Abstract
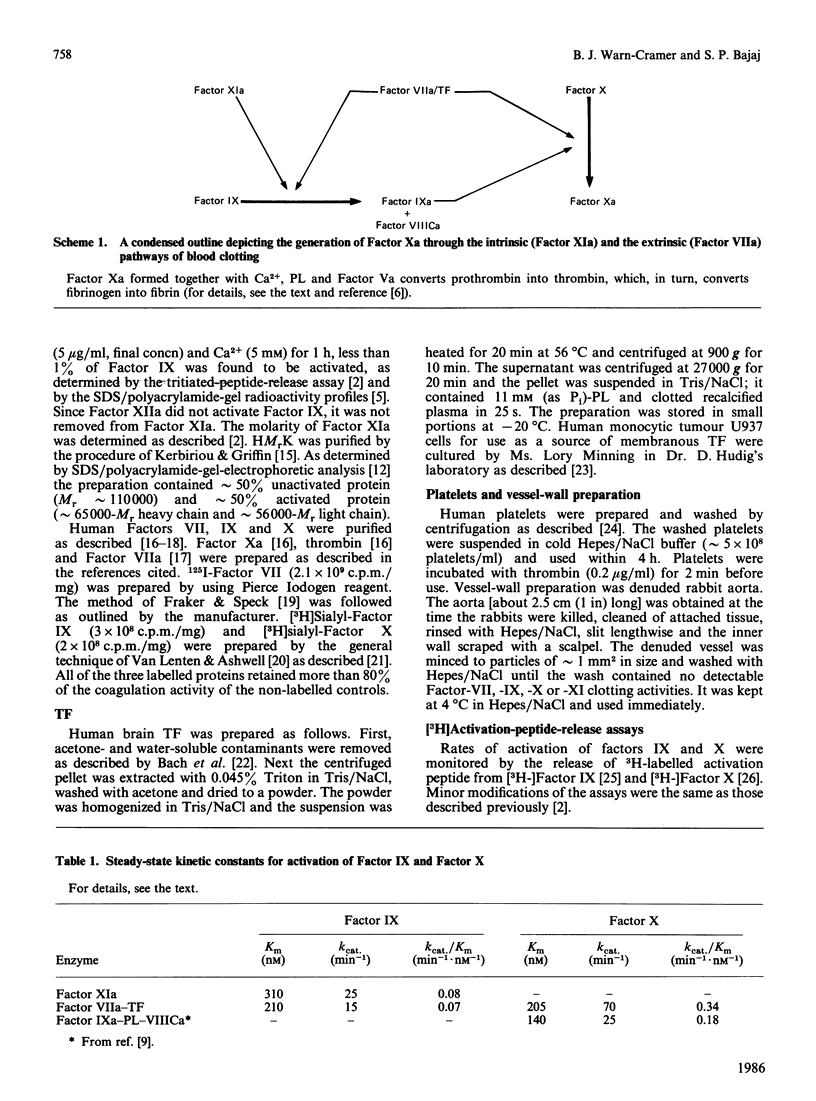
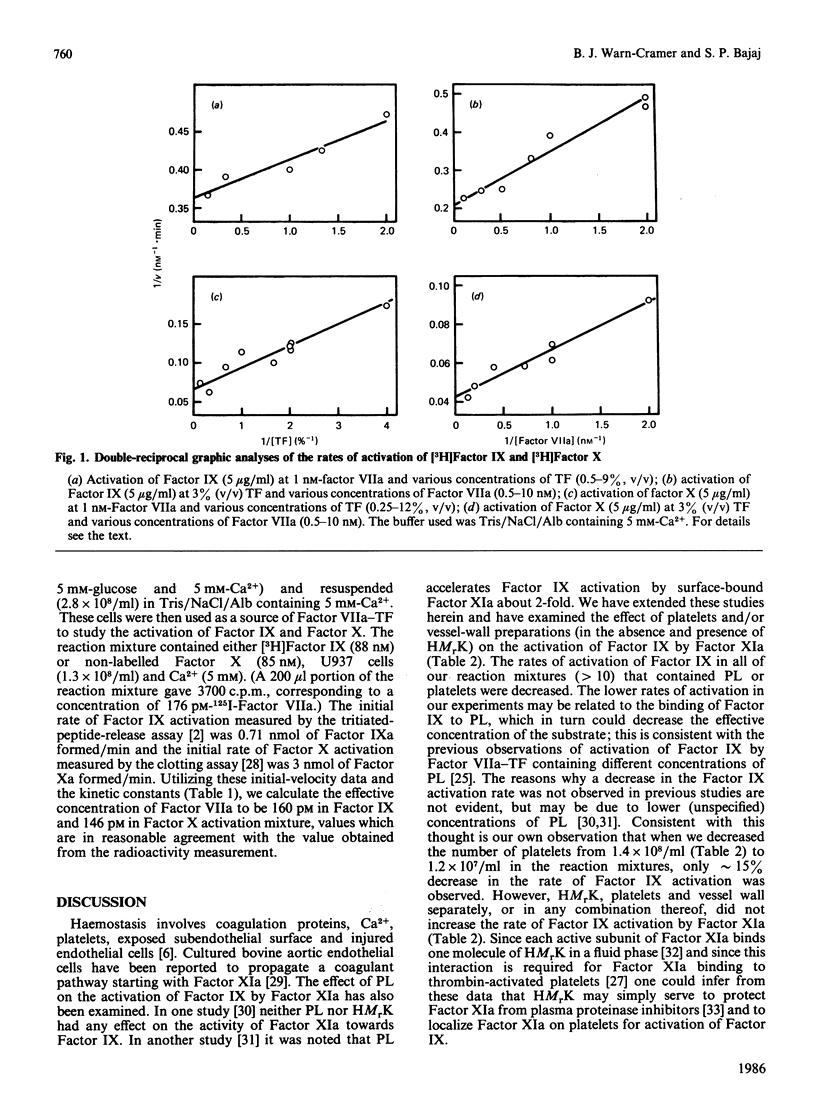
A study to compare the kinetics of activation of factor IX by Factor XIa/Ca2+ and by Factor VIIa/tissue factor/Ca2+ has been undertaken. When purified human proteins, detergent-extracted brain tissue factor and tritiated-activation-peptide-release assays were utilized, the kinetic constants obtained were: Km = 310 nM, kcat. = 25 min-1 for Factor XIa and Km = 210 nM, kcat. = 15 min-1 for Factor VIIa. The kinetic constants for the activation of Factor X by Factor VIIa/brain tissue factor were: Km = 205 nM, kcat. = 70 min-1. Predicted rates for the generation of Factor IXa and Factor Xa were obtained when human monocytic tumour U937 cells (source of tissue factor) and Factor VIIa were used to form the activator. In other experiments, inclusion of high-Mr kininogen did not increase the activation rates of Factor IX by Factor XIa in the presence or absence of platelets and/or denuded rabbit aorta. These kinetic data strongly indicate that both Factor XIa and Factor VIIa play physiologically significant roles in the activation of Factor IX.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach R., Nemerson Y., Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981 Aug 25;256(16):8324–8331. [PubMed] [Google Scholar]
- Bajaj S. P., Byrne R., Nowak T., Castellino F. J. Interaction of manganese with bovine factor X. J Biol Chem. 1977 Jul 25;252(14):4758–4761. [PubMed] [Google Scholar]
- Bajaj S. P. Cooperative Ca2+ binding to human factor IX. Effects of Ca2+ on the kinetic parameters of the activation of factor IX by factor XIa. J Biol Chem. 1982 Apr 25;257(8):4127–4132. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Brown S. F. Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem. 1981 Jan 10;256(1):253–259. [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Prodanos C. A simplified procedure for purification of human prothrombin, factor IX and factor X. Prep Biochem. 1981;11(4):397–412. doi: 10.1080/00327488108065531. [DOI] [PubMed] [Google Scholar]
- Bajaj S. P., Rapaport S. I., Russell W. A. Redetermination of the rate-limiting step in the activation of factor IX by factor XIa and by factor VIIa/tissue factor. Explanation for different electrophoretic radioactivity profiles obtained on activation of 3H- and 125I-labeled factor IX. Biochemistry. 1983 Aug 16;22(17):4047–4053. doi: 10.1021/bi00286a009. [DOI] [PubMed] [Google Scholar]
- Bouma B. N., Vlooswijk R. A., Griffin J. H. Immunologic studies of human coagulation factor XI and its complex with high molecular weight kininogen. Blood. 1983 Nov;62(5):1123–1131. [PubMed] [Google Scholar]
- Broze G. J., Jr Binding of human factor VII and VIIa to monocytes. J Clin Invest. 1982 Sep;70(3):526–535. doi: 10.1172/JCI110644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Davie E. W. Human factor XII (Hageman factor). Methods Enzymol. 1981;80(Pt 100):198–211. doi: 10.1016/s0076-6879(81)80018-3. [DOI] [PubMed] [Google Scholar]
- Hudig D., Bajaj S. P. Tissue factor-like activity of the human monocytic tumor cell line U937. Thromb Res. 1982 Aug 1;27(3):321–332. doi: 10.1016/0049-3848(82)90079-2. [DOI] [PubMed] [Google Scholar]
- Hultin M. B. Role of human factor VIII in factor X activation. J Clin Invest. 1982 Apr;69(4):950–958. doi: 10.1172/JCI110534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. M., Nemerson Y. Blood coagulation. Annu Rev Biochem. 1980;49:765–811. doi: 10.1146/annurev.bi.49.070180.004001. [DOI] [PubMed] [Google Scholar]
- Kerbiriou D. M., Griffin J. H. Human high molecular weight kininogen. Studies of structure-function relationships and of proteolysis of the molecule occurring during contact activation of plasma. J Biol Chem. 1979 Dec 10;254(23):12020–12027. [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Activation of human factor XI (plasma thromboplastin antecedent) by factor XIIa (activated Hageman factor). Biochemistry. 1977 Dec 27;16(26):5831–5839. doi: 10.1021/bi00645a030. [DOI] [PubMed] [Google Scholar]
- Leytus S. P., Chung D. W., Kisiel W., Kurachi K., Davie E. W. Characterization of a cDNA coding for human factor X. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3699–3702. doi: 10.1073/pnas.81.12.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhalter C., Schiffman S., Deutsch E. Phospholipids accelerate factor IX activation by surface bound factor XIa. Br J Haematol. 1984 Feb;56(2):261–271. doi: 10.1111/j.1365-2141.1984.tb03954.x. [DOI] [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K., Kisiel W., Sasagawa T., Howald W. N., Kwa E. Y., Weinstein B. Complete amino acid sequence of the light chain of human blood coagulation factor X: evidence for identification of residue 63 as beta-hydroxyaspartic acid. Biochemistry. 1983 Jun 7;22(12):2875–2884. doi: 10.1021/bi00281a016. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. A pathway of coagulation on endothelial cells. J Cell Biochem. 1985;28(4):253–264. doi: 10.1002/jcb.240280403. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L. V., Bajaj S. P. Purification of human factor VII utilizing O-(diethylaminoethyl)-Sephadex and Sulfopropyl-Sephadex chromatography. Anal Biochem. 1984 Feb;136(2):357–361. doi: 10.1016/0003-2697(84)90230-6. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Schapira M., James H. L., Cohen A. B., Colman R. W. Inactivation of factor XIa by plasma protease inhibitors: predominant role of alpha 1-protease inhibitor and protective effect of high molecular weight kininogen. J Clin Invest. 1982 Apr;69(4):844–852. doi: 10.1172/JCI110524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg S. A., Nemerson Y., Zur M. Kinetics of the activation of bovine coagulation factor X by components of the extrinsic pathway. Kinetic behavior of two-chain factor VII in the presence and absence of tissue factor. J Biol Chem. 1977 Dec 10;252(23):8481–8488. [PubMed] [Google Scholar]
- Sinha D., Seaman F. S., Koshy A., Knight L. C., Walsh P. N. Blood coagulation factor XIa binds specifically to a site on activated human platelets distinct from that for factor XI. J Clin Invest. 1984 Jun;73(6):1550–1556. doi: 10.1172/JCI111361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usharani P., Warn-Cramer B. J., Kasper C. K., Bajaj S. P. Characterization of three abnormal factor IX variants (Bm Lake Elsinore, Long Beach, and Los Angeles) of hemophilia-B. Evidence for defects affecting the latent catalytic site. J Clin Invest. 1985 Jan;75(1):76–83. doi: 10.1172/JCI111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lenten L., Ashwell G. Studies on the chemical and enzymatic modification of glycoproteins. A general method for the tritiation of sialic acid-containing glycoproteins. J Biol Chem. 1971 Mar 25;246(6):1889–1894. [PubMed] [Google Scholar]
- Walsh P. N., Bradford H., Sinha D., Piperno J. R., Tuszynski G. P. Kinetics of the Factor XIa catalyzed activation of human blood coagulation Factor IX. J Clin Invest. 1984 May;73(5):1392–1399. doi: 10.1172/JCI111343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn-Cramer B. J., Bajaj S. P. Stoichiometry of binding of high molecular weight kininogen to factor XI/XIa. Biochem Biophys Res Commun. 1985 Dec 17;133(2):417–422. doi: 10.1016/0006-291x(85)90922-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yoshitake S., Schach B. G., Foster D. C., Davie E. W., Kurachi K. Nucleotide sequence of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985 Jul 2;24(14):3736–3750. doi: 10.1021/bi00335a049. [DOI] [PubMed] [Google Scholar]
- Zur M., Nemerson Y. Kinetics of factor IX activation via the extrinsic pathway. Dependence of Km on tissue factor. J Biol Chem. 1980 Jun 25;255(12):5703–5707. [PubMed] [Google Scholar]
- van der Graaf F., Greengard J. S., Bouma B. N., Kerbiriou D. M., Griffin J. H. Isolation and functional characterization of the active light chain of activated human blood coagulation factor XI. J Biol Chem. 1983 Aug 25;258(16):9669–9675. [PubMed] [Google Scholar]


