Abstract
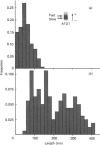
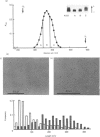
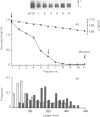
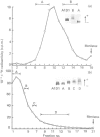
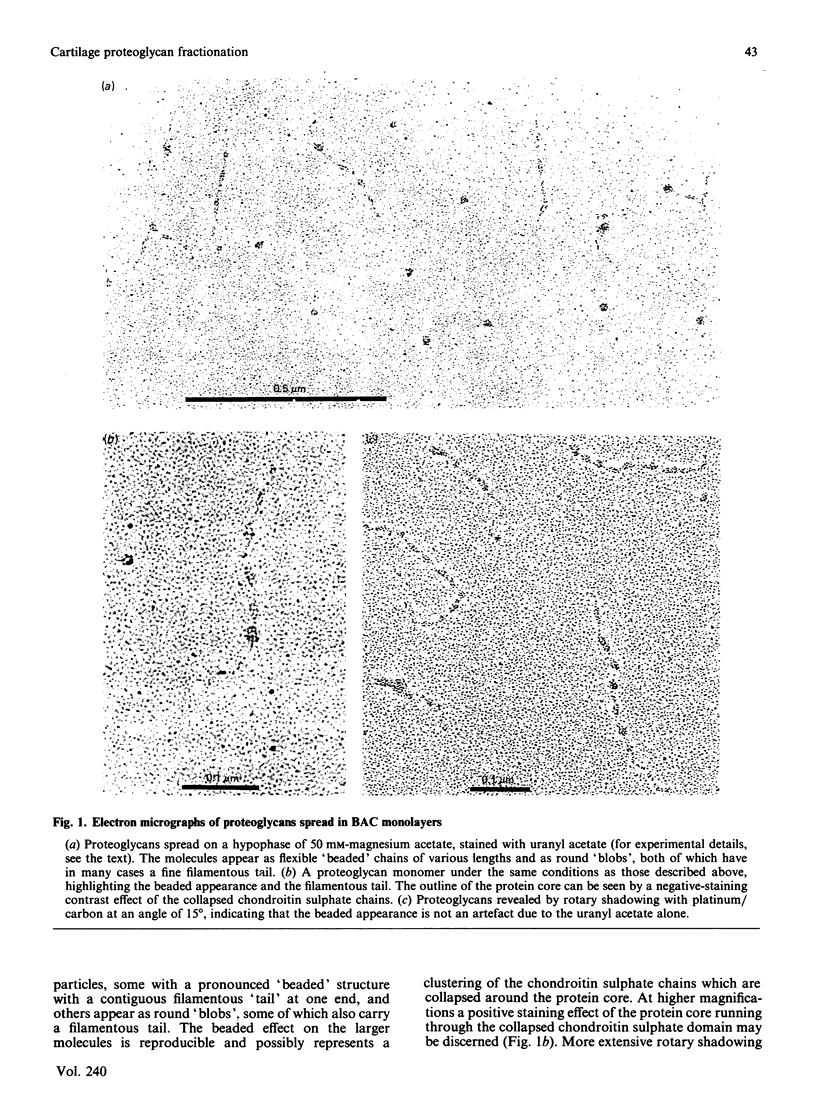
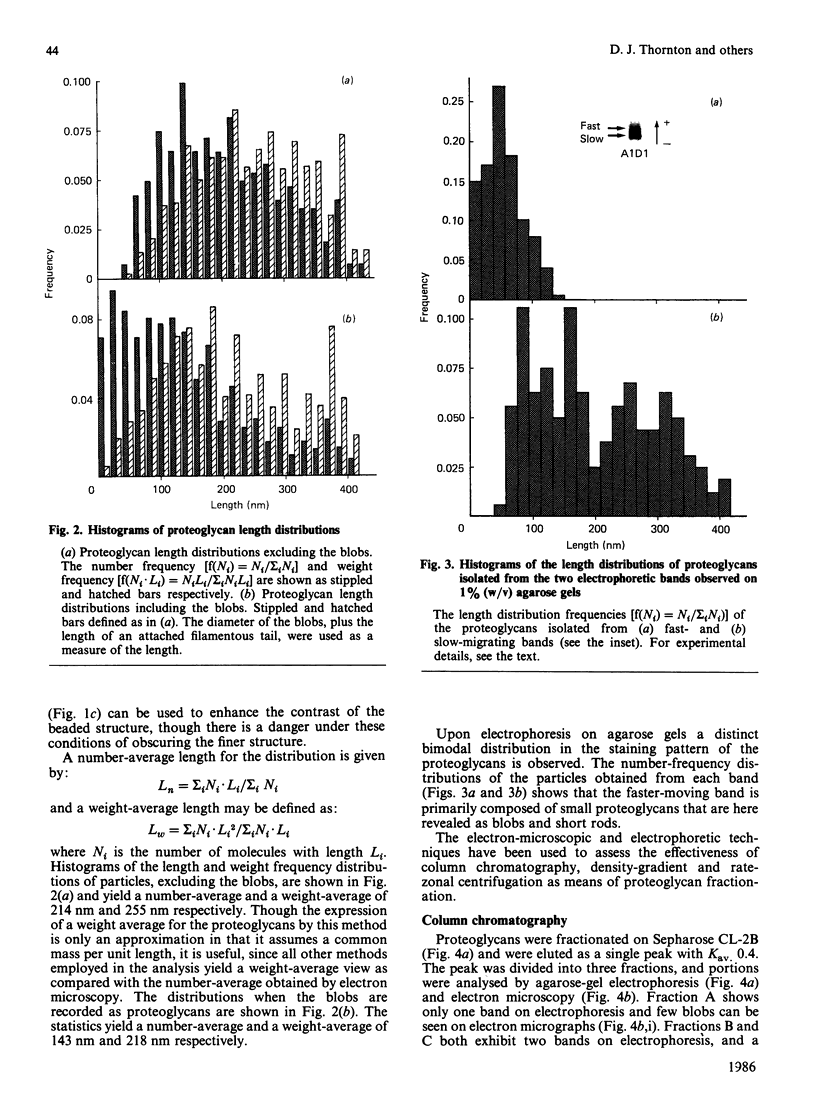
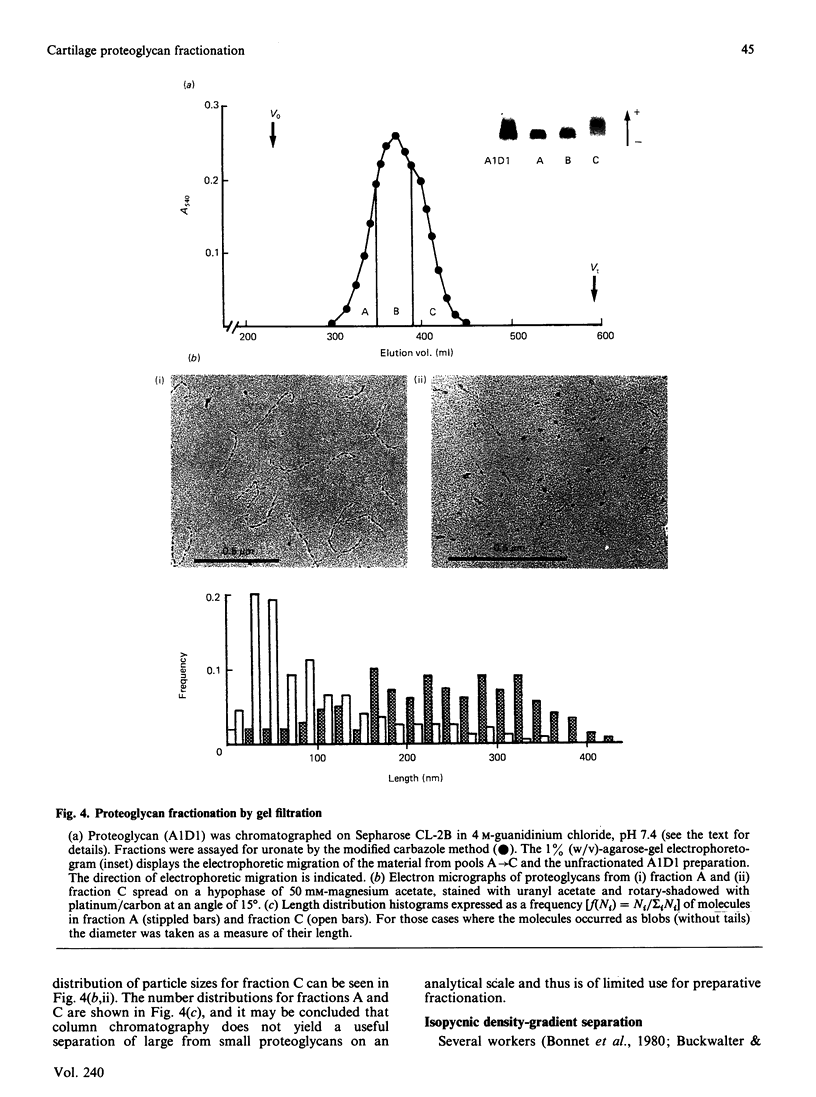
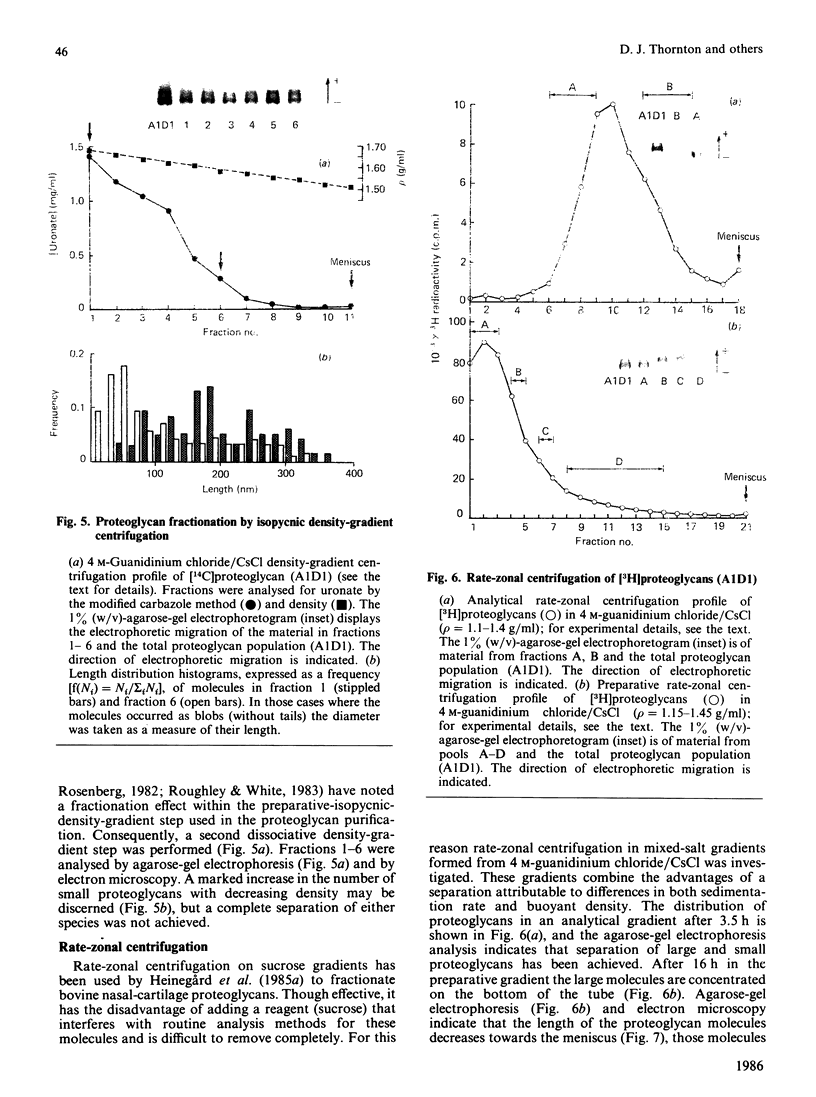
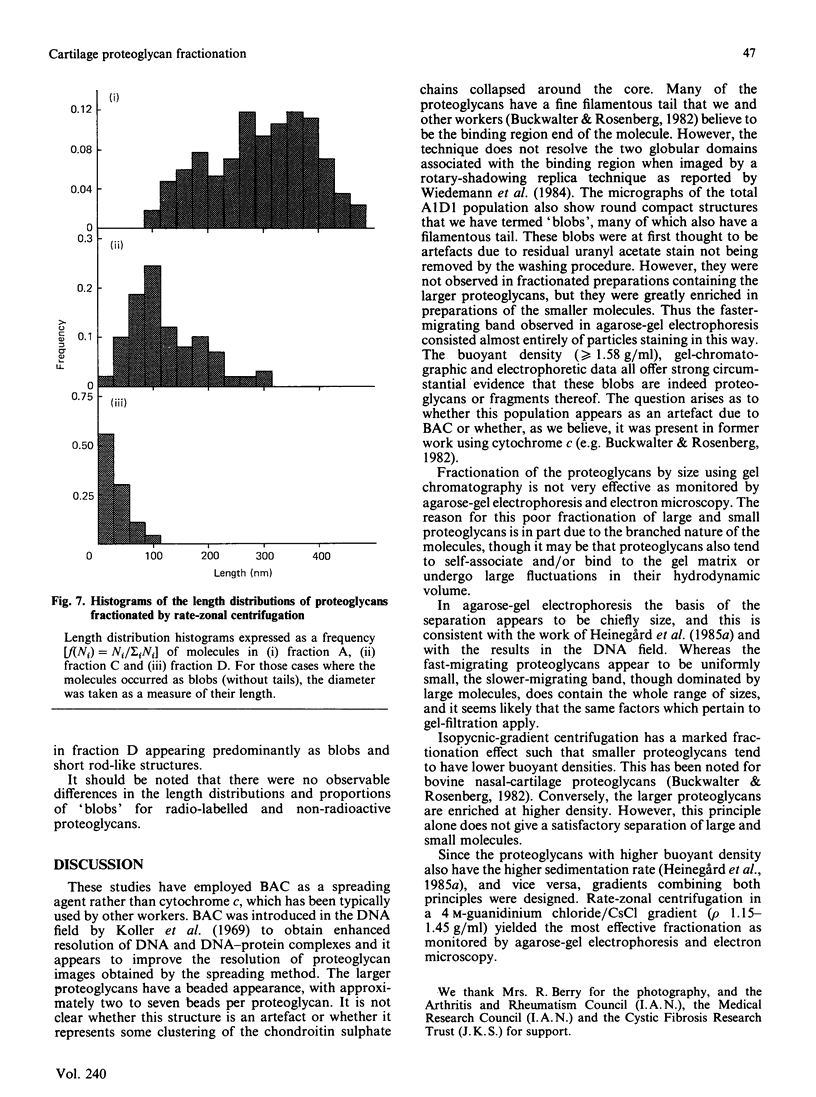
Proteoglycans (A1D1) extracted from bovine femoral-head cartilage were examined by electron microscopy using benzyldimethylammonium chloride as a spreading agent. The preparation contained a mixture of particles, some with a 'beaded' structure and a contiguous filamentous 'tail' at one end and others which appeared as round 'blobs', some of which also had filamentous tails. Previous electron-microscopic studies of proteoglycan monomers have indicated that their length distributions were apparently unimodal, a finding that contrasted with agarose/polyacrylamide-gel-electrophoresis results, which generally indicated two bands. In the present study proteoglycans isolated from the slowly migrating electrophoretic band were shown to be predominantly the larger molecules of beaded appearance, whereas the rapidly migrating proteoglycans were predominantly molecules with the 'blob-like' appearance. Gel-filtration, isopycnic-density-gradient-centrifugation and rate-zonal-centrifugation techniques were evaluated as means of proteoglycan fractionation by electron microscopy and agarose-gel electrophoresis. Rate-zonal centrifugation in mixed-salt gradients of caesium chloride/4 M-guanidinium chloride yielded the most effective fractionation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bonnet F., Périn J. P., Jollès P. Proteoglycan complex and proteoglycan subunit polydispersity. Study by isopycnic centrifugation in cesium sulfate density gradients. Biochim Biophys Acta. 1980 May 29;623(1):57–68. doi: 10.1016/0005-2795(80)90007-0. [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Rosenberg L. C. Electron microscopic studies of cartilage proteoglycans. Direct evidence for the variable length of the chondroitin sulfate-rich region of proteoglycan subunit core protein. J Biol Chem. 1982 Aug 25;257(16):9830–9839. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Lohmander S., Thyberg J. Cartilage proteoglycan aggregates. Electron-microscopic studies of native and fragmented molecules. Biochem J. 1978 Dec 1;175(3):913–919. doi: 10.1042/bj1750913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y., Hedbom E., Wieslander J., Larsson B. Assay of proteoglycan populations using agarose-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Nov 15;151(1):41–48. doi: 10.1016/0003-2697(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Wieslander J., Sheehan J., Paulsson M., Sommarin Y. Separation and characterization of two populations of aggregating proteoglycans from cartilage. Biochem J. 1985 Jan 1;225(1):95–106. doi: 10.1042/bj2250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. H., Osdoby P., Caplan A. I., Hascall V. C. Electron microscopic and biochemical studies of proteoglycan polydispersity in chick limb bud chondrocyte cultures. J Biol Chem. 1978 Jul 10;253(13):4721–4729. [PubMed] [Google Scholar]
- Kitchen R. G., Cleland R. L. Dilute solution properties of proteoglycan fractions from bovine nasal cartilage. Biopolymers. 1978 Mar;17(3):759–783. doi: 10.1002/bip.1978.360170316. [DOI] [PubMed] [Google Scholar]
- Lang D., Mitani M. Simplified quantitative electron microscopy of biopolymers. Biopolymers. 1970;9(3):373–379. doi: 10.1002/bip.1970.360090310. [DOI] [PubMed] [Google Scholar]
- Lyon M., Greenwood J., Sheehan J. K., Nieduszynski I. A. Isolation and characterization of high-buoyant-density proteoglycans from bovine femoral-head cartilage. Biochem J. 1983 Aug 1;213(2):355–362. doi: 10.1042/bj2130355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieduszynski I. A., Sheehan J. K., Phelps C. F., Hardingham T. E., Muir H. Equilibrium-binding studies of pig laryngeal cartilage proteoglycans with hyaluronate oligosaccharide fractions. Biochem J. 1980 Jan 1;185(1):107–114. doi: 10.1042/bj1850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Roughley P. J., Mason R. M. The electrophoretic heterogeneity of bovine nasal cartilage proteoglycans. Biochem J. 1976 Aug 1;157(2):357–367. doi: 10.1042/bj1570357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. The use of caesium sulphate density gradient centrifugation to analyse proteoglycans from human articular cartilages of different ages. Biochim Biophys Acta. 1983 Aug 23;759(1-2):58–66. doi: 10.1016/0304-4165(83)90189-7. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Nieduszynski I. A., Phelps C. F. Self-association of proteoglycan subunits from pig laryngeal cartilage. Biochem J. 1978 Apr 1;171(1):109–114. doi: 10.1042/bj1710109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren R., Jamieson A. M., Blackwell J., Carrino D. A., Caplan A. I. Light scattering studies of chick limb bud proteoglycans. J Biol Chem. 1982 Aug 10;257(15):8627–8629. [PubMed] [Google Scholar]
- Stanescu V., Stanescu R. The distribution of proteoglycans of high electrophoretic mobility in cartilages from different species and of different ages. Biochim Biophys Acta. 1983 Jun 9;757(3):377–381. doi: 10.1016/0304-4165(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Swann D. A., Powell S., Sotman S. The heterogeneity of cartilage proteoglycans. Isolation of different types of proteoglycans from bovine articular cartilage. J Biol Chem. 1979 Feb 10;254(3):945–954. [PubMed] [Google Scholar]
- Wiedemann H., Paulsson M., Timpl R., Engel J., Heinegård D. Domain structure of cartilage proteoglycans revealed by rotary shadowing of intact and fragmented molecules. Biochem J. 1984 Nov 15;224(1):331–333. doi: 10.1042/bj2240331. [DOI] [PMC free article] [PubMed] [Google Scholar]