Abstract
Background Cerebrovascular accidents (CVAs), or strokes, are major global health concerns associated with oxidative stress, inflammation, and gastrointestinal complications. This study aimed to explore the impact of postbiotic supplementation in CVA patients, specifically in terms of oxidative stress, inflammation, and clinical outcomes, as an alternative to probiotics with potential advantages. Method A prospective, single-center, randomized, controlled trial was conducted with 120 CVA patients in Iran. These patients were admitted to the ICU to assess the severity of their strokes. Patients were randomly assigned to receive either postbiotic supplementation (n = 60) or a placebo (n = 60). Various biomarkers related to oxidative stress, inflammation, and clinical outcomes were assessed. Data on demographic characteristics, nosocomial infections, and laboratory measurements were collected. Gut microbiota analysis was also performed on fecal samples. Results After the 7-day intervention, postbiotic supplementation resulted in significant improvements in inflammatory markers, oxidative stress, and a reduced incidence of pneumonia compared with those in the control group, with the postbiotic group demonstrating notable decreases in the serum IL-1β levels (-1.79; 95% CI: = -2.9 to -0.64, p = 0.002 ), MDA levels (-30.5; 95% CI: -54.8 to -6.1, p = 0.015), Hs-CRP levels (-0.67; 95% CI:-1.1 to -0.26 mg/dl, p = 0.001) and TAC levels (62.5; 95%CI: 34.1 to 90.9, p < 0.001) compared with those in the placebo group. However, no significant differences in other clinical outcomes, including the NIHSS score, NUTRIC score, and APACHE II score, or the gut microbiota profile, were observed between the two groups. Conclusion Postbiotic supplementation improved the levels of inflammatory factors and oxidative stress markers and reduced the risk of pneumonia in CVA patients. Trial registration This trial is registered in the Iranian Registry of Clinical Trials (registration code IRCT20180712040438N7), Registration date 06122022.
Keywords: Postbiotics, Oxidative stress, Inflammation, Clinical outcomes, Cerebrovascular accident, Gut microbiota
Subject terms: Cardiology, Medical research, Risk factors, Cardiovascular diseases
Introduction
Cerebrovascular accidents (CVAs), commonly known as strokes, are a leading cause of illness and death globally1. A stroke is a catastrophic event that disrupts blood flow to the brain, leading to a series of events, including oxidative stress and inflammation, both of which contribute to neuronal damage and hinder recovery2. While current treatments focus on minimizing brain injury and restoring blood flow, there is a growing interest in exploring therapies that target these downstream mechanisms3. Furthermore, individuals who have experienced severe stroke may face gastrointestinal complications, such as diarrhea, constipation, and infection. These issues can arise within 1 to 2 weeks after nutritional assistance is started, primarily due to significant dysfunction in the gastrointestinal system4,5. Along with possible changes in the acute phase of stroke, the gut microbiota could develop during the acute phase and result in a poor prognosis6. A recent study revealed that modifying the gut microbiota could influence the outcome of a stroke by affecting neuroendocrine and metabolic pathways, such as the serotoninergic system, γ-aminobutyric acid (GABA), plasma cortisol, and catecholamine7,8.
Probiotics, defined as beneficial live microorganisms that can exert positive effects when consumed in adequate amounts, have been extensively studied in this regard. Several studies have demonstrated that probiotic administration can effectively alter the gut microbial community structure, reduce pathogenic toxins, reduce abdominal distension, and lower inflammation, consequently reducing CVA complications9–12. However, probiotic interventions have limitations, including the need for continuous consumption, potential side effects, and variable efficacy among different probiotic strains13,14.
In recent years, postbiotics, defined as nonviable microbial products or components of microbial cells, have emerged as promising alternatives to probiotics for modulating the gut microbiota and promoting health15. Unlike probiotics, probiotics offer the advantages of stability, extended shelf-life, and a reduced risk of side effects16. Studies have shown that probiotics can effectively modulate the gut microbiota composition, enhance barrier function, and modulate host immune responses, suggesting their potential as therapeutic agents for various gastrointestinal and metabolic disorders in CVA patients17. Postbiotics, which demonstrate immunomodulatory, anti-inflammatory, and antioxidant properties, may surpass probiotics in benefits due to their longer shelf life, reduced side effects, and ability to enhance gut health and immune function, making them especially beneficial for those facing health challenges18. Given the potential benefits of probiotics and their role in modulating host physiology, exploring their impact on CVA patients, a population that often faces significant challenges in managing oxidative stress and inflammation, is essential. This study addresses the gap in understanding the specific benefits of postbiotics over probiotics, emphasizing their advantages for individuals facing health challenges, particularly those with CVA, who struggle with oxidative stress and inflammation.
There are currently no clinical trials evaluating the effects of Postbiotics on clinical outcomes in CVA patients, highlighting a significant research gap. While we can’t directly compare our findings to previous postbiotic studies due to this lack of research, we can look to probiotic studies for context. Many studies indicate that probiotics positively impact clinical outcomes in various patient groups, with some showing significant improvements in critically ill patients, while others report no effect19,20.
This study addresses the lack of randomized controlled trials (RCTs) on postbiotics in CVA patients by evaluating both short-term effects and long-term outcomes. It is the first randomized, double-blind, placebo-controlled trial assessing postbiotic supplementation in this population. We will measure biomarkers related to oxidative stress, inflammation, and clinical outcomes to explore the potential benefits of postbiotic supplementation for CVA patients.
Methods
Study design
The study was a prospective single-center, randomized, controlled trial carried out in patients with CVA from December 2023 to March 2024 in the Intensive Care Unit (ICU) of Imam-Reza Hospital in Kermanshah, Iran. The research protocol received approval from the Research Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1401.327). This clinical trial was registered in the Iranian Registry of Clinical Trials under the registration number IRCT20180712040438N7; date: 06-12-2022. Prior to participation in the study, written informed consent was obtained from all patients or their legal representatives.
Sample size and participants
The sample size was determined on the basis of 80% power and a 5% alpha error to detect the inflammatory effect through changes in the NIHSS score following postbiotic supplementation19. The participants in this study were selected through a random assignment process, which involved a total of 120 patients divided into two groups: the postbiotic group (n = 60) and the control group (n = 60). This allocation was achieved via a computer-generated random list, ensuring a 1:1 ratio with block sizes of two, four, or six. To minimize bias, all involved parties—including physicians, patients, nurses, and study personnel—were blinded to the treatment assignments.
The eligibility criteria for participation included individuals aged 18–80 years who were admitted to the ICU and had confirmed stroke, as evidenced by a brain CT or MRI conducted within the previous 72 h. The exclusion criteria included individuals with severe renal failure, severe hepatic failure, or severe neurodegenerative diseases or those who were pregnant or lactating. Potential biases in participant selection may arise from the exclusion criteria, which could limit the generalizability of the findings to a broader population.
Interventions
The study was carried out by a nutritionist (A R) under the supervision of an expert neurologist. Compliance was assessed on the basis of the usage of the sachet at the end of the trial, with patients being required to consume at least 90% of the sachet within the study. Additionally, all patients received their routine and usual treatment approach under the supervision of their neurologist. The treatment allocations were kept undisclosed from both patients and researchers until the completion of data analysis. The postbiotic sachet used in the study is similar to boxes with codes A and B, ensuring identical appearance properties. The blinding of patients, researchers, physicians, laboratory staff, and other personnel involved in the study was maintained until the trial’s completion and data analysis.
Patients who met the inclusion criteria were randomly assigned to one of 2 treatment groups: (1) placebo or (2) probiotics. The patients in the intervention group were administered a 2000 mg dose of postbiotic supplement containing short-chain fatty acids and exopolysaccharides of Lactobacillus paracasei from Postbiotics Immune, Probioway Co., Ltd. Meanwhile, the second group received a placebo containing maltodextrin once a day. The sachet was prescribed for seven days, following the guidelines established in previous studies21, and was administered to the patient through a feeding tube. Researchers monitored each patient’s medication intake and documented any missed or refused doses to evaluate compliance. A patient was deemed compliant if they took more than 80% of the prescribed study medication.
Data collection
This study collected information on demographic data, patient characteristics, nosocomial infections, hospital and ICUstays, and the clinical characteristics of stroke patients. Laboratory data were measured on the first day of admission and on day 7 or as needed. Various measurements and questionnaires have been used to assess nutritional status, disease severity, organ function, mortality rate, and clinical status. The length of hospital stays, length of ICU stays, and Glasgow Outcome Scale (GCS) score for the assessment of patient recovery were collected on the day of hospital discharge. Blood pressure was recorded from patient data in the ICU.
To minimize the risk of hyperglycemia from overfeeding in ICU patients, we set a baseline energy requirement of 25 kcal/kg body weight.
Hematological and inflammatory marker measurements
To assess inflammation, blood samples were collected after an 8-hour fast on two occasions: upon admission (within the first 24 h) and on the seventh day after admission. High-density lipoprotein (HDL), triglyceride, serum cholesterol, and low-density lipoprotein (LDL) levels were evaluated via an enzyme method. The total antioxidant capacity (TAC) was assessed via a commercial kit (Kiazist, Hamedan, Iran). High-sensitivity C-reactive protein (hs-CRP) levels were determined via standard ELISA kits from Tehran, Iran22,23. The inflammatory markers IL-10 and IL-6 were measured via ELISA kits.
The concentration of Malondialdehyde (MDA) was determined via a colorimetric method. Blood urea nitrogen (BUN) levels were measured through an enzymatic conductivity rate method, whereas serum creatinine (Cr) was assessed via the kinetic Jaffe rate method.
Outcomes
To assess the risk of mortality and disease severity, the APACHE II questionnaire was administered at the start and after the intervention, and the The Sequential Organ Failure Assessment (SOFA) questionnaire was used to evaluate organ function on days 1 and 7 of the study. Patients were monitored for 28 days after the intervention to determine the mortality rate. Clinical data were evaluated via National Institutes of Health Stroke Scale (NIHSS) scores on the first day of admission and on day 7 or at discharge for discharged patients early.
The mNUTRIC score, ranging from 0 to 9 points, was calculated for each patient within 24 h of ICU admission on the basis of five key variables: age, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, SOFA score, number of comorbidities, and length of hospital stay prior to ICU admission. In this study, due to the lack of IL-6 records in the database, a previously validated modified version of the mNUTRIC score was utilized for the sample calculations24.
To ensure an accurate diagnosis of hospital-acquired pneumonia (HAP), we implemented a multistep approach. First, the attending physicians confirmed that pneumonia onset occurred after day 2 of hospitalization and within 30 days of admission. Next, they evaluated patients for at least one of the following criteria: a focal infiltrate visible on chest X-ray, abnormal white blood cell counts (either elevated > 10,000 or low < 3,000 cells/mm3), and body temperature outside the normal range (fever > 38.5 °C or hypothermia < 35 °C). This approach ensures the differentiation of HAP from pneumonia potentially present upon admission.
Gut microbiota analysis
Fecal samples were collected in sterile containers, and DNA was extracted from these samples via the QIAamp Fast DNA Stool Mini Kit, with confirmation via agarose gel electrophoresis. Hypervariable regions 3 and 4 of the 16 S ribosomal RNA gene were amplified via specific primers, followed by paired-end sequencing on the MiSeq platform. The alpha and beta diversity of the gut community was evaluated via the Shannon index and inverse Simpson index, respectively, and was visualized through principal coordinate analysis (PCoA) plots based on unweighted UniFrac distances. The Firmicutes to Bacteroidetes (F/B) ratio was computed to analyze the microbial composition. To determine the F/B ratio, colony-forming unit (CFU) counts were obtained via the use of universal primers targeting the bacterial 16 S rRNA gene along with specific primers for Firmicutes and Bacteroidetes.
Statistical analysis
The quantitative data are presented as the means ± standard deviations (SD), whereas the qualitative data are expressed as frequencies (%). Normality was assessed via the Shapiro‒Wilk test. Statistical tests such as the chi-square test, paired samples t test, ANOVA, post hoc tests, and analysis of covariance (ANCOVA) were utilized for parametric variables to control for certain confounding factors, including baseline values, weight, GCS, NIHSS, and APACHE II score, with a significance level of less than 0.05 considered statistically significant. The APACHE II, SOFA, and NIHSS scores were designated primary outcomes, whereas inflammatory biomarkers were defined as secondary outcomes. All analyses were conducted on an intention-to-treat (ITT) basis via the statistical package for social sciences (SPSS, Inc., Chicago, IL, USA) software version 18, with significance set at P values below 0.05 and 95% confidence intervals (CIs).
Results
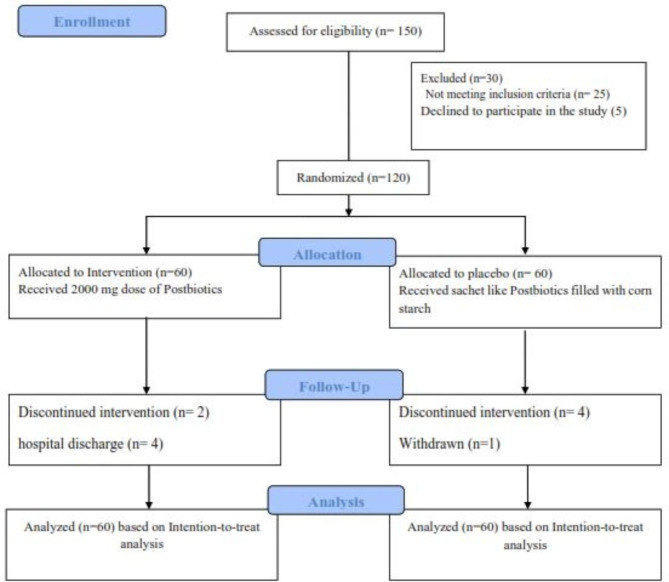
The study flowchart is depicted in Fig. 1. A total of 150 CVA patients were assessed for inclusion in the study. Of these, 120 patients who met the inclusion criteria were randomly assigned to two research groups, with 60 patients in each. Four patients from the postbiotic group (due to hospital discharge and death) and five from the control group (due to death and personal reasons) did not complete the study. Consequently, 111 patients (56 in the postbiotic group and 55 in the control group) completed the trial (Fig. 1). Both groups demonstrated more than 84% adherence to the dosage (Table 1). No adverse effects were reported during the intervention, indicating its safety. The results indicate that most patients tolerated the intervention well. Baseline demographic and medical data are shown in Table 1, with no significant differences between the groups in terms of age, weight, or comorbid conditions such as diabetes and cardiovascular disease. The mean age of the patients was 60.06 ± 10.48 years, with 90 (86%) being male. Additionally, Table 1 presents the clinical characteristics of the patients upon ICU admission. Among these variables, the baseline levels of FBS, DBP, SBP, SOFA score, and GCS score did not significantly differ between the postbiotic and placebo groups (all p > 0.05). Furthermore, other indices, such as length of ICU stay and hospital stay, did not significantly differ between the groups at the time of ICU admission (p = 0.906 and p = 0.527, respectively).
Fig. 1.
Flowchart of the study.
Table 1.
General characteristics of the study subjects.
| Variable | Postbiotic group (n = 56) |
Placebo group (n = 55) |
P value |
|---|---|---|---|
| Age (years)a | 60.64 ± 11.85 | 59.64 ± 8.39 | 0.820 d |
| Weight (kg)a | 78.25 ± 10.98 | 78.64 ± 7.27 | 0.127 d |
| Male sex, n (%) | 50 (90) | 40 (80) | 0.176 c |
| Comorbid conditions, n (%) | |||
| Type 2 diabetes n (%) | 12 (21.5) | 12 (21.8) | 1.00 c |
| Cardiovascular diseases n (%) | 17 (30) | 8 (15) | 0.068 c |
| Glasgow Coma Scale (GCS) | 5.89 ± 1.61 | 6.07 ± 1.84 | 0.570 d |
| APACHE II score a | 11.88 ± 3.22 | 12.25 ± 3.69 | 0.574 d |
| FBS a | 126.21 ± 7.07 | 107.78 ± 21.4 | 0.906 d |
| SBP a | 128.86 ± 20.11 | 122.91 ± 15.17 | 0.097 d |
| DBP a | 82.64 ± 11.9 | 79.33 ± 13.5 | 0.174 d |
| Length of ICU stay, days a | 6.24 ± 1.1 | 6.34 ± 2.1 | 0.906 d |
| Hospital stays, days a | 9.9 ± 1.6 | 10.17 ± 1.8 | 0.527 d |
| Mortality, n (%) | 2 (3.6) | 4 (7.2) | 0.438 c |
| Compliance rate, (%) | 84 | 85 | 0.052 c |
MD: Mean/Median of difference.
a- Values are expressed as the mean (SD).
c- Chi-square test.
d- Independent samples t test.
At the beginning of the study, there were no notable differences in clinical indices, such as the NIHSS score, NUTRIC score, SOFA score, or APACHE II score, between the two study groups. Compared with that before the intervention, the NUTRIC score decreased significantly in the postbiotic group (p < 0.001), but this change was not significant compared with that in the placebo group. The SOFA score significantly decreased (-1.68; 95% CI: 2.3–1.03, p = 0.001) after the 7-day intervention, but the changes in the NIHSS score, NUTRIC score, and APACHE II score, although they decreased, did not reach a significant level between the two study groups after the 7-day intervention (Table 2).
Table 2.
Summary results for the primary outcomes.
| Variable | Postbiotic group (n = 56) |
Placebo group (n = 55) |
Differences (95% CI) P value |
|---|---|---|---|
|
NIHSS Baseline End |
7.02 ± 0.66 4.60 ± 0.62 |
7.31 ± 0.7 4.74 ± 7.52 |
-0.28 (-2.5 to 1.9), 0.805$ -0.14 (-2.4 to 2.2), 0.906# |
| MD (95% CI), P* | -2.42 (-2.53, -2.31) p < 0.001 | -2.54 (-3.03, -2.1) p < 0.001 | |
|
APACHE II score Baseline End |
11.88 ± 3.22 10.95 ± 3.24 |
12.25 ± 3.69 11.32 ± 3.68 |
-0.37 (-1.67 to 0.93), 0.574$ -0.38 (-1.66 to 0.94),0.576# |
| MD (95% CI), P* | -1.88 (-3.25, -0.48) 0.010 | 0.56 (-1.58, 2.56) 0.546 | |
|
NUTRIC score Baseline End |
5.63 ± 2.2 5.05 ± 2.02 |
5.69 ± 1.58 5.11 ± S 1.55 |
-0.06 (-1.18 to 1.04), 0.905$ -0.07 (-1.15 to 1.6), 0.609# |
| MD (95% CI), P* | -0.58 (-0.6, 0.52) p < 0.001 | 1.04 (-1.31, 3.59) 0.399 | |
|
SOFA score Baseline End |
5.38 ± 2.02 3.69 ± 1.06 |
5.44 ± 1.58 5.37 ± 1.84 |
-0.06 (-1.18 to 1.04), 0.906$ -1.68 (-2.3 to -1.03), 0.001# |
| MD (95% CI), P* | -1.69 (-2.4, -0.97) p < 0.001 | -0.07 (-1.14, 0.95) 0.897 |
Abbreviations: APACHE II; Acute Physiology and Chronic Health Evaluation II. NUTRIC score; NUT rition Risk in the Critically ill., SOFA; Sequential Organ Failure Assessment.
Mean (SD) and Mean difference (95% CI) are presented for data.
* P based on Paired samples t test.
$ P based on independent samples t test.
# P based on ANCOVA adjusted for baseline values, weight, and calorie intakes.
Table 3 outlines the pre- and postintervention inflammation markers in both groups. Neither the differences between the groups nor the variations within each group reached statistical significance for IL-10, BUN, and creatinine at the beginning of the study. However, after the 7-day intervention, the serum IL-1β, MDA, and Hs-CRP levels significantly decreased between the two study groups. Compared with those in the placebo group, significant decreases in the IL1-Beta concentration (-1.79; 95% CI: -2.9 to -0.64, p = 0.002), MDA level (-30.5; 95% CI: -54.8 to -6.1, p = 0.015), and Hs-CRP level (-0.67; 95% CI: -1.1 to -0.26 mg/dl, p = 0.001) were observed following postbiotic supplementation. Furthermore, postbiotic supplementation resulted in a significant increase in TAC levels (62.5, 95% CI:34.1 to -90.9, p < 0.001) compared with those in the placebo group.
Table 3.
Effect of Postbiotic s supplementation inflammation markers and clinical status of participants.
| Variable | Postbiotic group (n = 56) |
Placebo group (n = 55) |
Differences (95% CI) P value |
|---|---|---|---|
|
IL-10 Baseline End |
4.96 ± 0.38 5.56 ± 0.66 |
4.66 ± 1.87 6.06 ± 1.10 |
0.29 (-1.4 to 2.1), 0.744$ -0.49 (-2.1 to 1.03), 0.519# |
| MD (95% CI), P* | 0.60 (-0.7, 1.9) 0.356 | 1.39 (-0.41, 3.21) 0.128 | |
|
IL1-Beta (pg/mL) Baseline End |
5.97 ± 0.65 3.86 ± 0.68 |
5.40 ± 1.23 5.66 ± 1.74 |
0.56 (-0.57 to 1.7), 0.330$ -1.79 (-2.9 to -0.64), 0.002# |
| MD (95% CI), P* | -2.1 (-2.97, -1.23) p < 0.001 | 0.25 (-0.88, 1.39) 0.655 | |
|
TAC Baseline End |
147.51 ± 36.22 235.23 ± 28.86 |
144.03 ± 39.84 172.6 ± 26.2 |
3.5 (-14.8 to 21.1), 0.708$ 62.5 (34.1 to 90.9), 0.001# |
| MD (95% CI), P* | 87.7 (64.1, 110.7) p < 0.001 | 28.6 (-8.85, 48.4) 0.052 | |
|
MDA Baseline End |
161.08 ± 17.81 116.05 ± 16.81 |
163.23 ± 16.03 146.56 ± 16.8 |
-1.14 (-28.9 to 2.6), 0.935$ -30.5 (-54.8 to -6.1), 0.015# |
| MD (95% CI), P* | -45.05 (-62.25, -27.7) p < 0.001 | -15.6 (-35.58, 4.46) 0.128 | |
|
Hs-CRP (ng/dl) Baseline End |
4.19 ± 1.7 2.57 ± 0.81 |
3.87 ± 1.6 3.24 ± 1.28 |
0.32 (-0.3, 0.97), 0.314$ -0.67 (-1.1, -0.26), 0.001# |
| MD (95% CI), P* | -1.63 (-2.17, -1.09), p < 0.001 | -0.62 (-1.14, -0.1), 0.018 | |
|
BUN (ng/ml) Baseline End |
25.21 ± 8.02 30.11 ± 8.2 |
23.1 ± 5.7 28.1 ± 5.75 |
2.1 (-0.5, 4.7), 0.115$ 2.21 (-0.52, 3.5), 0.097# |
| MD (95% CI), P* | 4.8 (4.79, 4.93), 0.001 | 5.01 (6.11, 3.2), 0.001 | |
|
Cr (ng/dl) Baseline End |
0.9 ± 0.4 1.10 ± 0.44 |
0.98 ± 0.30 1.15 ± 0.32 |
-0.07 (-0.2, 0.07), 0.310$ -0.05 (-0.3, 0.01), 0.225$ |
| MD (95% CI), P* | 0.20 (0.09, 0.21), 0.019 | 0.17 (0.08, 0.22), 0.021 |
Abbreviation: IL-10: Interleukin 10, IL-1 Beta: Interleukin 1 Beta, TAC: Total Antioxidant Capacity, MDA: Malondialdehyde, Hs-CRP: High-sensitivity C-reactive Protein, BUN: Blood Urea Nitrogen, Cr: Creatinine, GCS: Glasgow Coma Scale, NIHSS: National Institutes of Health Stroke Scale, APACHE II: Acute Physiology and Chronic Health Evaluation II.
Mean (SD) and Mean difference (95% CI) are presented for data.
* P based on Paired samples t test.
$ P based on independent samples t test.
# P based on ANCOVA adjusted for baseline values, weight, GCS, NIHSS, and APACHE II score.
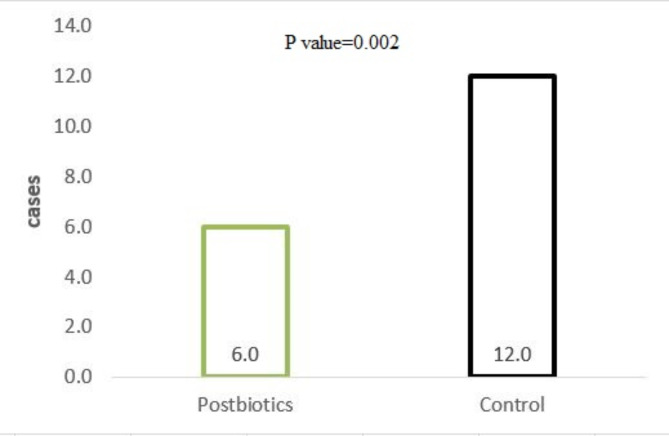
During the patients’ ICU stay and follow-up period, 28 patients (24%) developed pneumonia, with 6 out of 55 (10%) in the postbiotic group and 11 out of 55 (19%) in the control group (Fig. 2). A statistically significant difference was observed between the two study groups (p = 0.002).
Fig. 2.
Number of patients who developed pneumonia.
The gut microbiota profiles are presented in Table 4. Bacterial phylum analysis revealed that although the F/B ratio decreased (-0.19; 95% CI: -0.25 to 0.05, p = 0.595), the difference was not statistically significant compared with that of the control group. Furthermore, the Shannon diversity index, which considers species abundance and distribution, indicated that the microbial variety in the postbiotic patient group was notably greater than that in the control group (0.39; 95% CI:0.22 to 0.54, p < 0.001); although it decreased, it did not reach a significant level between the two study groups after the 7-day intervention.
Table 4.
Effect of Postbiotic s supplementation o gut microbiota profiles.
| Variable | Postbiotic group (n = 56) |
Placebo group (n = 55) |
Differences (95% CI) P value |
|---|---|---|---|
|
Shannon diversity index Baseline End |
2.87 ± 1.1 3.26 ± 1.02 |
2.99 ± 1.01 2.89 ± 1.1 |
-0.11 (-0.5 to 0.3), 0.562$ 0.36 (-0.7 to 0.8), 0.105# |
| MD (95% CI), P* | 0.39 (0.22, 0.54) p < 0.001 | 1.04 (-1.31, 3.59) 0.399 | |
|
Simpson diversity index Baseline End |
0.43 ± 0.22 0.53 ± 0.21 |
0.49 ± 0.23 0.59 ± 0.23 |
-0.05 (-0.14 to 0.02), 0.192$ -0.06 (-0.14 to 0.02),0.158# |
| MD (95% CI), P* | -0.9 (-0.08 ,0.1) p < 0.001 | 0.56 (-1.58, 2.56) 0.546 | |
|
Firmicutes/Bacteroidetes (F/B) ratio Baseline End |
5.38 ± 1.20 5.19 ± 1.61 |
5.44 ± 1.58 5.37 ± 1.84 |
0.11 (-2.4 to 2.7), 0.930$ 0.56 (-2.6 to 1.6), 0.605# |
| MD (95% CI), P* | -0.19 (-0.25, 0.5) 0.595 | -0.07 (-1.13, 0.99) 0.892 |
LPS: Lipopolysaccharide, mean (SD) and Mean difference (95% CI) are presented for data.
* P based on Paired samples t-test.
$ P based on independent samples t-test.
# P based on ANCOVA adjusted for baseline values, weight, PAL, and calorie intakes.
Discussion
In this RCT, for the first time, we investigated the effects of postbiotic treatment on various biomarkers related to oxidative stress, inflammation, and clinical outcomes in CVA patients. We found that daily postbiotic supplementation for 7 days improved inflammatory factor levels, oxidative stress marker levels and only SOFA scores in patients with CVA in the ICU, whereas these effects on other clinical outcomes, including the National Institutes of Health Stroke Scale (NIHSS) score, NUTRIC score, APACHE II score and gut microbiota profile, were not statistically significant. The occurrence of pneumonia during the patients’ intensive care unit (ICU) stay and follow-up period is another important outcome. The incidence of pneumonia was significantly lower in the postbiotic group than in the control group, indicating a potential benefit of postbiotic supplementation in reducing the risk of pneumonia in CVA patients.
Our results revealed a significant decrease in the serum IL-1beta, MDA, and Hs-CRP levels following postbiotic supplementation compared with those in the placebo group, suggesting a potential beneficial effect of the intervention in reducing inflammation. These results are particularly noteworthy, as they indicate a positive impact on markers associated with inflammation and oxidative stress following postbiotic supplementation compared with the placebo group, which is consistent with previous studies that have demonstrated the anti-inflammatory properties of similar interventions18,25. Furthermore, postbiotic supplementation resulted in a significant increase in TAC levels compared with those in the placebo group, indicating an improvement in the body’s ability to counteract oxidative stress. When these results are compared with those of previous studies, our findings align with research that reported similar reductions in inflammation markers following interventions targeting the modulation of the gut microbiota26. These consistent findings across studies reinforce the potential of postbiotic supplementation in mitigating inflammation and oxidative stress, highlighting the reproducibility of the observed effects. Postbiotics have been shown to possess anti-inflammatory properties by inhibiting the activation of inflammatory pathways and reducing the production of proinflammatory cytokines15,27. These effects can help attenuate the inflammatory response associated with CVA and improve clinical outcomes. The increase in TAC observed in the postbiotic group indicates increased antioxidant capacity. Postbiotics have been shown to exert antioxidant effects by scavenging free radicals, reducing oxidative stress, and upregulating endogenous antioxidant defense mechanisms28. These antioxidant properties may play a role in protecting against oxidative damage and promoting recovery in CVA patients.
Whereas gut dysbiosis is associated with poor prognosis in CVA patients29, little is known about the effects of postbiotic supplementation on other clinical outcomes, including SOFA, NIHSS, NUTRIC SCORE, and APACHE. To the best of our knowledge, no clinical trial has evaluated the effects of probiotics on this clinical outcome. Therefore, we cannot compare our results, but by comparing our results with those of previous probiotic studies, we can assess our results. Several previous studies have demonstrated the beneficial effects of probiotics on clinical outcomes in different patient populations. For example, a study by Johnstone et al. (2021) investigated the effects of probiotic supplementation in critically ill patients and reported significantly improved clinical outcomes20. In a significant clinical trial involving infants from rural India, the use of L. plantarum was found to reduce the likelihood of experiencing a combined outcome of infections and mortality30. However, another study by Mahmoodpoor et al. reported that probiotic supplementation has no effect on the above clinical outcomes19. Moreover, the effectiveness of probiotics may be diminished in older adults, as demonstrated by a recent rigorous study that revealed probiotics did not prevent C. difficile infections in individuals aged 65 and older who were hospitalized and receiving at least one antibiotic31. We found that postbiotic supplementation improved only the SOFA score, while these effects affected other clinical outcomes, including the National Institutes of Health Stroke Scale (NIHSS) score, NUTRIC score, and APACHE II score. The decrease in the SOFA score after postbiotic supplementation suggests a potential role in mitigating organ dysfunction and failure. Postbiotics have been shown to exert immunomodulatory effects by regulating the production of proinflammatory and anti-inflammatory cytokines, thereby modulating the immune response and reducing systemic inflammation6,15. This immunomodulatory action may contribute to the observed improvement in organ function in CVA patients3,17. Postbiotics, such as short-chain fatty acids and metabolites produced by beneficial gut bacteria, have been shown to increase nutrient absorption, promote gut barrier integrity, and modulate energy metabolism, all of which can contribute to improved nutritional outcomes7,19. The varying outcomes could be attributed to the diverse dosages of probiotic supplements, the different clinical settings of individuals, including varying severity, and the timelines for endpoint assessment among the participants in those studies.
The lower incidence of pneumonia in the postbiotic group is consistent with previous research demonstrating the potential of probiotics to prevent respiratory infections20. Recent meta-analyses indicate that the use of probiotics is linked to a significant reduction in ventilator-associated pneumonia (VAP), although there is no notable impact on overall mortality32. Conversely, Furthermore, the research conducted by Johnstone et al., which included over 2,600 participants, stands as the largest randomized trial to date. The findings indicated that probiotics were notably ineffective in decreasing the incidence of ventilator-associated pneumonia (VAP) among patients at high risk for this condition20. Observations suggest that probiotic therapy can be beneficial for carefully selected critically ill patients in surgical ICUs. Conversely, research indicates that probiotics should be avoided in immunosuppressed patients or those with severe acute pancreatitis33 ; however, such side effects are not observed with postbiotic supplementation. Postbiotics can increase the diversity and stability of the respiratory microbiota, strengthen the respiratory epithelial barrier, and stimulate local immune responses15,16. These mechanisms may contribute to a reduced risk of pneumonia in CVA patients receiving postbiotic supplementation. When these findings are compared with previous studies, it is important to note that the impact of postbiotic supplementation on CVA patients is an area of growing interest. While previous research has demonstrated the potential benefits of probiotics in various clinical settings, others have warned about the complications of probiotics, such as microbial transmission and infection19,20,34. However, probiotics do not have these disadvantages and were effective in our study. The current study contributes to the literature by providing valuable insights into the potential mechanisms and clinical implications of postbiotic supplementation in this patient population. Postbiotics may modulate the gut microbiota and its associated metabolic pathways, leading to downstream effects on systemic inflammation and oxidative stress18,25,35. These findings highlight the potential clinical implications of postbiotic supplementation as a novel approach for managing CVA patients, particularly in reducing the risk of complications such as pneumonia. Compared with that reported in previous studies, the incidence of pneumonia observed in the control group in our study was notably greater than the range reported in the literature. The reasons for this discrepancy are unclear but may be related to the unique characteristics of the patient population or the clinical setting in which our study was conducted.
Examination of the gut microbiota profiles revealed interesting patterns, as the group receiving probiotics presented a decreased F/B ratio and an increased Shannon diversity index. Although these differences were not statistically significant compared with those in the control group, they offer valuable insights into the potential impact of postbiotic supplementation on the composition of the gut microbiota in CVA patients. These findings are consistent with previous studies suggesting that probiotics can promote a more balanced and diverse gut microbiota composition, although this effect has not been observed in all previous studies27,36. An altered F/B ratio has been associated with various health conditions, such as asthma, gastrointestinal symptoms, and ICU mortality, in critically ill patients37. A diverse gut microbiota is linked to improved metabolic and immune function, which may contribute to the observed clinical benefits in CVA patients38,39. Research has shown that critically ill patients experience a reduction in beneficial gut bacteria, as reflected by a higher F/B ratio, an increase in potentially harmful microorganisms, and significant disruption of the balance of microbial communities40,41. These findings suggest that probiotics may not effectively address this clinically significant imbalance in the microbiota. However, it is possible that probiotics could prevent further dysbiosis without causing any complications.
Despite these significant findings, it is important to acknowledge the limitations of the current study. The relatively short intervention and follow-up periods may have restricted our ability to observe the long-term effects of postbiotic supplementation. Future research with extended intervention durations and follow-up assessments is needed to better understand the sustained impacts of postbiotics in CVA patients. Furthermore, conducting larger multicenter trials could strengthen the evidence regarding the clinical benefits of postbiotic supplementation in this population. Our study has several limitations, such as not evaluating the entire gut microbiome and SCFA levels. Additionally, the duration and dosage of the supplements used were not assessed, which could affect the generalizability of the findings. Considering the increasing use of the Firmicutes/Bacteroidetes ratio, analysis of the overall bacterial composition may be beneficial in future research. Additionally, further investigation into this supplementation is warranted.
In conclusion, this study underscores the significant potential of postbiotic supplementation to impact inflammation, oxidative stress, and clinical outcomes in CVA patients. These findings contribute to the growing body of evidence that positions postbiotics as a promising therapeutic strategy in the management of CVA. However, further research is essential to elucidate the underlying mechanisms and long-term effects of postbiotic supplementation, ultimately aiming to improve clinical outcomes and enhance the quality of life for individuals affected by CVA. The implications of this research could pave the way for more effective interventions in clinical practice.
Acknowledgements
The authors would like to express their gratitude to the Clinical Research Development Center of Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran, for their valuable advice.
Author contributions
Jalal Moludi designed the study. Akram Rahimi conducted the entire clinical study and collected the data. All authors prepared the manuscript. Jalal Moludi performed the statistical analysis. All authors contributed to editing the manuscript.
This research received partial support from Kermanshah University of Medical Sciences (grant number 4010582).
Data availability
All data generated and analyzed during this study are included in the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Ethical approval
Our study adhered to the Helsinki Declaration of the World Medical Association (2000), was approved by the Research Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1401.327) and was registered in the Iranian Registry of Clinical Trials (registration code: IRCT20180712040438N7).
Consent for publication
All the authors endorse the submission of this work to the journal.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mukherjee, D. & Patil, C. G. Epidemiology and the global burden of stroke. World Neurosurg. 76 (6), S85–S90 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Yang, C. et al. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiology-Cell Physiol. 316 (2), C135–C153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mir, M. A., Al-Baradie, R. S. & Alhussainawi, M. D. Pathophysiology of Strokes. Recent Advances in Stroke Therapeutics 1st edn p. 25–80 (Nova Science Publishers, Inc, 2014).
- 4.Benakis, C. & Liesz, A. The gut-brain axis in ischemic stroke: its relevance in pathology and as a therapeutic target. Neurol. Res. Pract. 4 (1), 57 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doshi, V. et al. Complications in stroke patients: a study carried out at the Rehabilitation Medicine Service, Changi General Hospital. Singap. Med. J. 44 (12), 643–652 (2003). [PubMed] [Google Scholar]
- 6.Sun, H. et al. Gut microbiota dysbiosis in acute ischemic stroke associated with 3-month unfavorable outcome. Front. Neurol. 12, 799222 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, W., Dong, X. Y. & Huang, R. Gut microbiota in ischemic stroke: role of gut bacteria-derived metabolites. Translational Stroke Res. 14 (6), 811–828 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Mörkl, S. et al. Probiotics and the microbiota-gut-brain axis: focus on psychiatry. Curr. Nutr. Rep. 9, 171–182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moludi, J. et al. The effect of probiotic supplementation on depressive symptoms and quality of life in patients after myocardial infarction: results of a preliminary double-blind clinical trial. Psychosom. Med. 81 (9), 770–777 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Wu, H. & Chiou, J. Potential benefits of probiotics and prebiotics for coronary heart disease and stroke. Nutrients. 13 (8), 2878 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang, R. et al. Buyang Huanwu decoction combined with probiotics or prebiotics for functional recovery from stroke: a meta-analysis protocol for systematic review. Medicine. 100 (51), e28371 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salari, A. et al. Probiotics supplementation and cardiometabolic risk factors: a new insight into recent advances, potential mechanisms, and clinical implications. PharmaNutrition. 16, 100261 (2021). [Google Scholar]
- 13.Moludi, J. et al. Metabolic endotoxemia and cardiovascular disease: a systematic review about potential roles of prebiotics and probiotics. Clin. Exp. Pharmacol. Physiol. 47 (6), 927–939 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Suez, J. et al. The pros, cons, and many unknowns of probiotics. Nat. Med. 25 (5), 716–729 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Żółkiewicz, J. et al. Postbiotics—a step beyond pre-and probiotics. Nutrients. 12 (8), 2189 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wegh, C. A. et al. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 20 (19), 4673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozma, M. A. et al. Postbiotics as the key mediators of the gut microbiota-host interactions. Le Infezioni Med. 30 (2), 180 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourebaba, Y. et al. Postbiotics as Potential new Therapeutic Agents for Metabolic Disorders Management153p. 113138 (Biomedicine & Pharmacotherapy, 2022). [DOI] [PubMed]
- 19.Mahmoodpoor, A. et al. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: a prospective double‐blind randomized controlled trial. Nutr. Clin. Pract. 34 (1), 156–162 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Johnstone, J. et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. Jama. 326 (11), 1024–1033 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong, D. Y. et al. The effect of probiotics in stroke treatment. Evidence-based Complementary and Alternative Medicine, 2021. (2021).
- 22.Soleimani, D. et al. Effect of propolis Supplementation on Athletic Performance, body Composition, Inflammation, and Oxidative Stress Following Intense Exercise: A triple-blind Randomized Clinical Trial9p. 3631–3640 (Food science & nutrition, 2021). 7. [DOI] [PMC free article] [PubMed]
- 23.Behrooz, M. et al. Are spexin levels associated with metabolic syndrome, dietary intakes and body composition in children? Diabetes Res. Clin. Pract. 172, 108634 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Rahman, A. et al. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the modified NUTRIC nutritional risk assessment tool. Clin. Nutr. 35 (1), 158–162 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Chen, M. et al. JK5G postbiotics attenuate immune-related adverse events in NSCLC patients by regulating gut microbiota: a randomized controlled trial in China. Front. Oncol. 13, 1155592 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malagón-Rojas, J. N. et al. Postbiotics for preventing and treating common infectious diseases in children: a systematic review. Nutrients. 12 (2), 389 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, W. Y. et al. The function of mixed postbiotic PE0401 in improving Intestinal Health via elevating Anti-inflammation, anti-oxidation, epithelial tight Junction Gene expression and promoting beneficial Bacteria growth. J. Pure Appl. Microbiol., 16(3). (2022).
- 28.Guerrero-Encinas, I. et al. Protective effect of lacticaseibacillus casei CRL 431 postbiotics on mitochondrial function and oxidative status in rats with aflatoxin B 1–induced oxidative stress. Probiotics Antimicrob. Proteins, : pp. 1–11. (2021). [DOI] [PubMed]
- 29.Xia, G. H. et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front. Neurol. 10, 397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panigrahi, P. et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 548 (7668), 407–412 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Allen, S. J. et al. Lactobacilli and Bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 382 (9900), 1249–1257 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Ji, T. et al. Preventive effect of probiotics on ventilator-associated pneumonia: a meta-analysis of 2428 patients. Ann. Pharmacother. 55 (8), 949–962 (2021). [DOI] [PubMed] [Google Scholar]
- 33.Doron, S. & Snydman, D. R. Risk and safety of probiotics. Clin. Infect. Dis. 60 (suppl_2), S129–S134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsilika, M. et al. A four-probiotic preparation for ventilator-associated pneumonia in multi-trauma patients: results of a randomized clinical trial. Int. J. Antimicrob. Agents. 59 (1), 106471 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Moludi, J. et al. Probiotics Supplementation improves Quality of Life, clinical symptoms, and inflammatory status in patients with psoriasis. J. Drugs Dermatology: JDD. 21 (6), 637–644 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Li, H. Y. et al. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: a narrative review. Nutrients. 13 (9), 3211 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan, C. et al. Dysbiosis of gut microbiota and short-chain fatty acids in acute ischemic stroke and the subsequent risk for poor functional outcomes. J. Parenter. Enter. Nutr. 45 (3), 518–529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojima, M. et al. Metagenomic analysis reveals dynamic changes of whole gut microbiota in the acute phase of intensive care unit patients. Dig. Dis. Sci. 61, 1628–1634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchesi, J. R. et al. The gut microbiota and host health: a new clinical frontier. Gut. 65 (2), 330–339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vos, W. M. & de Vos, E. A. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr. Rev. 70 (suppl_1), S45–S56 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Haak, B. W. et al. Disruptions of anaerobic gut bacteria are associated with stroke and post-stroke infection: a prospective case–control study. Translational Stroke Res. 12, 581–592 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and analyzed during this study are included in the manuscript.