Abstract
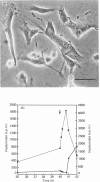
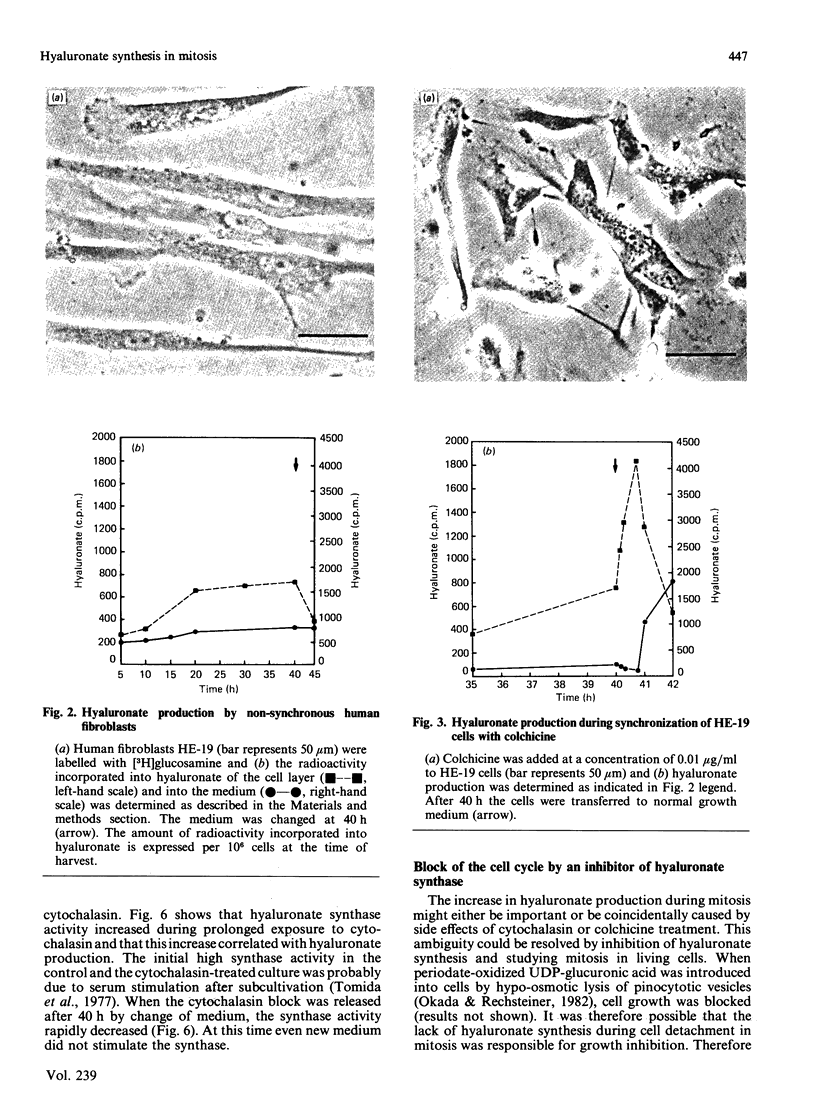
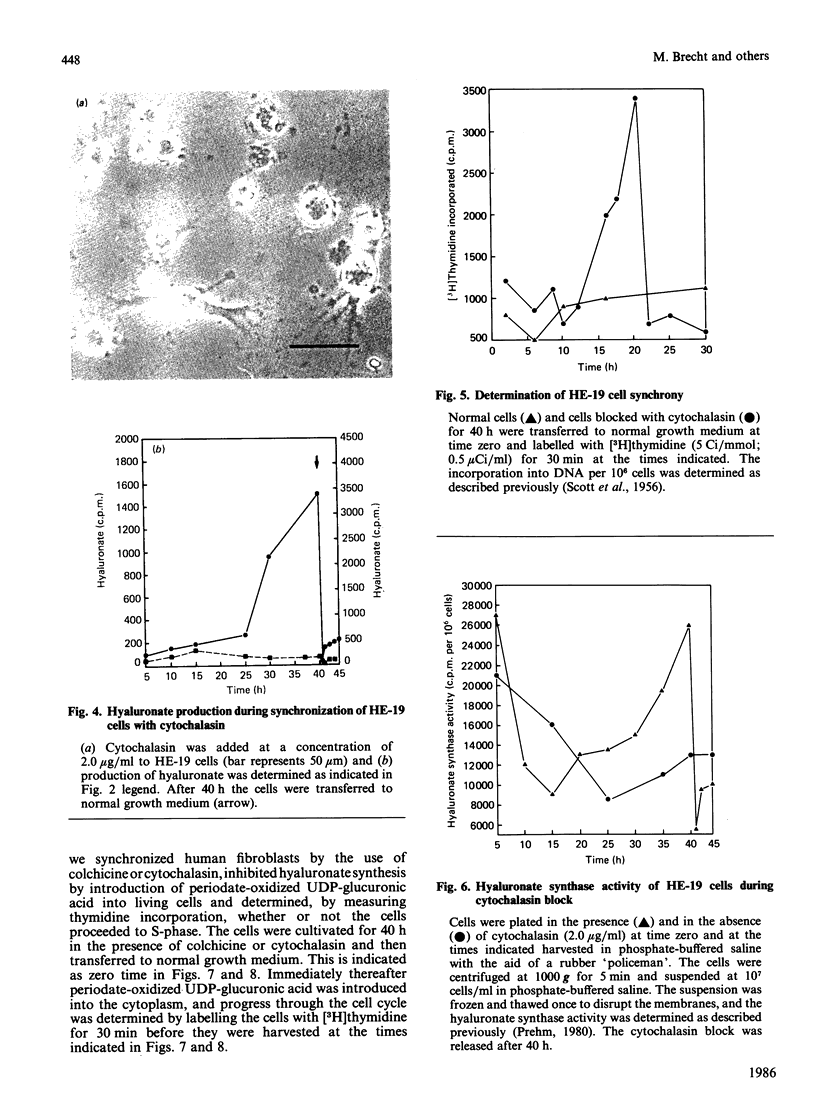
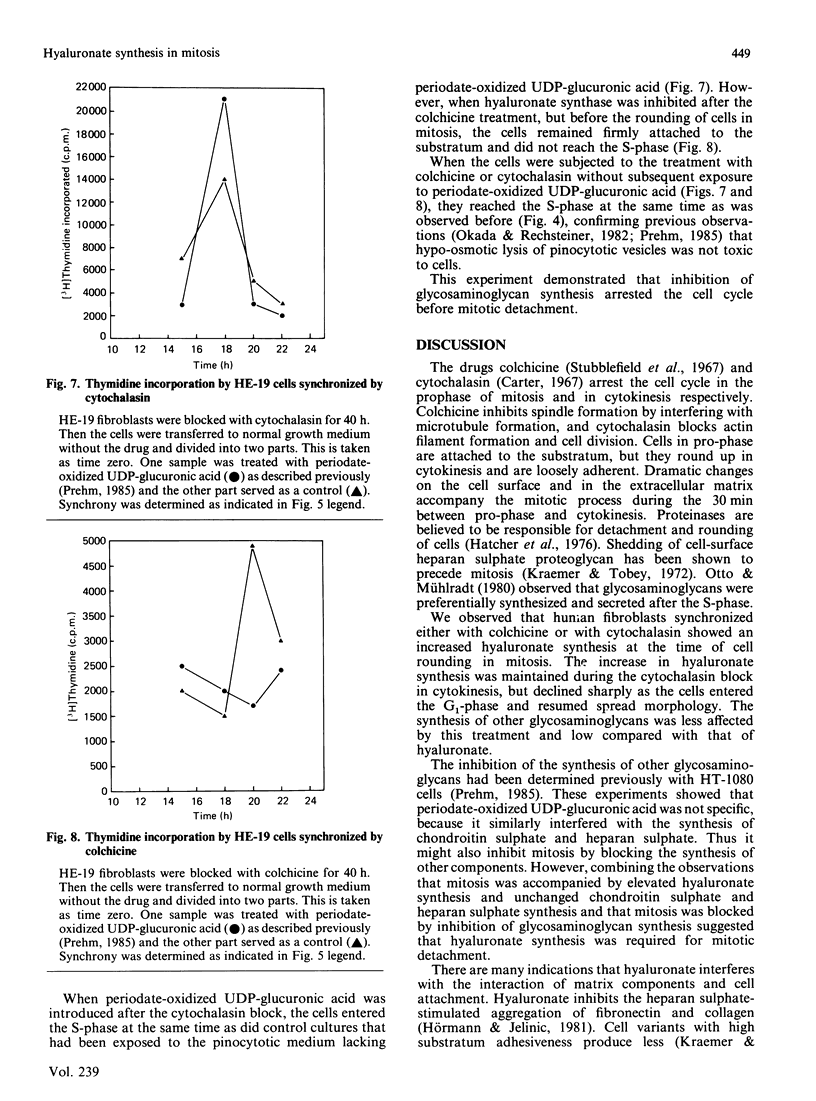
Human-embryo fibroblasts were synchronized by means of colchicine and cytochalasin, and the production of hyaluronate was determined by [3H]glucosamine incorporation and ion-exchange chromatography. Cells arrested by colchicine synthesized small amounts of hyaluronate, whereas cells blocked by cytochalasin were stimulated in hyaluronate production. When the colchicine block was released, there was an increased synthesis of hyaluronate, which appeared first in the cellular fraction and was then shed into the culture medium. After release of the cytochalasin block, the hyaluronate production declined to that found with unsynchronized cells. A comparable increase of hyaluronate synthase activity was observed during mitosis. When hyaluronate synthesis was blocked by periodate-oxidized UDP-glucuronic acid, the cells were arrested in mitosis before rounding of cells. These results suggest that hyaluronate synthesis is required for detachment and rounding of cells during mitosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abatangelo G., Cortivo R., Martelli M., Vecchia P. Cell detachment mediated by hyaluronic acid. Exp Cell Res. 1982 Jan;137(1):73–78. doi: 10.1016/0014-4827(82)90009-x. [DOI] [PubMed] [Google Scholar]
- Barnhart B. J., Cox S. H., Kraemer P. M. Detachment variants of Chinese hamster cells. Hyaluronic acid as a modulator of cell detachment. Exp Cell Res. 1979 Mar 15;119(2):327–332. doi: 10.1016/0014-4827(79)90360-4. [DOI] [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- Fisher M., Solursh M. The influence of the substratum on mesenchyme spreading in vitro. Exp Cell Res. 1979 Oct 1;123(1):1–13. doi: 10.1016/0014-4827(79)90416-6. [DOI] [PubMed] [Google Scholar]
- Hatcher V. B., Wertheim M. S., Rhee C. Y., Tsien G., Burk P. G. Relationship between cell surface protease activity and doubling time in various normal and transformed cells. Biochim Biophys Acta. 1976 Dec 21;451(2):499–510. doi: 10.1016/0304-4165(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Glycosaminoglycan synthesis by cultured human skin fibroblasts after transformation with simian virus 40. J Biol Chem. 1977 Jul 25;252(14):4777–4785. [PubMed] [Google Scholar]
- Hronowski L., Anastassiades T. P. The effect of cell density on net rates of glycosaminoglycan synthesis and secretion by cultured rat fibroblasts. J Biol Chem. 1980 Nov 10;255(21):10091–10099. [PubMed] [Google Scholar]
- Hörmann H., Jelinić V. Regulation by heparin and hyaluronic acid of the fibronectin-dependent association of collagen, Type III, with macrophages. Hoppe Seylers Z Physiol Chem. 1981 Jan;362(1):87–94. doi: 10.1515/bchm2.1981.362.1.87. [DOI] [PubMed] [Google Scholar]
- Knudson W., Biswas C., Toole B. P. Interactions between human tumor cells and fibroblasts stimulate hyaluronate synthesis. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6767–6771. doi: 10.1073/pnas.81.21.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer P. M., Barnhart B. J. Elevated cell-surface hyaluronate in substrate-attached cells. Exp Cell Res. 1978 Jun;114(1):153–157. doi: 10.1016/0014-4827(78)90047-2. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M., Tobey R. A. Cell-cycle dependent desquamation of heparan sulfate from the cell surface. J Cell Biol. 1972 Dec;55(3):713–717. doi: 10.1083/jcb.55.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuoka K., Mitsui Y., Murota S. Growth-coupled changes in glucosaminoglycans (heparan sulfate and hyaluronic acid) in normal and transformed human fibroblasts. Cell Biol Int Rep. 1985 Jun;9(6):577–586. doi: 10.1016/0309-1651(85)90023-2. [DOI] [PubMed] [Google Scholar]
- Okada C. Y., Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982 May;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- Otto A. M., Mühlradt P. F. Cell cycle dependent rate of labelling of cellular and secreted glycosaminoglycans in mouse embryonic fibroblasts. J Supramol Struct. 1980;13(3):281–294. doi: 10.1002/jss.400130302. [DOI] [PubMed] [Google Scholar]
- Prehm P. Hyaluronate is synthesized at plasma membranes. Biochem J. 1984 Jun 1;220(2):597–600. doi: 10.1042/bj2200597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Induction of hyaluronic acid synthesis in teratocarcinoma stem cells by retinoic acid. FEBS Lett. 1980 Mar 10;111(2):295–298. doi: 10.1016/0014-5793(80)80813-1. [DOI] [PubMed] [Google Scholar]
- Prehm P. Inhibition of hyaluronate synthesis. Biochem J. 1985 Feb 1;225(3):699–705. doi: 10.1042/bj2250699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Characterization of the synthase. Biochem J. 1983 Apr 1;211(1):181–189. doi: 10.1042/bj2110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P. Synthesis of hyaluronate in differentiated teratocarcinoma cells. Mechanism of chain growth. Biochem J. 1983 Apr 1;211(1):191–198. doi: 10.1042/bj2110191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT J. F., FRACCASTORO A. P., TAFT E. B. Studies in histochemistry. I. Determination of nucleic acids in microgram amounts of tissue. J Histochem Cytochem. 1956 Jan;4(1):1–10. doi: 10.1177/4.1.1. [DOI] [PubMed] [Google Scholar]
- Stubblefield E., Klevecz R., Deaven L. Synchronized mammalian cell cultures. I. Cell replication cycle and macromolecular synthesis following brief colcemid arrest of mitosis. J Cell Physiol. 1967 Jun;69(3):345–353. doi: 10.1002/jcp.1040690311. [DOI] [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Effects of adenosine 3':5'-cyclic monophosphate and serum on synthesis of hyaluronic acid in confluent rat fibroblasts. Biochem J. 1977 Mar 15;162(3):539–543. doi: 10.1042/bj1620539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomida M., Koyama H., Ono T. Induction of hyaluronic acid synthetase activity in rat fibroblasts by medium change of confluent cultures. J Cell Physiol. 1975 Aug;86(1):121–130. doi: 10.1002/jcp.1040860114. [DOI] [PubMed] [Google Scholar]
- Toole B. P., Biswas C., Gross J. Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6299–6303. doi: 10.1073/pnas.76.12.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. P. Hyaluronate turnover during chondrogenesis in the developing chick limb and axial skeleton. Dev Biol. 1972 Nov;29(3):321–329. doi: 10.1016/0012-1606(72)90071-1. [DOI] [PubMed] [Google Scholar]