Abstract
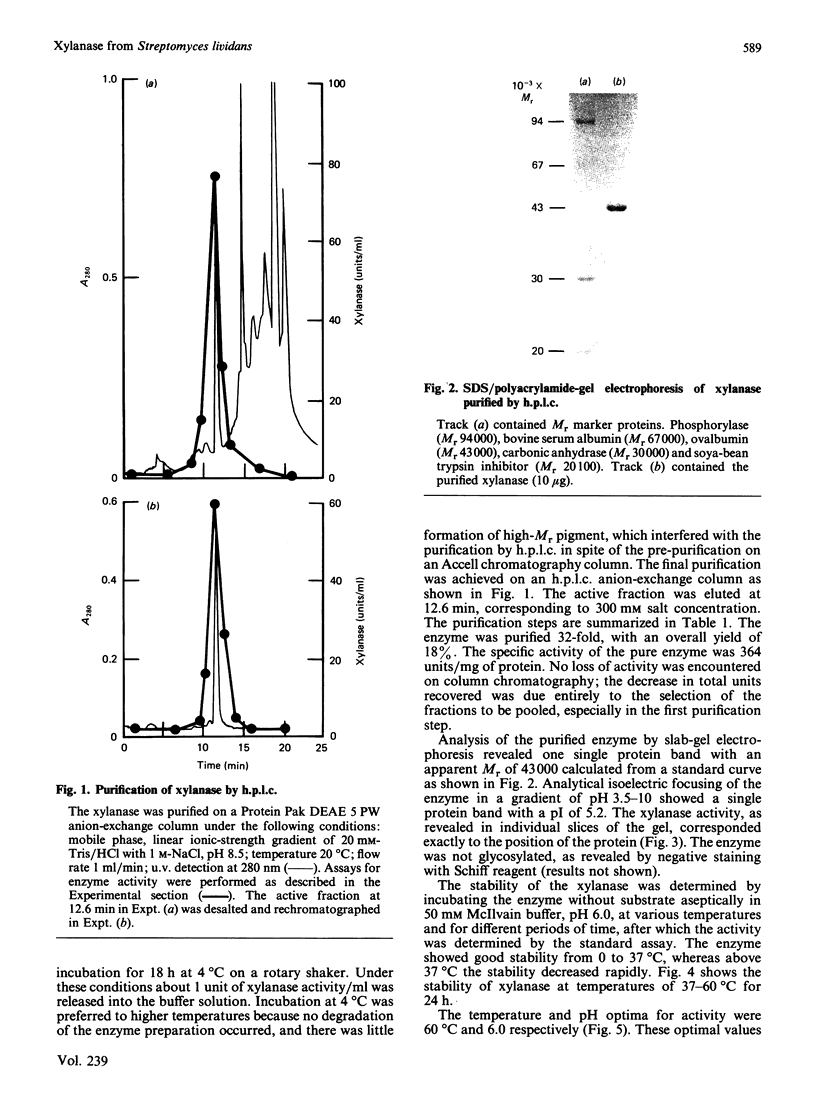
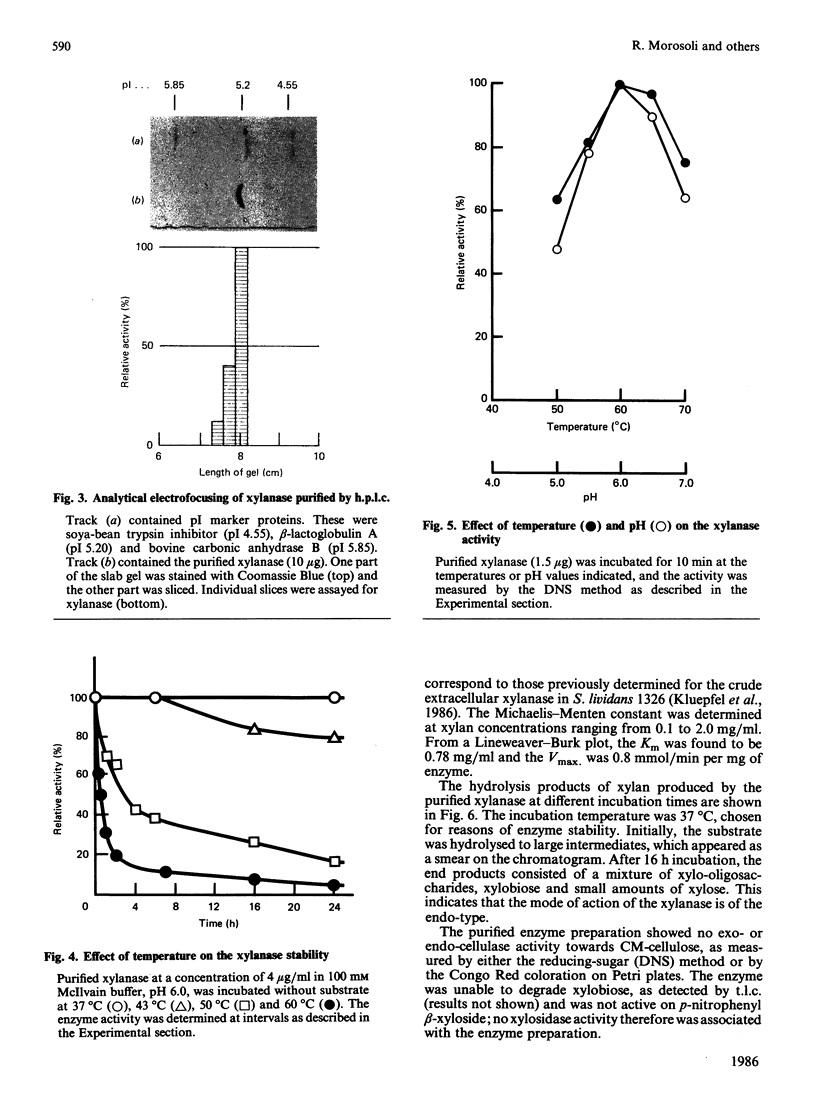
An extracellular xylanase produced by a cellulase-negative mutant strain of Streptomyces lividans 1326 was purified to homogeneity. The purified enzyme has an apparent Mr of 43,000 and pI of 5.2. The pH and temperature optima for the activity were 6.0 and 60 degrees C respectively, and the Km and Vmax. values, determined with a soluble oat spelts xylan, were 0.78 mg/ml and 0.85 mmol/min per mg of enzyme. The xylanase showed no activity towards CM-cellulose and p-nitrophenyl beta-D-xyloside. The enzyme degraded xylan, producing mainly xylobiose, a mixture of xylo-oligosaccharides and a small amount of xylose as end products. Its pattern of action on beta-1,4-D-xylan indicates that it is a beta-1,4-endoxylanase (EC 3.2.1.8).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier R., Desrochers M., Jurasek L., Paice M. G. Isolation and Characterization of a Xylanase from Bacillus subtilis. Appl Environ Microbiol. 1983 Aug;46(2):511–514. doi: 10.1128/aem.46.2.511-514.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Pettersson B. Purification and partial characterization of two acidic proteases from the white-rot fungus Sporotrichum pulverulentum. Eur J Biochem. 1982 Jun;124(3):635–642. doi: 10.1111/j.1432-1033.1982.tb06641.x. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- John M., Schmidt B., Schmidt J. Purification and some properties of five endo-1,4-beta-D-xylanases and a beta-D-xylosidase produced by a strain of Aspergillus niger. Can J Biochem. 1979 Feb;57(2):125–134. doi: 10.1139/o79-016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moldoveanu N., Kluepfel D. Comparison of beta-Glucosidase Activities in Different Streptomyces Strains. Appl Environ Microbiol. 1983 Jul;46(1):17–21. doi: 10.1128/aem.46.1.17-21.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paice M. G., Jurasek L., Carpenter M. R., Smillie L. B. Production, characterization, and partial amino acid sequence of xylanase A from Schizophyllum commune. Appl Environ Microbiol. 1978 Dec;36(6):802–808. doi: 10.1128/aem.36.6.802-808.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]