Summary
Background
Even with increasing access to rapid HIV diagnosis and early antiretroviral therapy (ART) initiation, infants living with HIV seem to have adverse outcomes. We assessed the probability of death, viral suppression, and other HIV-related events in the first three years of life among early-treated children with perinatally-acquired HIV in South Africa, Mozambique, and Mali.
Methods
We enrolled a cohort of infants who initiated ART within the initial 6 months of life and within 3 months of diagnosis. These children were monitored 2, 6, 12 and 24 weeks after enrolment, followed by biannual check-ups up to 4 years after enrolment. We assessed the probability of death, viral load (VL) suppression, severe immunosuppression (according to WHO guidelines), and engagement in care using Kaplan–Meier plots, and hazard ratios for these outcomes using multivariable Cox regression models.
Findings
Two hundred and fifteen infants were enrolled and monitored for a median of 34 months [IQR, 16.3; 44.1]. ART initiation occurred at a median of 34 days of age [IQR, 26.0; 73.0]. The probability of death at 1 year of ART was 10% (95% CI, 6–14), increased to 12% (95% CI, 8–17) at 2 and remained in 12% at 3 years. The main risk factor for HIV/AIDS-related mortality was baseline viral load [HR: 2.98 (95% CI, 1.25–7.12)]. Sixty-one of 146 (42%) children achieved sustained virological control below lower limit of detection for any ≥1 year period between enrolment and 4 years after enrolment. Viral suppression during follow-up was inversely associated with baseline viral load [Hazard Ratio (HR): 0.72 (95% CI, 0.58–0.89] and adverse maternal social events [HR: 0.26 (95% CI, 0.15–0.45)]. Adherence to ART was assessed as optimal in 81% of the visits. Female sex at birth, lower age at diagnosis and maternal adverse social life events were risk factors for low adherence [Odds ratio, OR 1.25 (95% CI, 1.00–1.56); 1.12 (95% CI, 1.01–1.27) and 2.52 (95% CI, 2.16–12.37), respectively].
Interpretation
Despite early ART, mortality remains high in infants. High baseline VL and adverse maternal social environment increased the risk of poor outcomes. Sustained supportive strategies are essential during and after pregnancy, to achieve better survival.
Funding
Early Treated Perinatally HIV Infected Individuals: Improving Children's Actual Life (EPIICAL) is a research consortium funded by ViiV Healthcare and led by Penta Foundation. The funder was not involved in the analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication. The corresponding authors had access to all data and take final responsibility for the decision to submit.
Keywords: HIV, Children, Africa, Antiretroviral treatment, Antiretroviral therapy
Research in context.
Evidence before this study
We searched PubMed and conference proceedings using the search terms “HIV”, “Antiretroviral Therapy (ART)”, and “early infant treatment” for English language articles published up to September 22nd, 2023. Since the development of ART, mortality among children living with HIV has fallen 10-fold. The CHER trial (NCT00102960) demonstrated that mortality declined from 16% to 4% in children treated at diagnosis instead of initiating treatment when symptoms or signs of immunosuppression occurred. Despite current guidelines recommending early treatment for children, mortality remains high. Even if ART is initiated within the first few days of birth, the risk of adverse outcomes in infants born with HIV in high-prevalence settings during first years of life remains excessive.
Added value of this study
This multicentre large cohort of 215 infants living with HIV diagnosed shortly after birth and followed for 3 years shows the disproportionate risk of mortality and adverse events of infants despite early ART. ART was started at a median of 34 days of life. The probability of death was 10% at 1 year and 12% at 2 and 3 years. Infants died predominantly in the first 6 months of life. Only 41% of participants achieved sustained virological control below lower limit of detection for any ≥1 year period between enrolment and 4 years after enrolment. Immunosuppression during the first months of life was common. Adherence to ART and engagement in care were suboptimal. We identified risk factors for mortality (baseline viral load, region, CD4 slope), for severe immunosuppression (baseline viral load, region, male sex, low adherence), for lack of virological suppression (baseline viral load, adverse social life events in the mother), and for low adherence (adverse social live events in the mother, female sex, early diagnosis). We also show the risk factors as a chain of events from birth leading to death.
Implications of all the available evidence
Key areas for intervention include reducing baseline viral load through stronger maternal or neonatal interventions, possibly including long-acting agents or broadly neutralizing antibodies. Also, strong, and sustained health and social support for mothers and children living with HIV during pregnancy and the infant's early life.
Introduction
In 2022, UNICEF estimated the number of children living with HIV (CLHIV) as 2.58 million, 85% in Sub-Saharan Africa. Children comprise 6.6% of the global population living with HIV, but account for 16% of HIV-associated deaths.1 Despite guidelines advocating for rapid HIV diagnosis and early antiretroviral therapy (ART) initiation in all infants2 only 57% of children are receiving ART, compared to 77% of adults.1
The CHER trial demonstrated that mortality declined from 16% to 4% in children treated at diagnosis instead of initiating treatment when symptoms or signs of immunosuppression occurred,3 but even if ART is initiated within the first few days of birth, the risk of adverse outcomes in infants born with HIV in high-prevalence settings during first years of life remains excessive.4, 5, 6, 7, 8 Risk factors associated with higher mortality and poor virological control include baseline VL,4,6,9 CD4%,4,7,9 birth weight, gestational age, sex, neonatal hospitalization,4 maternal age and adherence to ART,4,9 ART initiation >6 months,10 child adherence, and social factors as having refrigerator,5 among others.
Early treated infants have garnered interest for potential cure strategies.8,10,11 The Early Treated Perinatally HIV Infected Individuals: Improving Children's Actual Life (EPIICAL) Consortium was formed in 2016, aiming to identify candidates for proof-of-concept trials focused on an HIV cure. The first step towards this goal was establishing the Early Anti-Retroviral Treatment in Children (EARTH, NCT05784584) Cohort in South Africa, Mozambique, and Mali.
This study presents data on the probability of death, virological suppression, severe immunosuppression, and healthcare engagement in CLHIV who initiated ART early during their first years of life in the EARTH Cohort.
Methods
Study design and participants
EARTH Cohort is a prospective, multicentre study of early treated CLHIV followed for up to 4 years. This cohort was followed in 4 urban and 2 rural settings in Johannesburg, Cape Town, uMkhanyakude District in Kwa-Zulu Natal (South Africa), Maputo and Manhiça (Mozambique), and Bamako (Mali)—see Appendix for details on enrolling centres. Recruitment occurred between May 1st, 2018, and May 1st, 2021, and follow-up ended in February 2024.
We included infants infected very early in life (≤90 days) and who started ART very early (≤90 days after diagnosis). Inclusion criteria were age below 180 days of age at ART initiation with 2 positive molecular tests (DNA or RNA PCR) positive for HIV, including infants with perinatally-acquired HIV who started ART at ≤90 days after HIV diagnosis; or breastfed infants identified as having HIV ≤90 days of age and who started ART ≤90 days after diagnosis. Written informed consent from the caregivers/legal representatives was obtained. Exclusion criteria included second and successive HIV RNA PCR negative before initiation of ART, not being naïve for ART, not being able to do the follow-up, malignancy at diagnosis, current concomitant immunosuppressive therapy (including >15 days and >2 mg/kg/day of prednisone-equivalent), any situation that precludes inclusion in the study, at the discretion of the investigator, and withdrawal of consent. All mothers had access to public healthcare services, including a primary health-care program, prevention of vertical HIV transmission and ART programs.
An independent Endpoint Review Committee (ERC) was set up to consider if deaths were likely related to HIV, or if the relationship was not clear. The members (CF, HR, PA) were selected among paediatric HIV specialists who were members of the institutions of the EPIICAL Consortium, but not directly involved in the care of children enrolled in the cohort. All the experts had access to the information available in the database, plus additional, specific information if requested.
The independent Ethics and Institutional review boards of all the participating institutions and Countries approved the study.
Procedures
All infants were screened for HIV within 6 weeks after birth according to the local protocols. In South Africa, children were generally tested at birth using a centralized test detecting total nucleic acid, with families collecting results 1–4 weeks later. In Mozambique and Mali, children were tested between 4 and 6 weeks of age using point-of-care (POC) nucleic acid PCR, with results given on the same day. The methods for diagnosis and posterior VL analyses at each site are described in the Supplementary Material.
After confirmation of HIV diagnosis in a different blood sample, infants started ART immediately following national guidelines, and were enrolled in the study. Infants were followed two, six, 12 and 24 weeks after enrolment, and then every 6 months for up to 4 years. Clinical data, adherence and psychosocial events data, and blood for VL (quantification of HIV-RNA in plasma) and CD4 count, and percentage were collected at all the scheduled visits.
Additional biological samples were collected annually for further immunological and virological evaluation, including reservoir size. In case of missing visits, the study staff (including community-based fieldworkers) contacted the family, checked if the child was alive, and rebooked missed visits.
Study definitions
All-cause mortality
Mortality due to any cause.
HIV-related mortality
Mortality in a child attributable to HIV related diseases, according to the consensus of an external ERC comprising three external experts.
Virological suppression
Having ≥2 consecutive viral load (VL) measurements below the limit of detection across the sites (20 cp/mL in 5 sites, 150cp/mL in one site), in the tests performed at the visits.
Sustained virological control
Having at least 12 months of follow-up and VL below lower limit of detection during the last 12 months (having at least 3 tests, with a maximum interval between tests of 6 months).
Sustained immunological control
No significant immunosuppression during the last 12 months (minimum 3 tests, maximum interval between tests, every 6 months) according to WHO definition: CD4 percentage (%) >35% during first 11 months of life, >30% from 12 to 35 months, >25% from 36 to 59 months.12
Severe immunosuppression
Any values at or below age-related CD4 thresholds below which children have a greater than 5% chance of disease progression to severe clinical events or death in the next 12 months. This is, <25% for children <11 months, <20% for children from 12 to 35 months, and <15% from 36 to 59 months.12
Infant's optimal adherence in one specific visit
None of the following events: a) amount of medication more than foreseen in the container, b) run out of medicines, c) an ART interruption for a time more than 10% of the days between 2 visits or d) missing more than 10% of planned ART doses between visits, or e) a delay in collecting ART greater than 10% in the number of days between visits.
Adherence during follow-up
% of visits with optimal adherence to ART during the follow-up.
Engaged in care
Not being lost to follow-up (LTFU) over the study. LTFU included any patient missing visits during at least 1 year and who could not be reachable after a reasonable number of efforts. The event was assigned at the last visit performed.
Maternal adverse social life events
Any adverse change in employment (leading to unemployment and/or irregular work and/or lower pay for a job taken up), separation or relationship break-up, new partner, loss of home or move, death in the family, or other perceived adverse social life events during the study period (including domestic violence, alcohol or drug abuse, or unemployment perceived as adverse).
Maternal health issues
Any health problem affecting the mother during the follow-up
Weight/Height for age: Z-scores were calculated using Child Anthropometry z-Score calculator implemented in zscorer R package using WHO Growth Reference z-scores.
CD4 1-year slope
defined as the % CD4 change per year (cells/mm3/year) was calculated as the slope of a best-fit line obtained by linear regression of CD4 T cell percentage during one year against the time since ART initiation.
Endpoints
The primary endpoint was probability of all-cause mortality over time. The secondary endpoints were probabilities of HIV-related mortality, virological suppression, severe immunosuppression, engagement in care, and adherence over time.
Statistical analysis
This is a study with a convenience sample whose size was based on the ability to recruit participants fulfilling the inclusion criteria sites. We expected to recruit a minimum of 150 patients at all sites over the recruitment period. Assuming a 30% loss to follow-up, we expected a set of 100 patients to analyse at the end of the study.
Data was summarized in tables by average (standard deviations) or medians and interquartile ranges [IQR] for continuous variables and absolute counts and percentages for categorical variables. In summary tables of categorical variables, comparisons were assessed using Fisher's exact test and Mann–Whitney U test for continuous variables. To analyse the probability of each endpoint Kaplan–Meier curves were performed. A competing risk multivariable Cox regression was performed including unclear-relationship cause of death as a competing risk for the HIV-related death. LTFU was treated as censoring event. Each endpoint model was adjusted by baseline covariates (excluding the respective outcome) such as region, sex, baseline CD4, CD4% 1-year slope, age at diagnosis, age at ART, ART regimen, percentage of visits with suboptimal adherence during follow-up, and baseline children WHO reference weight-for-age (WAZ) and longitudinal time-dependent covariates such as measurements of VL or presence of mother's social or health issues. Characteristics of the variables, including units in the case of quantitative variables and levels in the case of qualitative variables are specified in Table 1. Selection of potential covariates was made according to mortality predictors shown in literature. Backward stepwise elimination was applied to reach the final multivariable model and Akaike Information Criterion (AIC) was used to identify the best fitting model. To assess the probability of having a suboptimal adherence over time (<90%), a multivariable binomial regression was performed including having suboptimal adherence as outcome weights as weighted by the number of visits. Covariates such as region, sex, age at ART, ART regimen, age at diagnosis, presence of mother's social or health issues, WAZ, baseline VL, and baseline CD4 were included in the adherence-related model.
Table 1.
Study population characteristics (N = 215).
| Characteristics | N, (%)–Median [IQR] |
|---|---|
| Enrolling site | |
| South Africa | 125/215 (58.3%) |
| Family Center for Research with Ubuntu, Tygerberg Hospital (Cape Town) | 50/215 (23.3%) |
| Perinatal Health Research Unit, Hospital Chris Baragwanath (Johannesburg) | 44/215 (20.5%) |
| Africa Health Research Institute (KwaZulu Natal) | 31/215 (14.4%) |
| Mozambique | 79/215 (36.7%) |
| Centro de Investigaçao en Saúde de Manhiça (Maputo) | 45/215 (20.9%) |
| Ariel Foundation (Maputo) | 34/215 (15.8%) |
| Mali | 11/215 (5.1%) |
| Gabriel Touré Hospital (Bamako, Mali) | 11/215 (5.1%) |
| Time of follow-up | |
| Months | 34.0 [16.3; 44.1] |
| Female | |
| N (%) | 101/215 (47.0%) |
| Preterm birth | |
| N (%) | 70/204 (34.3%) |
| Weight-for-age z-score | |
| Z-score | −1.16 [−2.15; −0.21] |
| Age at HIV diagnosis | |
| Days of life | 31.0 [0.0; 48.0] |
| Age at ART initiation | |
| Days of life | 34.0 [26.0; 73.0] |
| ART regimen initiation | |
| 3 TC + ABC + LPV/r | 140/215 (65.1%) |
| 3 TC + ABC + NVP | 3/215 (1.4%) |
| 3 TC + AZT + LPV/r | 38/215 (17.7%) |
| 3 TC + AZT + NVP | 34/215 (15.8%) |
| Children WHO Clinical stage at baseline | |
| Stage I | 194/214 (90.7%) |
| Stage II | 9/214 (4.2%) |
| Stage III | 6/214 (2.8%) |
| Stage IV | 5/214 (2.3%) |
| Baseline viral load (log10 copies/mL) | 4.95 [3.58; 5.82] |
| ≥5 logs | 104/211 (49.3%) |
| Baseline CD4 (Cell/mm3) | |
| Percentage CD4 | 37.1 [29.0; 45.4] |
| <15% | 10/202 (5.0%) |
| 15–25% | 15/202 (7.4%) |
| ≥25% | 177/202 (87.6%) |
| Baseline absolute CD4 count | |
| Cell/mm3 | 1797 [1046; 2624] |
IQR: interquartile range. ART: antiretroviral therapy. WHO: World Health Organization. 3 TC: lamivudine. ABC: abacavir. LPV/r: lopinavir-ritonavir. AZT: zidovudine. 13/215 (6%) patients presented missing baseline CD4%, and 4/215 (1.8%) patients presented missing VL at baseline.
To build the primary endpoint (mortality) multivariable model, a non-parametric missing value imputation was done. Continuous and categorical variables included in the model were imputed using Random Forest algorithm implemented in missForest R package. Imputation error had a 0.131 normalised root mean square error and a 0.005 proportion of falsely classified entries. A complete-case analysis was done excluding missing values were performed for the secondary endpoints. A p-value <0.05 was considered statistically significant. All analyses were performed using R (version 4.1.1).
Role of the funding source
EPIICAL is a consortium funded by ViiV Healthcare. The funder was not involved in the analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication. The corresponding authors had access to all data and take final responsibility for the decision to submit.
Results
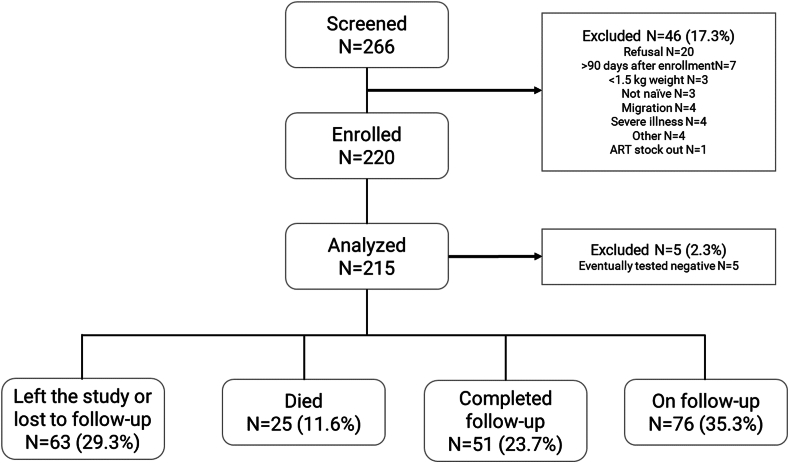
Of 266 infants initially approached; 215 participants were included in the analysis (Fig. 1). The median follow-up duration by the time of analysis was 34.0 months [16.3; 44.1], representing 528.9 person-years of follow-up. Maternal features are reported in Supplementary Table S1.
Fig. 1.
Cohort flowchart.
One hundred and one of 215 participants (47.0%) were females. The median age at HIV diagnosis was 31 days [0; 48] (Supplementary Figure S1), and the median age at ART initiation was 34 days [26; 73]. The baseline median WAZ was −1.16 [−2.15; −0.21], and median height for age z-score (HAZ) was −1.85 [−2.93; −0.86]. A total of 70 out of 204 (34.3%) infants were premature (<37 weeks of gestational age), and low birthweight (<2500 g) occurred in 48 out of 215 (22.3%) infants. Median baseline VL was 4.95 [3.58; 5.82] log10 copies/mL, and median baseline CD4% was 37.1% [29.0; 45.4] (Table 1). The most common first-line ART regimen at initiation was lamivudine (3 TC) + abacavir (ABC) + lopinavir/ritonavir (LPV/r) (Table 1). Twenty-two (10.2%) participants switched to dolutegravir (DTG) during the follow-up according to national policy in Mozambique.
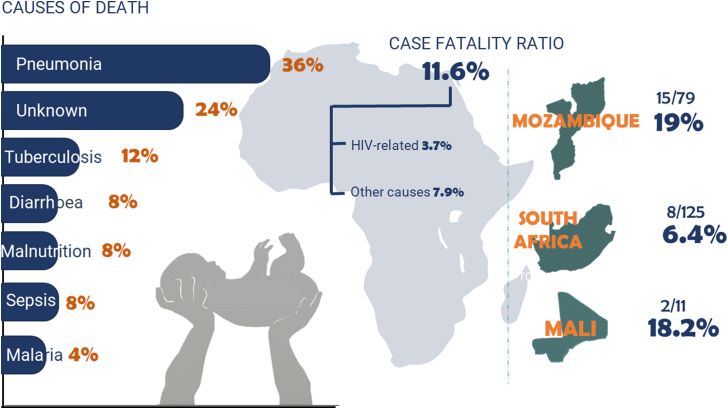
A total of 25 of 215 (11.6%) participants died during the follow-up. The mortality varied by region, with 8/125 (6.4%) in South Africa, 15/79 (19.4%) in Mozambique, and 2/11 (18.2%) in Mali (p = 0.019) (Fig. 2). The ERC attributed the cause of death to likely HIV-related causes in 8 of 25 (32%) infants, resulting in a likely HIV/AIDS-related CFR of 3.7% (8/215). The primary causes of death are reported in Supplementary Table S2. The median age of death was 5.3 months [3.0–9.6], ranging from 1.5 months to 22.3 months. Deaths occurred after a median of 2.8 months on ART [0.7–8.4], with a range of 0.3–21.1 months.
Fig. 2.
Mortality summary showing the causes of death and mortality according to HIV/AIDS relationship and region of recruitment.
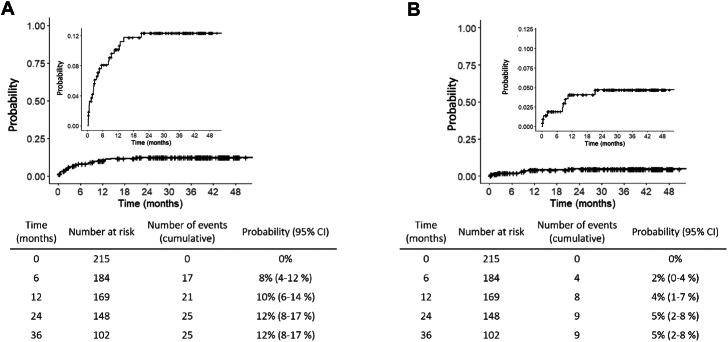
The probability of all-cause mortality 1 year after enrolment was 10% (95% CI, 6%–14%), and it increased to 12% (95% CI, 8%–17%) at 2 and remained in 12% 3 years after enrolment (Fig. 3). For HIV/AIDS-related mortality, the probability was 4% (95% CI, 1%–7%) at 1 year and 5% (95% CI, 2%–8%) at 2 and 3 years.
Fig. 3.
Kaplan–Meier function showing the probability of mortality. Panel A: All-cause mortality probability (larger panel: relative to 100% of the participants; smaller panel: zoom with a different scale Y-axis). Panel B: HIV-related mortality probability (larger panel: relative to 100% of the participants; smaller panel: zoom in the first part of the curve).
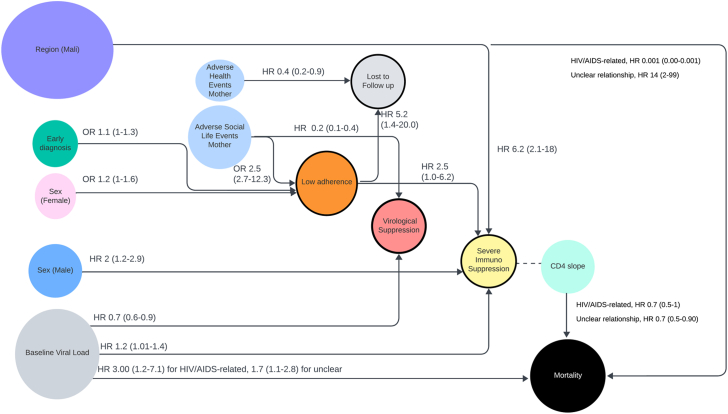
In a competing risk Cox model to analyse mortality risk factors, the best-fitting model included baseline VL, sex, CD4% slope during follow-up, age at ART initiation, and region. Only baseline VL, CD4% slope, and region were significant. Baseline VL was significantly associated with both likely HIV-related [HR, 2.98 (95% CI, 1.25–7.12)], and unclear relationship with HIV causes of death [HR, 1.75 (95% CI, 1.09–2.80)]. Likewise, CD4% slope was significantly associated with both causes of death [HR, 0.69 (95% CI, 0.46–1.03) and 0.68 (95% CI, 0.51–0.90)]. Patients from Mali had a higher probability of death with an unclear relationship with HIV compared to South Africa [HR, 13.8 (95% CI, 1.92–98.8)] (Supplementary Table S3, Fig. 4).
Fig. 4.
Significant associations between risk factors and endpoints. Numbers represent the hazard ratio (HR) and odds ratio (OR) (95% confidence interval).
For secondary outcomes, approximately half of the infants achieved virological suppression, with 98 out of 193 patients with enough data according to the suppression definition (50.7%) reaching this milestone at some point. For them, the median time to suppression was 5.5 months [2.07–15.57] (Supplementary Figure S2). The probability of achieving suppression at some point was 38% after 1 year of ART (95% CI, 30–44%), 50% at 2 years (95% CI, 42–57%), and 60% at 3 years (95% CI, 51–67%) (Supplementary Figure S3). However, at every visit, there was a proportion of children who changed from undetectable to detectable, across the study. This proportion varied, and the average was 23.9%. Most patients with available data who died (18/19, 94.7%) or were LTFU (44/58, 75.8%) had detectable VL in the previous visit (Supplementary Figure S4).
In the multivariable Cox model to predict suppression, covariables such as baseline VL and presence of maternal adverse social adverse events before suppression were included according to AIC selection method. Factors associated with the viral suppression included baseline VL [HR: 0.72 (95% CI, 0.58–0.89, p = 0.003)] and maternal adverse social life events after birth and before VL tests [HR: 0.26 (95% CI, 0.15–0.45, p < 0.001)].
Among patients with ≥1 year of follow-up and ≥2 VL measurements, 61 of 146 (41.8%) achieved sustained virological control for at least 1 year. However, only 27 of these 61 (45%) had optimal adherence in >90% of the visits. Interestingly, 22 of 61 (36.1%) did not achieve sustained immunological control to normal for age despite virological control (Supplementary Figure S5).
The median percentage of visits with optimal adherence to ART was 80.9% (IQR, 62.5–100%), 33/206 (16%) children with available data had less than 50% of visits with optimal adherence, and 97/206 (47.1%) had between 50% and 90%. Infants diagnosed early were more likely to have <90% visits with optimal adherence (OR 1.12 (95% CI, 1.01–1.27), p = 0.045). Females were more likely to have <90% visits with optimal adherence (OR 1.25 (95% CI, 1.00–1.56), p = 0.051). The presence of prior maternal adverse social life events was significantly associated with infant adherence <90% (OR: 2.52 (95% CI, 2.16–12.37, p < 0.0001).
Severe immunosuppression occurred in 104/208 (50%) of participants at some point during the study. The probability of having ever experienced severe immunosuppression at 1, 2, and 3 years of ART was 37% (95% CI, 29–43%), 46% (95% CI, 38–53%) and 50% (95% CI, 48–57%) respectively (Supplementary Figures S6 and S7). Associated factors included higher baseline VL (HR: 1.20 (95% CI, 1.01–1.43), p = 0.048), sex (males, HR: 1.94, (95% CI, 1.27–2.95; p = 0.002)), and the region of recruitment: patients from Mali were more likely to have severe immunosuppression than South Africa (HR: 6.26 (95% CI, 2.17–18.02), p = 0.001). The higher percentage of visits with suboptimal adherence, the higher probability of severe immunosuppression (HR: 2.51 (95% CI, 1.02–6.23), p = 0.046, Fig. 4).
Globally, 63/215 (29.3%) participants were LTFU. After 1, 2 and 3 years, the probability of engagement in care was 87% (95% CI, 83–92%), 82% (95% CI, 77–88%) and 71% (95% CI, 64–78%), respectively (Supplementary Figure S8). The percentage of visits with suboptimal adherence was significantly associated with the probability of LTFU (HR: 5.16 (95% CI, 1.38–19.4), p = 0.015). Maternal health issues were inversely associated with the probability of LTFU (HR: 0.43 (95% CI, 0.20–0.90), p = 0.026).
Discussion
The EARTH study examined the long-term outcomes for infants with perinatally-acquired HIV who began early ART in three African nations. Despite monitoring them two, six, 12 weeks and 24 weeks after enrolment, and then biannually for up to 4 years, the study revealed concerning mortality, loss to follow up and sub-optimal virological suppression rates, particularly within the initial six months of life.
The overall all-cause mortality proportion over the follow-up (median, 34 months) was 12%, higher than the 7% (95% CI 6–8) at 24 months reported in a meta-analysis of children living with HIV in sub-Saharan Africa from 2001 to 2016,13 or the 4% observed in the CHER trial over 40 months.3 However, the only infants eligible for CHER were those who did not meet CD4 and clinical criteria to start ART, so they were preselected to have lower risk of death. Also, the CHER trial and most studies in the metanalysis enrolled participants later in life (CHER, 7.4 [6.6–8.9] weeks, metanalysis, 4.4 years), so children with most advanced disease already died before getting onto ART. Our study showed mortality rates of 10% in the first year, in line with recent real-life paediatric cohorts showing 7%–18%.4, 5, 6, 7 Mortality seems to be lower in infants with optimal features, very early diagnosis and treatment and optimal follow-up enrolled in interventional studies: 3/73 (4%) at 48 weeks in infants treated before 14 days of life in South Africa,14 2/40 (5%) at 24 weeks in Botswana, and 2/54 (3.6%) at 24 weeks in the IMPAACT P1115 study, both receiving full ART within the first 48 h of life.15,16 In any case, while HIV care has improved globally, young infants and their families still face significant challenges.
Interestingly, only a third of deaths were directly attributed to HIV. However, risk factors were similar for both groups (HIV-related and unclear causes), including VL (albeit 3-fold for HIV-related compared to other) and CD4% slope, suggesting a potential role of HIV also in those children with unclear relationship with HIV. This may also underscore the social and health vulnerability of high-risk infants with HIV, potentially exacerbated by maternal adverse social events and poor adherence, which all contributing to poor virological suppression and severe immunosuppression (Fig. 4).
Baseline viral load consistently correlated with adverse outcomes, indicating the need for better interventions to decrease the peak of viremia. Better maternal ART adherence during pregnancy and after delivery may reduce VL in those infants who acquire infection despite maternal ARTs.17 Three-drugs ART from birth may be an option. Potent drugs such as DTG are desirable, although not currently available for infants under four weeks of age. Additional therapeutic approaches, such as early long-acting ART or broadly neutralizing antibodies, may also contribute to achieving earlier viral suppression and potentially reducing mortality.
Most deaths occurred within the first six months, emphasizing the importance of frequent follow-up, emotional and adherence support, and counselling during this critical period.18, 19, 20
In our study, only 50% of participants achieved viral suppression within two years. Also, as one fifth of children relapsed to detectable at every visit, only 42% maintained virological control during one year of follow-up, consistent with other cohorts.4,7,8
Interestingly, some children presented sustained undetectable VLs with poor adherence, which suggests potential spontaneous control. Female sex and maternal adverse social events, but also lower age at diagnosis were associated with poor adherence. Younger age at diagnosis being associated with worse adherence is a concerning finding but adds to the evidence of the considerable challenges for mothers of attaining optimal adherence with neonatal treatment even with early diagnosis.7
There are limitations to our study. Overall, the samples size is small, especially in some sites as Mali—which has an impact in the risk estimation. Mortality may be underestimated due to LTFU (29% of participants) with unknown outcomes. Also, not all the participants completed 4 years of follow-up at the time of the report, so mortality and LTFU may be underestimated, as well as other endpoints related with long-term follow-up. In studies preceding wide ART availability, 38% of children LTFU and traced had died.18 Virological suppression and severe immunosuppression were considered as terminal events, so we considered only the first time that the event happened, but those events theoretically might happen on-off during the follow-up. Our study considered only one primary cause of death, potentially overlooking other contributing factors. Also, classification into “HIV/AIDS likely related” and “unclear relationship with HIV/AIDS” may have a subjective component, and the ERC often lacked relevant information to classify confidently—which may impact the results. Some biases may happen, as channelling bias because participants were enrolled in a non-randomised fashion hence baseline differences existed; multi-country differences in HIV implementation programs are different hence bias may have been introduced. Self-reported measures such as those on perceived social issues, health problems, or adherence may introduce social-desirability bias. Variation in time of those issues was not included in the models. Spikes between visits’ tests could not be detected with this scheme. The median time of diagnosis and ART initiation was not the same, likely due to the verification of positive HIV status, counselling, family discussion and consent. We were not able to differentiate between infection in utero, during delivery or after delivery via breastfeeding. This may have influenced the lack of association of age at ART with the analysed endpoints. Finally, we have done imputation of missing data in the primary endpoint, whereas secondary endpoints were not imputed. Although the difference in the final numbers is likely to be small, this represents a limitation.
In summary, infants with perinatally acquired HIV, despite early ART initiation, face a high risk of death in their early months. The risk is higher in those disadvantaged by high baseline VL in a suboptimal health system, coupled with adverse social events among caregivers. These lead to a negative spiral of poor adherence to treatment, lack of virological suppression, immunosuppression, and death. Interventions should focus on strengthening health systems, reducing baseline viral loads, and providing strong health and social support to the mother and the child during pregnancy and the infant's early life.
Contributors
Alfredo Tagarro, Sara Dominguez and Álvaro Ballesteros accessed and verified the underlying data. Alfredo Tagarro and Pablo Rojo were responsible for the decision of submitting this manuscript.
Data sharing statement
Penta, as sponsor of the EARTH Study, is the sole and exclusive owner of Study Data and Study Results and all intellectual property rights pertaining to such Study Data and Study Results. Data sharing will be considered upon reasonable request, after consideration of a written scientific proposal by the requesting institution.
Declaration of interests
The following institutions, to which the author-researchers belong, received funding from the sponsor (Penta Foundation) to carry out this research, who in turn received a non-competitive grant from ViiV named Early Treated Perinatally HIV Infected Individuals: Improving Children's Actual Life (EPIICAL): Fundación de Investigación Biomédica Hospital 12 de Octubre, Stellenbosch University, University of the Witwatersrand, Africa Health Research Institute, Fundação Ariel Glaser contra o SIDA Pediátrico, Centro de Investigaçao em Saude de Manhiça, Instituto Nacional de Saúde, Bambino Gesù Children's Hospital, Ragon Institute of MGH, MIT, and Harvard, University College of London, Gianni Benzi Pharmacological Research Foundation, University of Miami, Centre Hospitalier Universitaire Gabriel Touré, ISGlobal, Columbia University Irving Medical Center, University of Rome “Tor Vergata”, Penta Foundation. The authors reported no other relationships/conditions/circumstances that present a potential conflict of interest for this research.
Elisa López reported being a full employee of ViiV Healthcare since April 2023.
Outside the submitted work, Tacilta Nhampossa reported a grant from EDCTP Career Development Fellowships. Proposal: TMA2017CDF-1927 (2019–2022). Sheila Fernández-Luis reported a grant from Secretariat of Universities and Research, Ministry of Enterprise and Knowledge of the Government of Catalonia and cofounded by European Social Fund. Paolo Palma reported a grant from NIH from 2020 to 2025 named PAVE, grant from NIH-NIAID (Targting HIV reservoirs in children with HIVIS-DNA and MVA-CMDR vaccines, and reported being the founder of Promiomics, a spin-off company of University Tor Vergata. Nicola Cotugno reported being the CRO and founder of Promiomics, a spin-off company of University Tor Vergata. Helena Rabie reported personal fees from ViiV community engagement meeting, personal fees from MSD Community engagement meeting.
Acknowledgements
ViiV funded the EPIICAL Consortium through a grant to the Penta Foundation.
The researchers thank the families for their participation, as well as all the individuals and committees involved in its evaluation and implementation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102648.
Appendix A. Supplementary data
References
- 1.Global and regional trends. https://data.unicef.org/topic/hivaids/global-regional-trends
- 2.2nd, editor. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. World Health Organization; Geneva: 2016. [PubMed] [Google Scholar]
- 3.Violari A., Cotton M.F., Gibb D.M., et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Millar J.R., Bengu N., Fillis R., et al. HIGH-FREQUENCY failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal NON-ADHERENCE. eClinicalMedicine. 2020;22 doi: 10.1016/j.eclinm.2020.100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ateba Ndongo F., Texier G., Ida Penda C., et al. Virologic response to early antiretroviral therapy in HIV-infected infants: evaluation after 2 Years of treatment in the pediacam study, Cameroon. Pediatr Infect Dis J. 2018;37:78–84. doi: 10.1097/INF.0000000000001745. [DOI] [PubMed] [Google Scholar]
- 6.Technau K.-G., Strehlau R., Patel F., et al. 12-month outcomes of HIV-infected infants identified at birth at one maternity site in Johannesburg, South Africa: an observational cohort study. Lancet HIV. 2018;5:e706–e714. doi: 10.1016/S2352-3018(18)30251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lain M.G., Vaz P., Sanna M., et al. Viral response among early treated HIV perinatally infected infants: description of a cohort in southern Mozambique. Healthcare. 2022;10:2156. doi: 10.3390/healthcare10112156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiau S., Abrams E.J., Arpadi S.M., Kuhn L. Early antiretroviral therapy in HIV-infected infants: can it lead to HIV remission? Lancet HIV. 2018;5:e250–e258. doi: 10.1016/S2352-3018(18)30012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiau S., Strehlau R., Shen Y., et al. Virologic response to very early HIV treatment in neonates. J Clin Med. 2021;10:2074. doi: 10.3390/jcm10102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiau S., Strehlau R., Technau K.-G., et al. Early age at start of antiretroviral therapy associated with better virologic control after initial suppression in HIV-infected infants. AIDS. 2017;31:355–364. doi: 10.1097/QAD.0000000000001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn L., Schramm D.B., Shiau S., et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV infected children when suppressed. AIDS. 2015;29:1053–1060. doi: 10.1097/QAD.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn L., Paximadis M., Da Costa Dias B., et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . 2007. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. [Google Scholar]
- 14.Ahmed I., Lemma S. Mortality among pediatric patients on HIV treatment in sub-Saharan African countries: a systematic review and meta-analysis. BMC Publ Health. 2019;19:149. doi: 10.1186/s12889-019-6482-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhn L., Strehlau R., Shiau S., et al. Early antiretroviral treatment of infants to attain HIV remission. eClinicalMedicine. 2020;18 doi: 10.1016/j.eclinm.2019.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maswabi K., Ajibola G., Bennett K., et al. Safety and efficacy of starting antiretroviral therapy in the first week of life. Clin Infect Dis. 2021;72:388–393. doi: 10.1093/cid/ciaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson B.S., Tierney C., Persaud D., et al. Infants receiving very early antiretroviral therapy have high CD4 counts in the first year of life. Clin Infect Dis. 2023;76:e744–e747. doi: 10.1093/cid/ciac695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel F., Shiau S., Strehlau R., et al. Low pretreatment viral loads in infants with HIV in an era of high-maternal antiretroviral therapy coverage. Pediatr Infect Dis J. 2021;40:55–59. doi: 10.1097/INF.0000000000002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kooten Niekerk N.K.M., Knies M.M., Howard J., et al. The first 5 Years of the family clinic for HIV at tygerberg hospital: family demographics, survival of children and early impact of antiretroviral therapy. J Trop Pediatr. 2006;52:3–11. doi: 10.1093/tropej/fmi047. [DOI] [PubMed] [Google Scholar]
- 20.Nachega J.B., Chaisson R.E., Goliath R., et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24:1273–1280. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.