Abstract
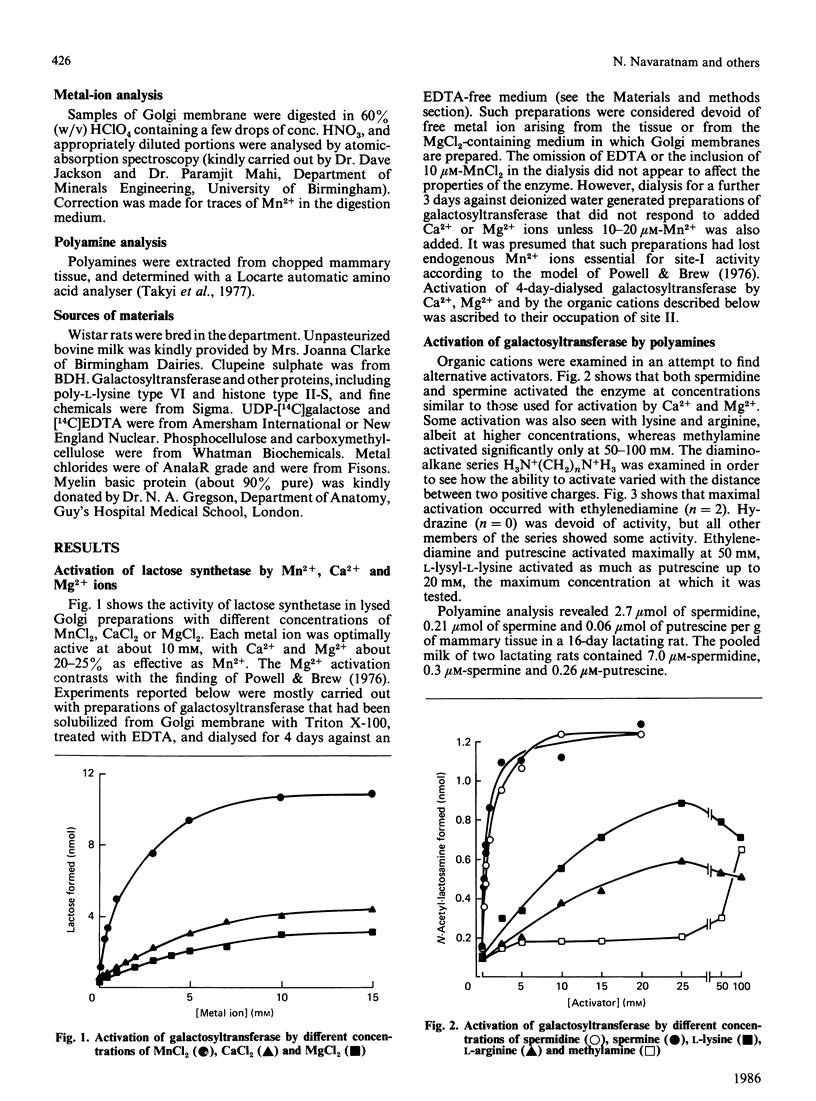
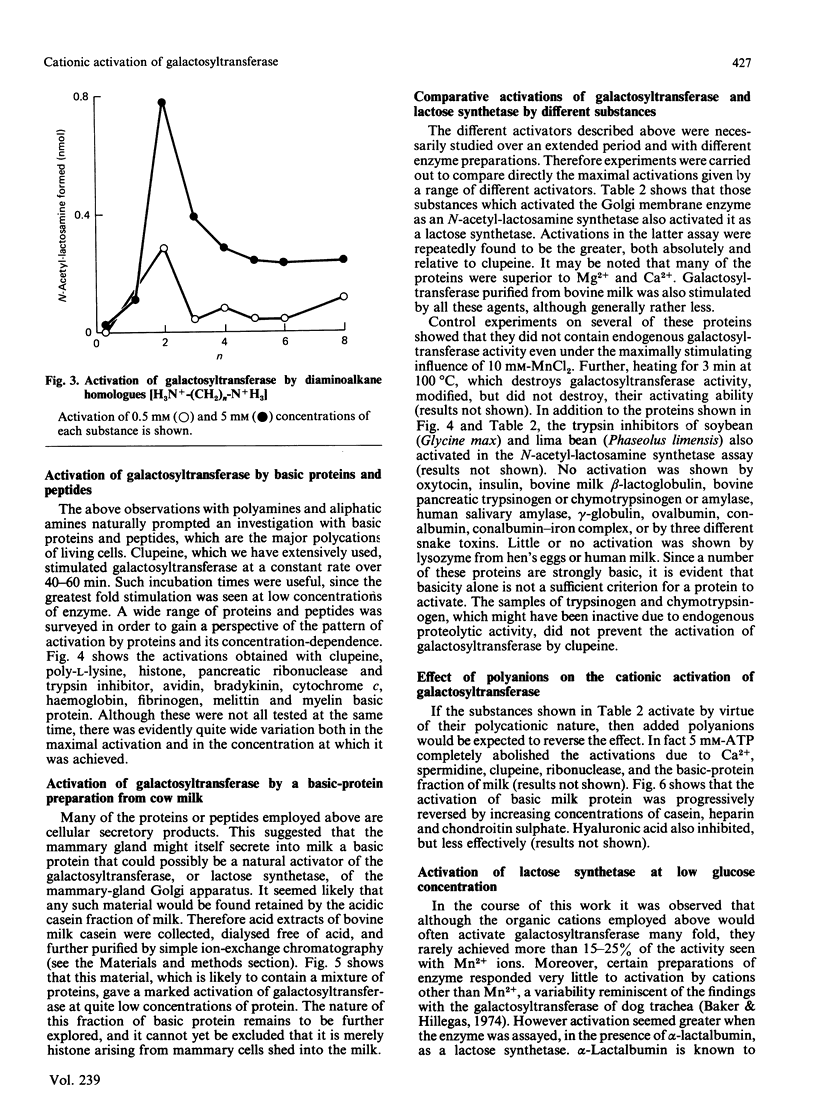
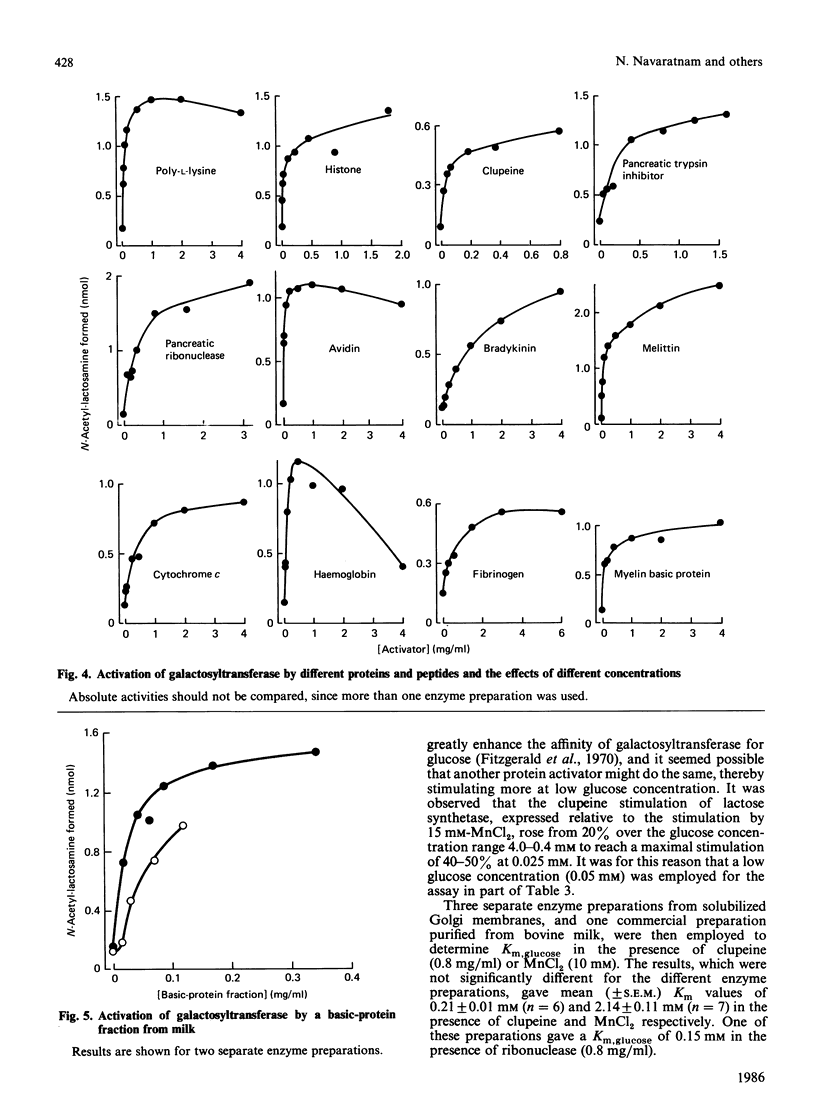
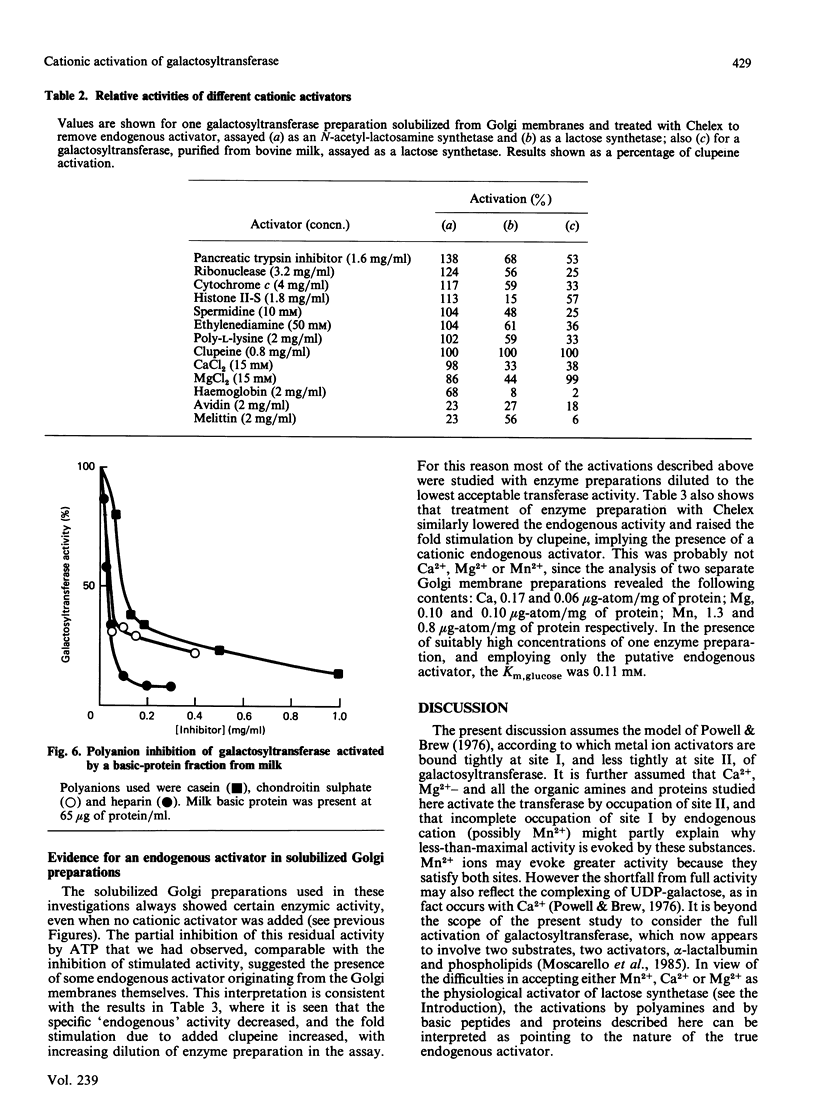
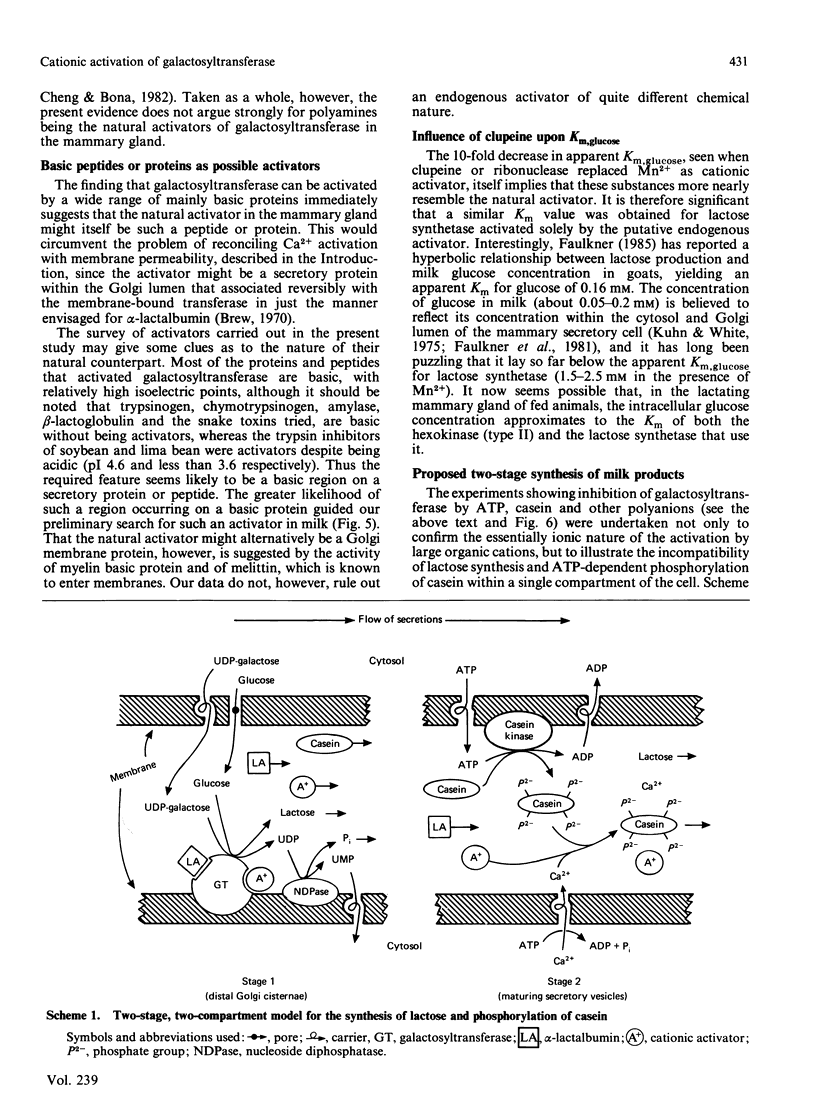
Galactosyltransferase (EC 2.4.1.22) requires bivalent metal ions for its activity. However, preparations of this enzyme solubilized from Golgi membranes of lactating rat mammary gland were shown to be activated not only by Mn2+, Ca2+ and Mg2+, but also by spermine, spermidine, lysyl-lysine, ethylenediamine and other diaminoalkanes, and by a range of basic proteins and peptides, including clupeine, histone, polylysine, ribonuclease, pancreatic trypsin inhibitor, cytochrome c, melittin, avidin and myelin basic protein. Both N-acetyl-lactosamine synthetase and lactose synthetase activities were enhanced. A basic protein fraction was isolated from bovine milk and shown to activate galactosyltransferase at low concentrations. The polyanions ATP, casein, chondroitin sulphate and heparin reversed the activation of galactosyltransferase by several of the above substances. Galactosyltransferase, assayed as a lactose synthetase, showed a 10-fold greater affinity for glucose when Mn2+ ions were replaced by clupeine or by ribonuclease as cationic activator. Evidence was obtained for the presence of an endogenous cationic activator in solubilized Golgi membrane preparations which evoked a similar low apparent Km,glucose. The findings are discussed in the light of cationic activations of glycosyltransferases generally, of the porous nature of the Golgi membrane, and of the unlikelihood of bivalent metal ions being the physiological activators of galactosyltransferase. It is suggested that the natural cationic activator of lactose synthetase may be a secretory protein acting in a manner analogous to the enzyme's activation by alpha-lactalbumin. A scheme is proposed for the two-stage synthesis of lactose and phosphorylation of casein within the cell, to accommodate the apparent incompatibility of these two processes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARGMANN W., KNOOP A. Uber die Morphologie der Milchsekretion; lichtund elektronenmikroskopische Studien an der Milchdrüse Ratte. Z Zellforsch Mikrosk Anat. 1959;49(3):344–388. [PubMed] [Google Scholar]
- Babad H., Hassid W. Z. Soluble uridine diphosphate D-galactose: D-glucose beta-4-D-galactosyltransferase from bovine milk. J Biol Chem. 1966 Jun 10;241(11):2672–2678. [PubMed] [Google Scholar]
- Baker A. P., Hillegass L. M. Enhancement of UDP-galactose:mucin galactosyltransferase activity by spermine. Arch Biochem Biophys. 1974 Dec;165(2):597–603. doi: 10.1016/0003-9861(74)90287-2. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Baumrucker C. R., Keenan T. W. Membranes of mammary gland. X. Adenosine triphosphate dependent calcium accumulation by Golgi apparatus rich fractions from bovine mammary gland. Exp Cell Res. 1975 Feb;90(2):253–260. doi: 10.1016/0014-4827(75)90314-6. [DOI] [PubMed] [Google Scholar]
- Bergqvist U., Samuelsson G., Uvnäs B. Chemical composition of basophil granules from isolated rat mast cells. Acta Physiol Scand. 1971 Nov;83(3):362–372. doi: 10.1111/j.1748-1716.1971.tb05089.x. [DOI] [PubMed] [Google Scholar]
- Berliner L. J., Wong S. S. Manganese (II) And spin-labeled uridine 5'-diphosphate binding to bovine galactosyltransferase. Biochemistry. 1975 Nov 4;14(22):4977–4982. doi: 10.1021/bi00693a029. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Hill R. L. Enzymatic properties of the beta-galactoside alpha 1 leads to 2 fucosyltransferase from porcine submaxillary gland. J Biol Chem. 1980 Jun 10;255(11):5373–5379. [PubMed] [Google Scholar]
- Bosmann H. B., Eylar E. H. Glycoprotein biosynthesis: the biosynthesis of the hydroxylysine-galactose linkage in collagen. Biochem Biophys Res Commun. 1968 Oct 24;33(2):340–346. doi: 10.1016/0006-291x(68)90790-0. [DOI] [PubMed] [Google Scholar]
- Brew K. Lactose synthetase: evolutionary origins, structure and control. Essays Biochem. 1970;6:93–118. [PubMed] [Google Scholar]
- Brosnan M. E., Farrell R., Wilansky H., Williamson D. H. Effect of starvation and refeeding on polyamine concentrations and ornithine decarboxylase antizyme in mammary gland of lactating rats. Biochem J. 1983 Apr 15;212(1):149–153. doi: 10.1042/bj2120149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan M. E., Ilic V., Williamson D. H. Regulation of the activity of ornithine decarboxylase and S-adenosylmethionine decarboxylase in mammary gland and liver of lactating rats. Effects of starvation, prolactin and insulin deficiency. Biochem J. 1982 Mar 15;202(3):693–698. doi: 10.1042/bj2020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick D. T., Kuhn N. J. Diurnal variation and response to food withdrawal of lactose synthesis in lactating rats. Biochem J. 1978 Jul 15;174(1):319–325. doi: 10.1042/bj1740319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P. W., Bona S. J. Mucin biosynthesis. Characterization of UDP-galactose: alpha-N-acetylgalactosaminide beta 3 galactosyltransferase from human tracheal epithelium. J Biol Chem. 1982 Jun 10;257(11):6251–6258. [PubMed] [Google Scholar]
- Deutscher S. L., Hirschberg C. B. Mechanism of galactosylation in the Golgi apparatus. A Chinese hamster ovary cell mutant deficient in translocation of UDP-galactose across Golgi vesicle membranes. J Biol Chem. 1986 Jan 5;261(1):96–100. [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner A., Chaiyabutr N., Peaker M., Carrick D. T., Kuhn N. J. Metabolic significance of milk glucose. J Dairy Res. 1981 Feb;48(1):51–56. doi: 10.1017/s0022029900021440. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. K., Brodbeck U., Kiyosawa I., Mawal R., Colvin B., Ebner K. E. Alpha-lactalbumin and the lactose synthetase reaction. J Biol Chem. 1970 Apr 25;245(8):2103–2108. [PubMed] [Google Scholar]
- Fleischer B., Smigel M. Solubilization and properties of galactosyltransferase and sulfotransferase activities of Golgi membranes in Triton X-100. J Biol Chem. 1978 Mar 10;253(5):1632–1638. [PubMed] [Google Scholar]
- Fraser I. H., Mookerjea S. Purification of membrane-bound galactosyltransferase from rat liver microsomal fractions. Biochem J. 1977 Jun 15;164(3):541–547. doi: 10.1042/bj1640541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainer H., Russell J. T., Loh Y. P. The enzymology and intracellular organization of peptide precursor processing: the secretory vesicle hypothesis. Neuroendocrinology. 1985 Feb;40(2):171–184. doi: 10.1159/000124070. [DOI] [PubMed] [Google Scholar]
- Gold A. H. On the possibility of metabolite control of liver glycogen synthetase activity. Biochemistry. 1970 Feb 17;9(4):946–952. doi: 10.1021/bi00806a034. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Androgen-dependent synthesis of basic secretory proteins by the rat seminal vesicle. Biochem J. 1976 Aug 15;158(2):271–282. doi: 10.1042/bj1580271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson S. The ATP-dependent concentration of calcium by a Golgi apparatus-rich fraction isolated from rat liver. J Cell Sci. 1978 Apr;30:117–128. doi: 10.1242/jcs.30.1.117. [DOI] [PubMed] [Google Scholar]
- Jakubowski H. Polyamines and yellow lupin aminoacyl-tRNA synthetases. Spermine and spermidine help to maintain the active structures of aminoacyl-tRNA synthetases. FEBS Lett. 1980 Jan 1;109(1):63–66. doi: 10.1016/0014-5793(80)81312-3. [DOI] [PubMed] [Google Scholar]
- Jarkovsky Z., Marcus D. M., Grollman A. P. Fucosyltransferase found in human milk. Product of the Lewis blood group gene. Biochemistry. 1970 Mar 3;9(5):1123–1128. doi: 10.1021/bi00807a011. [DOI] [PubMed] [Google Scholar]
- Jensen J. W., Schutzbach J. S. The biosynthesis of oligosaccharide-lipids. Partial purification and characterization of mannosyltransferase II. J Biol Chem. 1981 Dec 25;256(24):12899–12904. [PubMed] [Google Scholar]
- Keller S. J., Keenan T. W., Eigel W. N. Glycosylation of kappa-casein. I. Localization and characterization of sialyltransferase in bovine mammary gland. Biochim Biophys Acta. 1979 Feb 9;566(2):266–273. doi: 10.1016/0005-2744(79)90030-5. [DOI] [PubMed] [Google Scholar]
- Khatra B. S., Herries D. G., Brew K. Some kinetic properties of human-milk galactosyl transferase. Eur J Biochem. 1974 May 15;44(2):537–560. doi: 10.1111/j.1432-1033.1974.tb03513.x. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969 May;44(1):39–54. doi: 10.1677/joe.0.0440039. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Evidence for specific transport of uridine diphosphate galactose across the Golgi membrane of rat mammary gland. Biochem J. 1976 Jan 15;154(1):243–244. doi: 10.1042/bj1540243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. Milk glucose as an index of the intracellular glucose concentration of rat mammary gland. Biochem J. 1975 Oct;152(1):153–155. doi: 10.1042/bj1520153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson A. E., Suttie J. W. Vitamin K-dependent carboxylase: effect of Mn2+ and other divalent cations. FEBS Lett. 1980 Aug 25;118(1):95–98. doi: 10.1016/0014-5793(80)81226-9. [DOI] [PubMed] [Google Scholar]
- Marniemi J., Hänninen O. Radiochemical assay of UDP glucuronyltransferase (p-nitrophenol). FEBS Lett. 1973 Jun 1;32(2):273–276. doi: 10.1016/0014-5793(73)80851-8. [DOI] [PubMed] [Google Scholar]
- McKenzie L., Fitzgerald D. K., Ebner K. E. Lactose synthetase activities in rat and mouse mammary glands. Biochim Biophys Acta. 1971;230(3):526–530. doi: 10.1016/0304-4165(71)90183-8. [DOI] [PubMed] [Google Scholar]
- Mita M., Yasumasu I. Inhibition of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in sea urchin eggs by palymitoyl-coenzyme A and reversal by polyamines. Arch Biochem Biophys. 1980 Apr 15;201(1):322–329. doi: 10.1016/0003-9861(80)90517-2. [DOI] [PubMed] [Google Scholar]
- Mita M., Yasumasu I. Reversal of palmitoyl coenzyme A-caused inhibition of glucose-6-phosphate dehydrogenase by polyamines. Biochem Biophys Res Commun. 1979 Feb 28;86(4):961–967. doi: 10.1016/0006-291x(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic effects of -lactalbumin with N-acetylglucosamine and glucose as galactosyl group acceptors. J Biol Chem. 1971 Jun 25;246(12):3992–3998. [PubMed] [Google Scholar]
- Morrison J. F., Ebner K. E. Studies on galactosyltransferase. Kinetic investigations with N-acetylglucosamine as the galactosyl group acceptor. J Biol Chem. 1971 Jun 25;246(12):3977–3984. [PubMed] [Google Scholar]
- Moscarello M. A., Mitranic M. M., Vella G. Stimulation of bovine milk galactosyltransferase activity by bovine colostrum N-acetylglucosaminyltransferase I. Biochim Biophys Acta. 1985 Oct 4;831(2):192–200. doi: 10.1016/0167-4838(85)90035-4. [DOI] [PubMed] [Google Scholar]
- Mrsny R. J., Meizel S. Potassium ion influx and Na+,K+-ATPase activity are required for the hamster sperm acrosome reaction. J Cell Biol. 1981 Oct;91(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Myllylä R., Risteli L., Kivirikko K. I. Assay of collagen-galactosyltransferase and collagen-glucosyltransferase activities and preliminary characterization of enzymic reactions with transferases from chick-embryo cartilage. Eur J Biochem. 1975 Apr 1;52(3):401–410. doi: 10.1111/j.1432-1033.1975.tb04008.x. [DOI] [PubMed] [Google Scholar]
- Neville M. C., Selker F., Semple K., Watters C. ATP-dependent calcium transport by a Golgi-enriched membrane fraction from mouse mammary gland. J Membr Biol. 1981;61(2):97–105. doi: 10.1007/BF02007636. [DOI] [PubMed] [Google Scholar]
- Neville M. C., Watters C. D. Secretion of calcium into milk: review. J Dairy Sci. 1983 Mar;66(3):371–380. doi: 10.3168/jds.S0022-0302(83)81802-5. [DOI] [PubMed] [Google Scholar]
- Notides A. C., Williams-Ashman H. G. The basic protein responsible for the clotting of guinea pig semen. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1991–1995. doi: 10.1073/pnas.58.5.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe E. T., Hill R. L., Bell J. E. Active site of bovine galactosyltransferase: kinetic and fluorescence studies. Biochemistry. 1980 Oct 28;19(22):4954–4962. doi: 10.1021/bi00563a003. [DOI] [PubMed] [Google Scholar]
- Oppenheimer C. L., Hill R. L. Purification and characterization of a rabbit liver alpha 1 goes to 3 mannoside beta 1 goes to 2 N-acetylglucosaminyltransferase. J Biol Chem. 1981 Jan 25;256(2):799–804. [PubMed] [Google Scholar]
- Pascall J. C., Boulton A. P., Craig R. K. Characterisation of a membrane-bound serine-specific casein kinase isolated from lactating guinea-pig mammary gland. Eur J Biochem. 1981 Sep;119(1):91–99. doi: 10.1111/j.1432-1033.1981.tb05581.x. [DOI] [PubMed] [Google Scholar]
- Piller F., Cartron J. P. UDP-GlcNAc:Gal beta 1-4Glc(NAc) beta 1-3N-acetylglucosaminyltransferase. Identification and characterization in human serum. J Biol Chem. 1983 Oct 25;258(20):12293–12299. [PubMed] [Google Scholar]
- Powell J. T., Brew K. Metal ion activation of galactosyltransferase. J Biol Chem. 1976 Jun 25;251(12):3645–3652. [PubMed] [Google Scholar]
- Pâquet M. R., Moscarello M. A. A kinetic comparison of partially purified rat liver Golgi and rat serum galactosyltransferases. Biochem J. 1984 Mar 15;218(3):745–751. doi: 10.1042/bj2180745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggio H. A., Palade G. E. Sulfated compounds in the zymogen granules of the guinea pig pancreas. J Cell Biol. 1978 May;77(2):288–314. doi: 10.1083/jcb.77.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., McVicker T. A. Polyamine biogenesis in the rat mammary gland during pregnancy and lactation. Biochem J. 1972 Nov;130(1):71–76. doi: 10.1042/bj1300071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHATTON J. B., GRUENSTEIN M., SHAY H., WEINHOUSE S. ENZYMES OF GALACTOSE SYNTHESIS IN MAMMARY GLAND AND MAMMARY TUMORS OF THE RAT. J Biol Chem. 1965 Jan;240:22–28. [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Complete amino acid sequence of a basic proline-rich peptide, P-F, from human parotid saliva. J Biochem. 1983 Mar;93(3):883–888. doi: 10.1093/jb/93.3.883. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Further fractionation of basic proline-rich peptides from human parotid saliva and complete amino acid sequence of basic proline-rich peptide P-H. J Biochem. 1983 Dec;94(6):1991–1999. doi: 10.1093/oxfordjournals.jbchem.a134553. [DOI] [PubMed] [Google Scholar]
- Sanguansermsri J., György P., Zilliken F. Polyamines in human and cow's milk. Am J Clin Nutr. 1974 Aug;27(8):859–865. doi: 10.1093/ajcn/27.8.859. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. Immunoelectron microscopic exploration of the Golgi complex. J Histochem Cytochem. 1983 Aug;31(8):1049–1056. doi: 10.1177/31.8.6863900. [DOI] [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Glycoprotein biosynthesis: studies on thyroglobulin. Thyroid galactosyltransferase. J Biol Chem. 1968 Dec 25;243(24):6529–6537. [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Studies on the biosynthesis of the hydroxylysine-liked disaccharide unit of basement membranes and collagens. I. Kidney glucosyltransferase. J Biol Chem. 1971 Aug 25;246(16):4899–4909. [PubMed] [Google Scholar]
- Takyi E. E., Fuller D. J., Donaldson L. J., Thomas G. H. Deoxyribonucleic acid and polyamine synthesis in rat ventral prostrate. Effects of age of the intact rat and androgen stimulation of the castrated rat with testosterone, 5 alpha-dihydrotestosterone and 5 alpha-androstane-3 beta, 17 beta-diol. Biochem J. 1977 Jan 15;162(1):87–97. doi: 10.1042/bj1620087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N., Yanagisawa K., Makita A., Naiki M. Purification and properties of rat liver globotriaosylceramide synthase, UDP-galactose:lactosylceramide alpha 1-4-galactosyltransferase. J Biol Chem. 1985 Apr 25;260(8):4908–4913. [PubMed] [Google Scholar]
- Tartakoff A., Greene L. J., Palade G. E. Studies on the guinea pig pancreas. Fractionation and partial characterization of exocrine proteins. J Biol Chem. 1974 Dec 10;249(23):7420–7431. [PubMed] [Google Scholar]
- Virk S. S., Kirk C. J., Shears S. B. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem J. 1985 Mar 15;226(3):741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLINGS S. R., DEOME K. B. Milk protein droplet formation in the Golgi apparatus of the C3H/Crgl mouse mammary epithelial cells. J Biophys Biochem Cytol. 1961 Feb;9:479–485. doi: 10.1083/jcb.9.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELLINGS S. R., PHILP J. R. THE FUNCTION OF THE GOLGI APPARATUS IN LACTATING CELLS OF THE BALB/CCRGL MOUSE. AN ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDY. Z Zellforsch Mikrosk Anat. 1964 Jan 31;61:871–882. doi: 10.1007/BF00340040. [DOI] [PubMed] [Google Scholar]
- Waheed A., Hasilik A., von Figura K. UDP-N-acetylglucosamine:lysosomal enzyme precursor N-acetylglucosamine-1-phosphotransferase. Partial purification and characterization of the rat liver Golgi enzyme. J Biol Chem. 1982 Oct 25;257(20):12322–12331. [PubMed] [Google Scholar]
- Wallace A. V., Kuhn N. J. Incorporation into phospholipid vesicles of pore-like properties from Golgi membranes of lactating-rat mammary gland. Biochem J. 1986 May 15;236(1):91–96. doi: 10.1042/bj2360091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. W., Clegg R. A. Casein kinase activity in rat mammary gland Golgi vesicles. Demonstration of latency and requirement for a transmembrane ATP carrier. Biochem J. 1984 Apr 1;219(1):181–187. doi: 10.1042/bj2190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D. W., Clegg R. A. Casein kinase activity in rat mammary gland Golgi vesicles. Phosphorylation of endogenous caseins. Eur J Biochem. 1983 Dec 1;137(1-2):215–220. doi: 10.1111/j.1432-1033.1983.tb07817.x. [DOI] [PubMed] [Google Scholar]
- West D. W. Energy-dependent calcium sequestration activity in a Golgi apparatus fraction derived from lactating rat mammary glands. Biochim Biophys Acta. 1981 Apr 3;673(4):374–386. doi: 10.1016/0304-4165(81)90469-4. [DOI] [PubMed] [Google Scholar]
- White M. D., Kuhn N. J., Ward S. Mannitol and glucose movement across the Golgi membrane of lactating-rat mammary gland. Biochem J. 1981 Jan 15;194(1):173–177. doi: 10.1042/bj1940173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D., Kuhn N. J., Ward S. Permeability of lactating-rat mammary gland Golgi membranes to monosaccharides. Biochem J. 1980 Sep 15;190(3):621–624. doi: 10.1042/bj1900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D., Ward S., Kuhn N. J. Biosynthesis of galactinol by lactose synthetase. Int J Biochem. 1982;14(6):449–451. doi: 10.1016/0020-711x(82)90111-2. [DOI] [PubMed] [Google Scholar]
- White M. D., Ward S., Kuhn N. J. Composition, stability and electrolyte permeability of Golgi membranes from lactating-rat mammary gland. Biochem J. 1981 Dec 15;200(3):663–669. doi: 10.1042/bj2000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D., Ward S., Kuhn N. J. Pore properties of the Golgi membrane from lactating-rat mammary gland. Effects of pH and temperature and reconstitution into phospholipid vesicles. Biochem J. 1984 Jan 1;217(1):297–301. doi: 10.1042/bj2170297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding F. B., Morgan G. Calcium localization in lactating rabbit mammary secretory cells. J Ultrastruct Res. 1978 Jun;63(3):323–333. doi: 10.1016/s0022-5320(78)80056-2. [DOI] [PubMed] [Google Scholar]


