Abstract
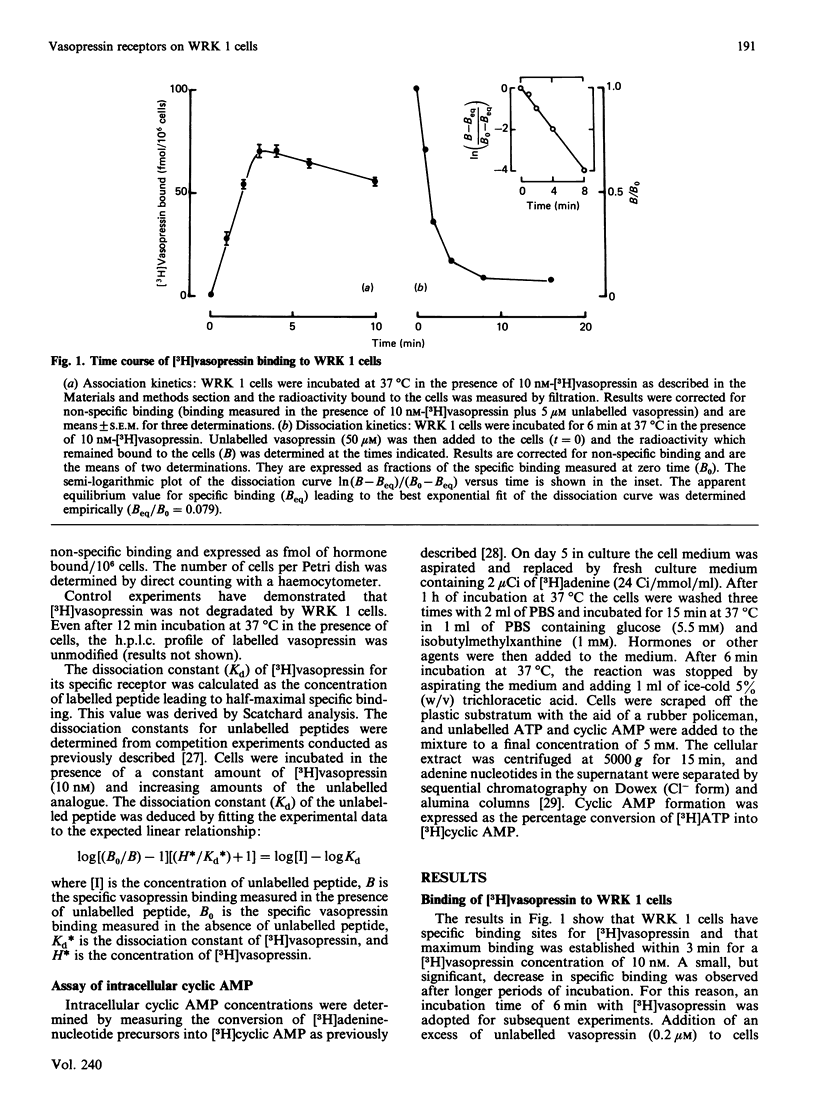
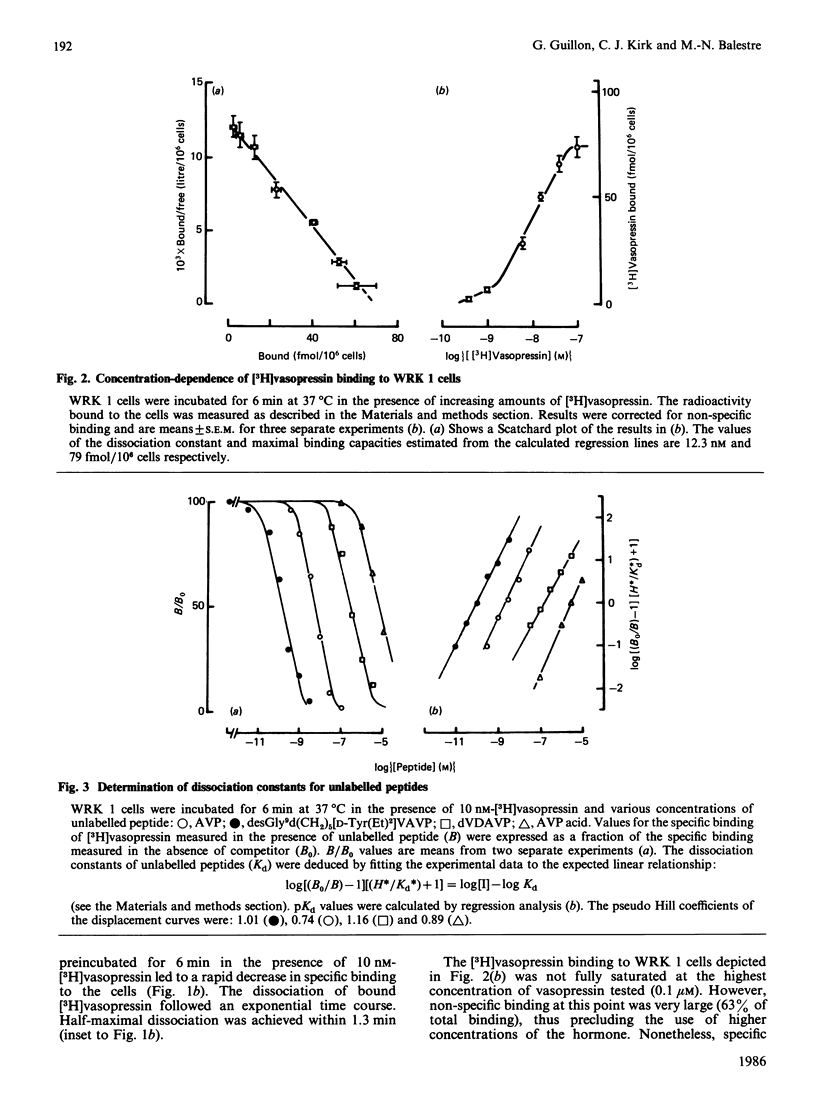
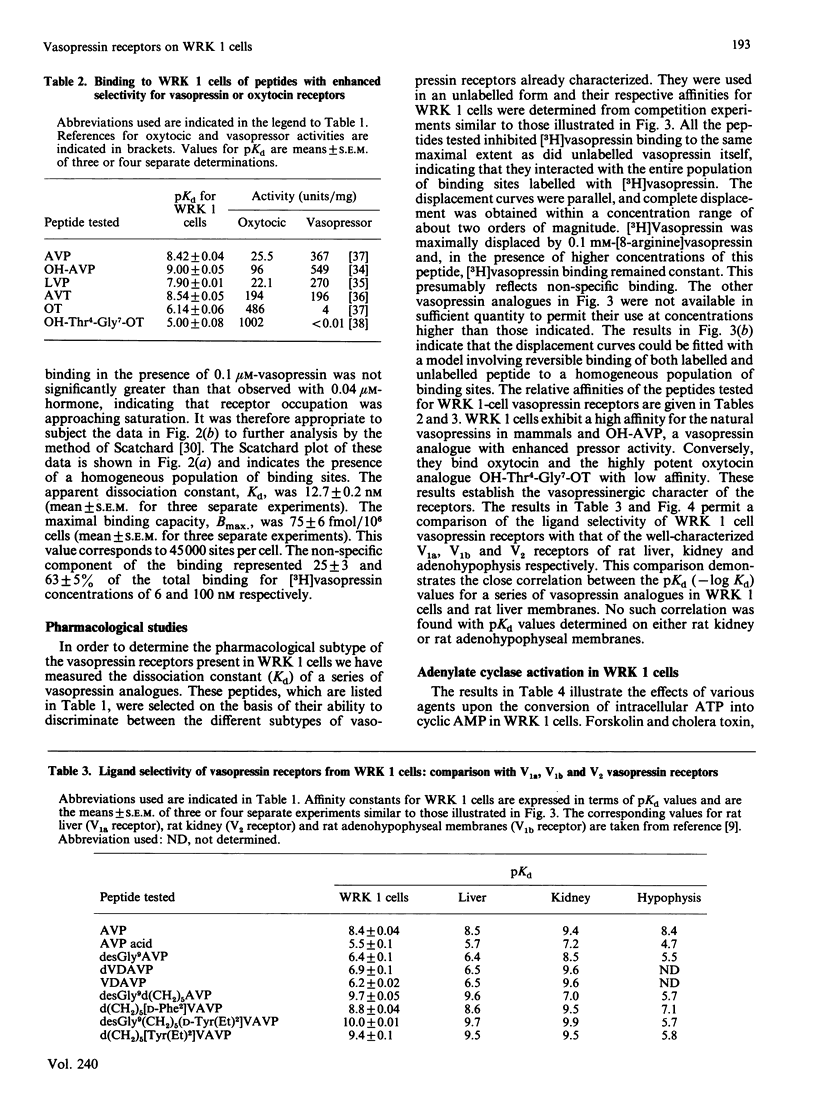
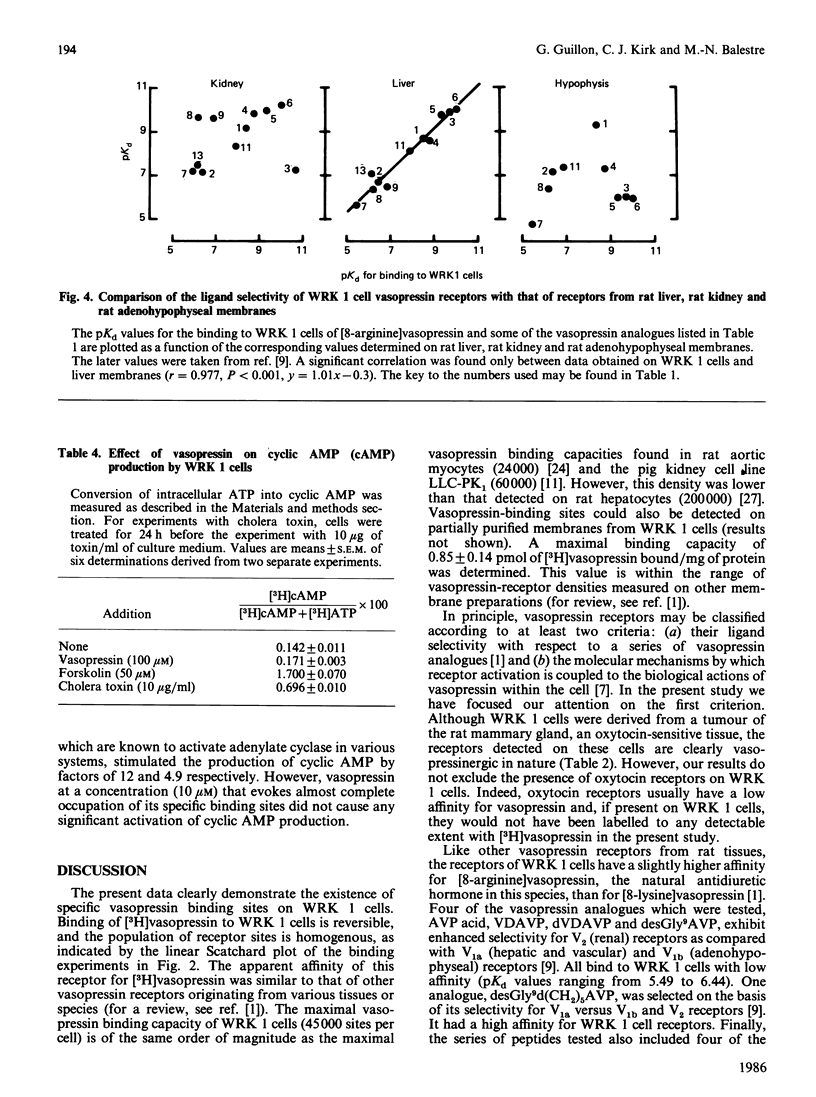
WRK 1, a cloned cell line derived from a rat mammary tumour, carries specific vasopressin-binding sites. Specific binding of 2-tyrosine-3H-labelled [8-lysine]vasopressin ([3H]vasopressin) was time-dependent, saturable and reversible. Scatchard-plot analysis of hormone binding indicated the presence of a single class of receptors with an equilibrium dissociation constant of 12.7 +/- 0.2 nM. The maximal binding capacity was 75 +/- 6 fmol/10(6) cells, which corresponds to approx. 45,000 sites per cell. Oxytocin and a highly potent oxytocin analogue were able to inhibit completely [3H]vasopressin binding, but, in this respect, they were far less potent than vasopressin. This clearly demonstrates the vasopressinergic nature of this receptor. Pharmacological studies using a series of 14 vasopressin or oxytocin analogues indicated that the ligand selectivity of the vasopressin receptor found on WRK 1 cells resembles that of the rat hepatocyte. This signifies that this vasopressin receptor is of the V1a subtype. This conclusion was confirmed by the observation that vasopressin did not influence the production of intracellular cyclic AMP in WRK 1 cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera G., Harwood J. P., Wilson J. X., Morell J., Brown J. H., Catt K. J. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem. 1983 Jul 10;258(13):8039–8045. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bockaert J., Roy C., Rajerison R., Jard S. Specific binding of (3H) lysine-vasopressin to pig kidney plasma membranes. Relationship of receptor occupancy to adenylate cyclase activation. J Biol Chem. 1973 Sep 10;248(17):5922–5931. [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Gaillard R. C., Schoenenberg P., Favrod-Coune C. A., Muller A. F., Marie J., Bockaert J., Jard S. Properties of rat anterior pituitary vasopressin receptors: relation to adenylate cyclase and the effect of corticotropin-releasing factor. Proc Natl Acad Sci U S A. 1984 May;81(9):2907–2911. doi: 10.1073/pnas.81.9.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon G., Couraud P. O., Butlen D., Cantau B., Jard S. Size of vasopressin receptors from rat liver and kidney. Eur J Biochem. 1980 Oct;111(1):287–294. doi: 10.1111/j.1432-1033.1980.tb06104.x. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Bone E. A., Palmer S., Michell R. H. The role of phosphatidylinositol 4,5 bisphosphate breakdown in cell-surface receptor activation. J Recept Res. 1984;4(1-6):489–504. doi: 10.3109/10799898409042569. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Guillon G., Balestre M. N., Creba J. A., Michell R. H., Jard S. Hormone-mediated inositol lipid breakdown in hepatocytes and WRK1 cells: relationship to receptor function. Biochimie. 1985 Oct-Nov;67(10-11):1161–1167. doi: 10.1016/s0300-9084(85)80115-2. [DOI] [PubMed] [Google Scholar]
- Kirk C. J., Guillon G., Balestre M. N., Jard S. Stimulation, by vasopressin and other agonists, of inositol-lipid breakdown and inositol phosphate accumulation in WRK 1 cells. Biochem J. 1986 Nov 15;240(1):197–204. doi: 10.1042/bj2400197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Lowbridge J., Manning M., Haldar J., Sawyer W. H. Synthesis and some pharmacological properties of [4-threonine, 7-glycine]oxytocin, [1-(L-2-hydroxy-3-mercaptopropanoic acid), 4-threonine, 7-glycine]oxytocin (hydroxy[Thr4, Gly7]oxytocin), and [7-Glycine]oxytocin, peptides with high oxytocic-antidiuretic selectivity. J Med Chem. 1977 Jan;20(1):120–123. doi: 10.1021/jm00211a025. [DOI] [PubMed] [Google Scholar]
- Lowbridge J., Manning M., Haldar J., Sawyer W. [1-(L-2-hydroxy-3-mercaptopropanoic acid)] analogues of arginine-vasopressin, [8-D-arginine]vasopressin, and [4-valine,8-D-arginine]vasopressin. J Med Chem. 1977 Sep;20(9):1173–1176. doi: 10.1021/jm00219a012. [DOI] [PubMed] [Google Scholar]
- Manning M., Coy E. J., Acosta M., Sawyer W. H. Solid-phase synthesis and some pharmacological properties of deamino-4-threonine analogs of the vasopressins and vasotocin and (deamino)arginine-vasotocin. J Med Chem. 1973 Jul;16(7):836–839. doi: 10.1021/jm00265a020. [DOI] [PubMed] [Google Scholar]
- Manning M., Coy E. J., Sawyer W. H. The deamino derivatives of (4-threonine)-oxytocin and (4-threonine)-mesotocin; analogs possessing a surprising spectrum of diminished pharmacological activities. Experientia. 1971;27(11):1372–1374. doi: 10.1007/BF02136746. [DOI] [PubMed] [Google Scholar]
- Manning M., Olma A., Klis W., Kolodziejczyk A., Nawrocka E., Misicka A., Seto J., Sawyer W. H. Carboxy terminus of vasopressin required for activity but not binding. Nature. 1984 Apr 12;308(5960):652–653. doi: 10.1038/308652a0. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):123–138. doi: 10.1098/rstb.1981.0177. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Kidwell W. R., Lippman M. E. Neurohypophysial-hormone-responsive cell line derived from a dimethylbenzanthracene-induced rat mammary tumour. Biochem J. 1980 May 15;188(2):437–441. doi: 10.1042/bj1880437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco M. E., Lippman M. E. A new model system for studying the phosphatidylinositol cycle. J Cell Physiol. 1982 Jul;112(1):148–153. doi: 10.1002/jcp.1041120122. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Lippmann M. E., Knazek R., Kidwell W. R. Vasopressin stimulation of acetate incorporation into lipids in a dimethylbenz(a)anthracene-induced rat mammary tumor cell line. Cancer Res. 1978 Nov;38(11 Pt 2):4101–4104. [PubMed] [Google Scholar]
- Monaco M. E. The phosphatidylinositol cycle in WRK-1 cells. Evidence for a separate, hormone-sensitive phosphatidylinositol pool. J Biol Chem. 1982 Mar 10;257(5):2137–2139. [PubMed] [Google Scholar]
- Monaco M. E., Woods D. Characterization of the hormone-sensitive phosphatidylinositol pool in WRK-1 cells. J Biol Chem. 1983 Dec 25;258(24):15125–15129. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Payet N., Isler H. Adrenal glomerulosa mitotic stimulation by posterior pituitary hormones. Cell Tissue Res. 1976 Sep 6;172(1):93–101. doi: 10.1007/BF00226051. [DOI] [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Pradelles P., Morgat J. L., Fromageot P., Camier M., Bonne D., Cohen P., Bockaert J., Jard S. Tritium labelling of 8-lysine vasopressin and its purification by affinity chromatography on sepharose bound neurophysins. FEBS Lett. 1972 Oct 1;26(1):189–192. doi: 10.1016/0014-5793(72)80570-2. [DOI] [PubMed] [Google Scholar]
- Roy C., Ausiello D. A. Characterization of (8-lysine) vasopressin binding sites on a pig kidney cell line (LLC-PK1). Evidence for hormone-induced receptor transition. J Biol Chem. 1981 Apr 10;256(7):3415–3422. [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Takhar A. P., Kirk C. J. Stimulation of inorganic-phosphate incorporation into phosphatidylinositol in rat thoracic aorta mediated through V1-vasopressin receptors. Biochem J. 1981 Jan 15;194(1):167–172. doi: 10.1042/bj1940167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali F. A. Effects of oxytocin and vasopressin on ganglionic transmission at the rabbit superior cervical ganglion. Pharmacol Res Commun. 1984 Jan;16(1):55–62. [PubMed] [Google Scholar]
- Weiss S., Sebben M., Bockaert J. Corticotropin-peptide regulation of intracellular cyclic AMP production in cortical neurons in primary culture. J Neurochem. 1985 Sep;45(3):869–874. doi: 10.1111/j.1471-4159.1985.tb04074.x. [DOI] [PubMed] [Google Scholar]


