesian analysis, BF01 = 1.736, error % = 0.007), again indicating an elimination of the directed forgetting cost effect (Fig 3A). Additionally, verbal memory was marginally better following the forget instruction compared to the remember instruction for List2 (t(38) = 1.914, p = 0.064, d = 0.61) (Fig 3A).
Fig 3. The dominance of negative visual context over neutral verbal memory persisted to the following day.
(A) Verbal memory strength remained similar for words under the remember and the forget conditions for the List1 and enhanced after the instruction to forget the words for List2. (B) Resistance of negative visual memory to indirect modulation also persisted to the next day. There was a general primacy effect for negative contextual pictures, with pictures from List1 remembered better than pictures from List2 under both types of instructions. Error bars represent ±1 Standard error of the mean (S.E.M.) *p<0.05.
Negative visual memory
The resistance of negative visual memory to indirect modulation also persisted to the next day (F(1,38) = 0.319, p = 0.575, η2p = 0.008; further supported by a complimentary Bayesian analysis, BF01 = 2.412, error % = 1.564). In addition, there was a general primacy effect for negative visual memory, with better visual memory strength for List1 relative to List2 under both types of instructions (F(1,38) = 6.135, p = 0.018, η2p = 0.139) (Fig 3B).
Discussion
The results emphasize the dominance of negative visual context memory over neutral content memory. Under negative context, the instruction to remember the words did not improve verbal memory relative to the instruction to forget the words. Additionally, negative visual memory was not affected by indirect modulation, with comparable visual memory strength under either instruction. Taken together, the current results suggest that negative context impairs memory of embedded neutral details and is resistant to indirect memory modulation.
The suggestion that negative visual context impairs related neutral memory is in line with previous findings. Negative events usually attract more attention than neutral events, leading to increased neural activity associated with the perception and encoding of the negative event, resulting in better memory [46, 47]. Even when negative and neutral information is attended similarly, the encoding of negative arousing memories is often prioritized at the expense of related neutral memory encoding [48–50]. Interestingly, negative context can also strengthen related neutral memories [51–53], for example when neutral information is linked to a threating context [54, 55]. Of note, while negative context reduced verbal memory strength for the remember condition, it did not affect verbal memory strength for the forget condition. These results are consistent with similar findings from the DF item-method paradigm [56, 57], suggesting that negative context disrupts memory upregulation (remember condition) and not downregulation (forget condition). While we did not find a DF cost effect for memory recall, this effect is mostly preserved for memory recognition [56, 57], indicating that negative context might impair memory retrieval more than memory encoding.
Negative visual context could also strengthen verbal memory following instructions to forget the words. Several underlying brain mechanisms may account for these effects, and should be further examined in future studies. Enhanced memory after intentional forgetting relates to a decrease in frontoparietal EEG alpha power [58, 59], possibly reflecting a reset in memory encoding [45]. Negative visual stimuli similarly induce a decrease in alpha power [60–63]. Additionally, negative stimuli can promote amygdala driven hippocampal inhibition [64–66], similar to hippocampal downregulation elicited by the forget instruction [1, 2]. The results of the current study suggest that intentional forgetting together with negative context synergistically promotes subsequent memory, while such an effect is not evident under neutral context [11].
The current results demonstrate the dominance of negative visual memory over neutral verbal memory, with neutral verbal memory strength impaired by negative visual context and negative visual context resistant to indirect memory modulation. In addition, this dominance may have abolished the correlation between negative visual memory strength and neutral verbal memory strength. Negative visual memories engage sensory and medial temporal lobe areas to support better encoding and memory vividness, often on the expense of neutral memory processing [23, 67]. Negative memories dominate through arousal and valence driven processes [68–71]. High arousal triggers automatic noradrenergic response in the amygdala to alter hippocampal activity in favor of the arousing memory, while negative valence activates a controlled fronto-hippocampal process to prioritize negative memory. These visual memories are forgotten more slowly compared to neutral visual memories, through support of amygdala driven item–emotion bindings and repeated negative memory reactivation [23, 72]. Here, we demonstrate a post-consolidation advantage for negative visual memory encoded in the first part of the learning session. In psychopathology distressing visual memories are especially persistent, and recurrent involuntary retrieval of aversive imagery can last many years after experiencing a traumatic event [65, 73].
Nevertheless, it is important to note the limitations of the current study. We examined the dominance of pictures with negative valence; however, it is possible that arousal induced by the negative pictures affected the current results. While high arousal is believed to prioritize memory encoding of the arousing event over related content [47], negative valence is thought to enhance memory for the aversive event [74]. Future studies should disentangle the contribution of valence and arousal on negative visual memory dominance. Additionally, we found that negative visual memories were resilient to indirect memory modulation on recognition memory tests, nevertheless it is possible that our method indirectly affected negative visual memory recall [75, 76]. Finally, this study included only words and pictures, portraying a simplified model of everyday life events. Future studies should explore the dominance of negative visual memories in more ecological settings such as augmented and virtual reality [77, 78].
Conclusions
Our results exhibit the superiority of negative visual context memory over neutral content memory, and negative visual memory durability to indirect memory modulation. These findings further emphasize the current challenges to effectively downregulate and modulate pathological distressing visual memories such as intrusive memories [65, 73]. Future research could test alternative memory modulation methods to overcome the noted dominance and resistance of negative visual memories. For example, positive verbal memories can attenuate negative emotional responses [79, 80], and possibly alter negative visual memory strength. Additionally, non-invasive brain stimulation can modulate human learning and memory [81–83]. Recent studies have shown that such neuromodulation using repetitive transcranial magnetic stimulation (rTMS) can reduce the intensity of reactivated intrusive memories and fear memories in non-clinical populations [84, 85]. This study was conducted in healthy participants rather than clinical populations. It remains to be determined whether similar effects would occur for example in patients with PTSD. Such patients are likely to experience greater long term memory impairment under negative context, compared to healthy participants, as they perform poorer on working memory tasks with negative emotional context [86–88], and have difficulties in suppressing unwanted memories [89, 90]. Elevating the capability of PTSD patients to withstand emotional distress and intentionally control memories, by targeting reward and prefrontal networks, could be an important step in overcoming the dominance of negative memories in psychopathology [25, 91–93]. Taken together, we suggest that interventions in patients, geared to modulate negative visual memories, would require multiple sessions spanning a longer duration. Therefore, the development of strategies combining behavioral and neuromodulation approaches described here may open new avenues for alleviating the burden of maladaptive negative memories in clinical populations.
Acknowledgments
We thank Dahlia Perez for helpful feedback on the manuscript.
Data Availability
We have uploaded all the data and analysis scripts to a public repository (https://osf.io/vm74g/).
Funding Statement
“The study was supported by the Israel Science Foundation (ISF 526/17) and the European Research Council (ERC-2019-COG 866093). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript”.
References
- 1.Anderson MC, Hanslmayr S. Neural mechanisms of motivated forgetting. Vol. 18, Trends in Cognitive Sciences. Elsevier Ltd; 2014. p. 279–92. doi: 10.1016/j.tics.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson MC, Hulbert JC. Active Forgetting: Adaptation of Memory by Prefrontal Control. Vol. 72, Annual Review of Psychology. Annual Reviews Inc.; 2021. p. 1–36. doi: 10.1146/annurev-psych-072720-094140 [DOI] [PubMed] [Google Scholar]
- 3.Otani H, Libkuman TM, Goernert PN, Kato K, Migita M, Freehafer SE, et al. Emotion, directed forgetting, and source memory. Br J Psychol. 2012. Aug;103(3):343–58. doi: 10.1111/j.2044-8295.2011.02078.x [DOI] [PubMed] [Google Scholar]
- 4.Bastin C, Feyers D, Majerus S, Balteau E, Degueldre C, Luxen A, et al. The neural substrates of memory suppression: A fMRI exploration of directed forgetting. PLoS One. 2012. Jan 6;7(1):e29905. doi: 10.1371/journal.pone.0029905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallant SN, Dyson BJ. Neural modulation of directed forgetting by valence and arousal: An event-related potential study. Brain Res. 2016;1648:306–16. doi: 10.1016/j.brainres.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 6.Bailey K, Chapman P. When can we choose to forget? An ERP study into item-method directed forgetting of emotional words. Brain Cogn. 2012;78(2):133–47. doi: 10.1016/j.bandc.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Hulbert JC, Henson RN, Anderson MC. Inducing amnesia through systemic suppression. Nat Commun. 2016. Mar 15;7(1):1–9. doi: 10.1038/ncomms11003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis-Peacock JA, Norman KA. Competition between items in working memory leads to forgetting. Nat Commun. 2014. Dec 18;5(1):1–10. doi: 10.1038/ncomms6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pertzov Y, Manohar S, Husain M. Rapid forgetting results from competition over time between items in visual working memory. J Exp Psychol Learn Mem Cogn. 2017. Apr 1;43(4):528–36. doi: 10.1037/xlm0000328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tozios CJI, Fukuda K. Indirect, but Not Direct, Down-Regulation of Visual Long- Term Memory Encoding Through Strategic Biasing of Attentional Allocation. J Exp Psychol Gen. 2019; doi: 10.1037/xge0000712 [DOI] [PubMed] [Google Scholar]
- 11.Kozak S, Herz N, Bar-Haim Y, Censor N. Indirect modulation of human visual memory. Sci Rep. 2021. Mar 31;11(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall KJ, Fawcett EJ, Hourihan KL, Fawcett JM. Emotional memories are (usually) harder to forget: A meta-analysis of the item-method directed forgetting literature. Psychon Bull Rev. 2021. Aug 1;28(4):1313–26. doi: 10.3758/s13423-021-01914-z [DOI] [PubMed] [Google Scholar]
- 13.Chen C, Liu C, Huang R, Cheng D, Wu H, Xu P, et al. Suppression of aversive memories associates with changes in early and late stages of neurocognitive processing. Neuropsychologia. 2012;50(12):2839–48. doi: 10.1016/j.neuropsychologia.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 14.Nørby S, Lange M, Larsen A. Forgetting to forget: On the duration of voluntary suppression of neutral and emotional memories. Acta Psychol (Amst). 2010;133(1):73–80. doi: 10.1016/j.actpsy.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Nowicka A, Marchewka A, Jednoróg K, Tacikowski P, Brechmann A. Forgetting of emotional information is hard: An fMRI study of directed forgetting. Cereb Cortex. 2011;21(3):539–49. doi: 10.1093/cercor/bhq117 [DOI] [PubMed] [Google Scholar]
- 16.Butler AJ, James KH. The neural correlates of attempting to suppress negative versus neutral memories. Cogn Affect Behav Neurosci. 2010. Jun;10(2):182–94. doi: 10.3758/CABN.10.2.182 [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Liu P, Xiao X, Li X, Zeng C, Qiu J, et al. Different neural substrates underlying directed forgetting for negative and neutral images: An event-related potential study. Brain Res. 2012;1441:53–63. doi: 10.1016/j.brainres.2011.10.042 [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Liu P, Cui Q, Wei D, Li W, Qiu J, et al. Directed Forgetting of Negative Self-Referential Information Is Difficult: An fMRI Study. PLoS One. 2013. Oct 4;8(10). doi: 10.1371/journal.pone.0075190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfonso P, Menor J. ERP and behavioural measures of cognitive effort associated to forget negative and neutral words. Brain Cogn. 2021;148. doi: 10.1016/j.bandc.2020.105672 [DOI] [PubMed] [Google Scholar]
- 20.Simeonov L, Peniket M, Das R. No-think, No drink? Assessing the ability of reconsolidation interference by intentional forgetting to suppress alcohol memories in hazardous drinkers. Behav Res Ther. 2022;152. doi: 10.1016/j.brat.2022.104055 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Lin W, Liu C, Luo Y, Wu J, Bayley PJ, et al. Memory consolidation reconfigures neural pathways involved in the suppression of emotional memories. Nat Commun. 2016. Nov 29;7(1):1–12. doi: 10.1038/ncomms13375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herz N, Bar-Haim Y, Holmes EA, Censor N. Intrusive memories: A mechanistic signature for emotional memory persistence. Behav Res Ther. 2020;135. [DOI] [PubMed] [Google Scholar]
- 23.Bowen HJ, Kark SM, Kensinger EA. NEVER forget: negative emotional valence enhances recapitulation. Vol. 25, Psychonomic Bulletin and Review. Springer New York LLC; 2018. p. 870–91. doi: 10.3758/s13423-017-1313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engen HG, Anderson MC. Memory Control: A Fundamental Mechanism of Emotion Regulation. Vol. 22, Trends in Cognitive Sciences. Elsevier Current Trends; 2018. p. 982–95. doi: 10.1016/j.tics.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costanzi M, Cianfanelli B, Santirocchi A, Lasaponara S, Spataro P, Rossi-Arnaud C, et al. Forgetting unwanted memories: Active forgetting and implications for the development of psychological disorders. Vol. 11, Journal of Personalized Medicine. Multidisciplinary Digital Publishing Institute; 2021. p. 241. doi: 10.3390/jpm11040241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nørby S. Forgetting and emotion regulation in mental health, anxiety and depression. Vol. 26, Memory. Routledge; 2018. p. 342–63. [DOI] [PubMed] [Google Scholar]
- 27.Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nat Neurosci. 2013. Feb 28;16(2):146–53. doi: 10.1038/nn.3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Vol. 18, Trends in Cognitive Sciences. 2014. p. 596–604. doi: 10.1016/j.tics.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 29.Phelps EA, Hofmann SG. Memory editing from science fiction to clinical practice. Vol. 572, Nature. Nature Publishing Group; 2019. p. 43–50. doi: 10.1038/s41586-019-1433-7 [DOI] [PubMed] [Google Scholar]
- 30.Spillers GJ, Unsworth N. Are the costs of directed forgetting due to failures of sampling or recovery? Exploring the dynamics of recall in list-method directed forgetting. Mem Cogn. 2011;39(3):403–11. doi: 10.3758/s13421-010-0038-z [DOI] [PubMed] [Google Scholar]
- 31.Blaskovich B, Szőllősi Á, Gombos F, Racsmány M, Simor P. The Benefit of Directed Forgetting Persists After a Daytime Nap: The Role of Spindles and Rapid Eye Movement Sleep in the Consolidation of Relevant Memories. Sleep. 2017. Mar 1;40(3). doi: 10.1093/sleep/zsw076 [DOI] [PubMed] [Google Scholar]
- 32.Pitarque A, Satorres E, Escudero J, Algarabel S, Bekkers O, Meléndez JC. Motivated forgetting reduces veridical memories but slightly increases false memories in both young and healthy older people. Conscious Cogn. 2018. Mar 1;59:26–31. [DOI] [PubMed] [Google Scholar]
- 33.Lang PJ, Bradley MM, Cuthbert &, Greenwald M, Dhman A, Vaid D, et al. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. International Affective Picture System (IAPS. 1997.
- 34.Carretié L, Tapia M, López-Martín S, Albert J. EmoMadrid: An emotional pictures database for affect research. Motiv Emot. 2019. Dec 1;43(6):929–39. [Google Scholar]
- 35.Marchewka A, Żurawski Ł, Jednoróg K, Grabowska A. The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behav Res Methods. 2014;46(2):596–610. doi: 10.3758/s13428-013-0379-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merlhiot G, Mermillod M, Le Pennec JL, Mondillon L. Introduction and validation of the Natural Disasters Picture System (NDPS). PLoS One. 2018. Aug 1;13(8). doi: 10.1371/journal.pone.0201942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haberkamp A, Glombiewski JA, Schmidt F, Barke A. The DIsgust-RelaTed-Images (DIRTI) database: Validation of a novel standardized set of disgust pictures. Behav Res Ther. 2017;89:86–94. doi: 10.1016/j.brat.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 38.Sahakyan L, Kelley CM. A Contextual Change Account of the Directed Forgetting Effect. J Exp Psychol Learn Mem Cogn. 2002;28(6):1064–72. doi: 10.1037//0278-7393.28.6.1064 [DOI] [PubMed] [Google Scholar]
- 39.Hupbach A, Sahakyan L. Additional boundary condition for list-method directed forgetting: The effect of presentation format. J Exp Psychol Learn Mem Cogn. 2014. Mar;40(2):596–601. doi: 10.1037/a0034978 [DOI] [PubMed] [Google Scholar]
- 40.Lang, P. J., Bradley, M. M., & Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida, Gainesville, FL. 2008;
- 41.Onoz B, Oguz B. Assessment of Outliers in Statistical Data Analysis. In: Integrated Technologies for Environmental Monitoring and Information Production. Springer Netherlands; 2003. p. 173–80. [Google Scholar]
- 42.Sahakyan L, Delaney PF, Foster NL, Abushanab B. List-method directed forgetting in cognitive and clinical research: A theoretical and methodological review. In: Psychology of Learning and Motivation—Advances in Research and Theory. Academic Press Inc.; 2013. p. 131–89. [Google Scholar]
- 43.Bäuml KH, Pastötter B, Hanslmayr S. Binding and inhibition in episodic memory-Cognitive, emotional, and neural processes. Vol. 34, Neuroscience and Biobehavioral Reviews. Pergamon; 2010. p. 1047–54. doi: 10.1016/j.neubiorev.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 44.Delaney PF, Barden EP, Smith WG, Wisco BE. What can directed forgetting tell us about clinical populations? Vol. 82, Clinical Psychology Review. 2020. doi: 10.1016/j.cpr.2020.101926 [DOI] [PubMed] [Google Scholar]
- 45.Pastötter B, Tempel T, Bäuml KHT. Long-term memory updating: The reset-of-encoding hypothesis in list-method directed forgetting. Vol. 8, Frontiers in Psychology. Frontiers Media S.A.; 2017. doi: 10.3389/fpsyg.2017.02076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talmi D, McGarry LM. Accounting for immediate emotional memory enhancement. J Mem Lang. 2012;66(1):93–108. [Google Scholar]
- 47.Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspect Psychol Sci. 2011. Mar 1;6(2):114–33. doi: 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talmi D, Schimmack U, Paterson T, Moscovitch M. The role of attention and relatedness in emotionally enhanced memory. Emotion. 2007;7(1):89–102. doi: 10.1037/1528-3542.7.1.89 [DOI] [PubMed] [Google Scholar]
- 49.Steinmetz KRM, Kensinger EA. The emotion-induced memory trade-off: More than an effect of overt attention? Mem Cogn. 2013;41(1):69–81. doi: 10.3758/s13421-012-0247-8 [DOI] [PubMed] [Google Scholar]
- 50.Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and β-adrenergic-dependent. Proc Natl Acad Sci U S A. 2003. Nov 11;100(23):13626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proc Natl Acad Sci U S A. 2006. Jan 31;103(5):1599–604. doi: 10.1073/pnas.0506308103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallant SN, Dyson BJ, Yang L. Local context effects during emotional item directed forgetting in younger and older adults. Memory. 2017. Sep 14;25(8):1129–38. doi: 10.1080/09658211.2016.1274036 [DOI] [PubMed] [Google Scholar]
- 53.Knight M, Mather M. Reconciling Findings of Emotion-Induced Memory Enhancement and Impairment of Preceding Items. Emotion. 2009. Dec;9(6):763–81. doi: 10.1037/a0017281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis FC, Somerville LH, Ruberry EJ, Berry ABL, Shin LM, Whalen PJ. A Tale of Two Negatives: Differential Memory Modulation by Threat-Related Facial Expressions. Emotion. 2011;11(3):647–55. doi: 10.1037/a0021625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Y, Zhao Y, Ybarra O, Stephan WG, Yang Q. Enhanced memory for both threat and neutral information under conditions of intergroup threat. Front Psychol. 2015;6(NOV). doi: 10.3389/fpsyg.2015.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu TL, Chen NF, Cheng S kuen. Selective rehearsal is affected by the emotionality of the encoding context in item-method directed forgetting: An event-related potential study. Biol Psychol. 2017. Feb 1;123:15–24. doi: 10.1016/j.biopsycho.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 57.Pierguidi L, Righi S, Gronchi G, Marzi T, Caharel S, Giovannelli F, et al. Emotional contexts modulate intentional memory suppression of neutral faces: Insights from ERPs. Int J Psychophysiol. 2016;106:1–13. doi: 10.1016/j.ijpsycho.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 58.Bäuml KH, Hanslmayr S, Pastötter B, Klimesch W. Oscillatory correlates of intentional updating in episodic memory. Neuroimage. 2008. Jun 1;41(2):596–604. doi: 10.1016/j.neuroimage.2008.02.053 [DOI] [PubMed] [Google Scholar]
- 59.Hanslmayr S, Volberg G, Wimber M, Oehler N, Staudigl T, Hartmann T, et al. Prefrontally driven downregulation of neural synchrony mediates goal-directed forgetting. J Neurosci. 2012;32(42):14742–51. doi: 10.1523/JNEUROSCI.1777-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strube A, Rose M, Fazeli S, Büchel C. Alpha-to-beta- and gamma-band activity reflect predictive coding in affective visual processing. Sci Rep. 2021;11(1). doi: 10.1038/s41598-021-02939-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schubring D, Schupp HT. Emotion and Brain Oscillations: High Arousal is Associated with Decreases in Alpha- And Lower Beta-Band Power. Cereb Cortex. 2021;31(3):1597–608. doi: 10.1093/cercor/bhaa312 [DOI] [PubMed] [Google Scholar]
- 62.Schubring D, Schupp HT. Affective picture processing: Alpha- and lower beta-band desynchronization reflects emotional arousal. Psychophysiology. 2019. Aug 1;56(8). doi: 10.1111/psyp.13386 [DOI] [PubMed] [Google Scholar]
- 63.Schubring D, Kraus M, Stolz C, Weiler N, Keim DA, Schupp H. Virtual reality potentiates emotion and task effects of alpha/beta brain oscillations. Brain Sci. 2020;10(8):1–19. doi: 10.3390/brainsci10080537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bisby JA, Burgess N. Differential effects of negative emotion on memory for items and associations, and their relationship to intrusive imagery. Vol. 17, Current Opinion in Behavioral Sciences. Elsevier Ltd; 2017. p. 124–32. doi: 10.1016/j.cobeha.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brewin CR, Gregory JD, Lipton M, Burgess N. Intrusive Images in Psychological Disorders: Characteristics, Neural Mechanisms, and Treatment Implications. Psychol Rev. 2010. Jan;117(1):210–32. doi: 10.1037/a0018113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bisby JA, Horner AJ, Hørlyck LD, Burgess N. Opposing effects of negative emotion on amygdalar and hippocampal memory for items and associations. Soc Cogn Affect Neurosci. 2016;11(6):981–90. doi: 10.1093/scan/nsw028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murty VP, Ritchey M, Adcock RA, LaBar KS. Reprint of: fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2011. Mar 1;49(4):695–705. doi: 10.1016/j.neuropsychologia.2011.02.031 [DOI] [PubMed] [Google Scholar]
- 68.Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proc Natl Acad Sci U S A. 2004. Mar 2;101(9):3310–5. doi: 10.1073/pnas.0306408101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rouhani N, Niv Y, Frank MJ, Schwabe L. Multiple routes to enhanced memory for emotionally relevant events. Vol. 27, Trends in Cognitive Sciences. 2023. p. 867–82. doi: 10.1016/j.tics.2023.06.006 [DOI] [PubMed] [Google Scholar]
- 70.Kang C, Wang Z, Surina A, Lü W. Immediate emotion-enhanced memory dependent on arousal and valence: The role of automatic and controlled processing. Acta Psychol (Amst). 2014;150:153–60. doi: 10.1016/j.actpsy.2014.05.008 [DOI] [PubMed] [Google Scholar]
- 71.Fastenrath M, Coynel D, Spalek K, Milnik A, Gschwind L, Roozendaal B, et al. Dynamic modulation of amygdala-hippocampal connectivity by emotional arousal. J Neurosci. 2014;34(42):13935–47. doi: 10.1523/JNEUROSCI.0786-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yonelinas AP, Ritchey M. The slow forgetting of emotional episodic memories: An emotional binding account. Vol. 19, Trends in Cognitive Sciences. 2015. p. 259–67. doi: 10.1016/j.tics.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iyadurai L, Visser RM, Lau-Zhu A, Porcheret K, Horsch A, Holmes EA, et al. Intrusive memories of trauma: A target for research bridging cognitive science and its clinical application. Vol. 69, Clinical Psychology Review. 2019. p. 67–82. doi: 10.1016/j.cpr.2018.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bowen HJ, Kark SM, Kensinger EA. NEVER forget: negative emotional valence enhances recapitulation. Psychon Bull Rev. 2018. Jun 1;25(3):870–91. doi: 10.3758/s13423-017-1313-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu Z, Anderson MC, Wang Y. Inducing forgetting of unwanted memories through subliminal reactivation. Nat Commun. 2022;13(1). doi: 10.1038/s41467-022-34091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Z, Wang Y. Forgetting Unrelated Episodic Memories Through Suppression-Induced Amnesia. J Exp Psychol Gen. 2020; doi: 10.1037/xge0000782 [DOI] [PubMed] [Google Scholar]
- 77.Smith SA. Virtual reality in episodic memory research: A review. Vol. 26, Psychonomic Bulletin and Review. Springer New York LLC; 2019. p. 1213–37. doi: 10.3758/s13423-019-01605-w [DOI] [PubMed] [Google Scholar]
- 78.Vinci C, Brandon KO, Kleinjan M, Brandon TH. The clinical potential of augmented reality. Vol. 27, Clinical Psychology: Science and Practice. Blackwell Publishing Inc.; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Speer ME, Mauricio R. Reminiscing about positive memories buffers acute stress responses. Nat Hum Behav. 2017. Apr 24;1(5):1–9. doi: 10.1038/s41562-017-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xia T, Yao Z, Guo X, Liu J, Chen D, Liu Q, et al. Updating memories of unwanted emotions during human sleep. Curr Biol. 2023. Jan 23;33(2):309–320.e5. doi: 10.1016/j.cub.2022.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yeh N, Rose NS. How can transcranial magnetic stimulation be used to modulate episodic memory?: A systematic review and meta-analysis. Vol. 10, Frontiers in Psychology. Frontiers Media S.A.; 2019. doi: 10.3389/fpsyg.2019.00993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim K, Ekstrom AD, Tandon N. A network approach for modulating memory processes via direct and indirect brain stimulation: Toward a causal approach for the neural basis of memory. Vol. 134, Neurobiology of Learning and Memory. 2016. p. 162–77. doi: 10.1016/j.nlm.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 83.Borgomaneri S, Battaglia S, Sciamanna G, Tortora F, Laricchiuta D. Memories are not written in stone: Re-writing fear memories by means of non-invasive brain stimulation and optogenetic manipulations. Vol. 127, Neuroscience and Biobehavioral Reviews. 2021. p. 334–52. doi: 10.1016/j.neubiorev.2021.04.036 [DOI] [PubMed] [Google Scholar]
- 84.Herz N, Bar-Haim Y, Tavor I, Tik N, Sharon H, Holmes EA, et al. Neuromodulation of Visual Cortex Reduces the Intensity of Intrusive Memories. Cereb Cortex. 2022;32(2):408–17. doi: 10.1093/cercor/bhab217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borgomaneri S, Battaglia S, Garofalo S, Tortora F, Avenanti A, di Pellegrino G. State-Dependent TMS over Prefrontal Cortex Disrupts Fear-Memory Reconsolidation and Prevents the Return of Fear. Curr Biol. 2020. Sep 21;30(18):3672–3679.e4. doi: 10.1016/j.cub.2020.06.091 [DOI] [PubMed] [Google Scholar]
- 86.Mirabolfathi V, Moradi AR, Jobson L. Influence of affective distractors on working memory capacity in relation to symptoms of posttraumatic stress disorder. Appl Cogn Psychol. 2019. Sep 1;33(5):904–10. [Google Scholar]
- 87.Zhang JN, Xiong KL, Qiu MG, Zhang Y, Xie B, Wang J, et al. Negative emotional distraction on neural circuits for working memory in patients with posttraumatic stress disorder. Brain Res. 2013;1531:94–101. doi: 10.1016/j.brainres.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 88.Schweizer S, Dalgleish T. The impact of affective contexts on working memory capacity in healthy populations and in individuals with PTSD. Emotion. 2016;16(1):16–23. doi: 10.1037/emo0000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang W, Liu P, Zhuang K, Wei D, Anderson MC, Qiu J. Behavioral and neural correlates of memory suppression in subthreshold depression. Psychiatry Res—Neuroimaging. 2020;297. doi: 10.1016/j.pscychresns.2020.111030 [DOI] [PubMed] [Google Scholar]
- 90.Catarino A, Küpper CS, Werner-Seidler A, Dalgleish T, Anderson MC. Failing to Forget: Inhibitory-Control Deficits Compromise Memory Suppression in Posttraumatic Stress Disorder. Psychol Sci. 2015. May 8;26(5):604–16. doi: 10.1177/0956797615569889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dutcher JM, Creswell JD. The role of brain reward pathways in stress resilience and health. Vol. 95, Neuroscience and Biobehavioral Reviews. Pergamon; 2018. p. 559–67. [DOI] [PubMed] [Google Scholar]
- 92.Kozak S, Dezachyo O, Stanford W, Bar-Haim Y, Censor N, Dayan E. Elevated integration within the reward network underlies vulnerability to distress. Cereb Cortex. 2022;1–28. [DOI] [PubMed] [Google Scholar]
- 93.Mary A, Dayan J, Leone G, Postel C, Fraisse F, Malle C, et al. Resilience after trauma: The role of memory suppression. Science (80-). 2020. Feb 14;367(6479). doi: 10.1126/science.aay8477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have uploaded all the data and analysis scripts to a public repository (https://osf.io/vm74g/).