Abstract
The molecular properties of the haemagglutinin of Ricinus communis (RCA I or RCA 120) were evaluated by analytical ultracentrifugation, light-scattering, c.d. and fluorescence. The native molecule had a fairly expanded structure (f/f0 = 1.43) and dissociated into two subunits of equal size in 6 M-guanidinium chloride. This native structure was stable in alkali (up to pH 11) and resistant to thermal denaturation at neutrality. A pH-triggered change in the haemagglutinin conformation was observed and characterized by analytical ultracentrifugation, c.d. and fluorescence between pH 7 and 4.5, the range in which its affinity for galactosides decreased [Yamasaki, Absar & Funatsu (1985) Biochim, Biophys. Acta 828, 155-161]. These results are discussed in relation to those reported in the literature for other lectins and more especially ricin, for which a pH-dependent conformation transition has been observed in the same range of low pH.
Full text
PDF
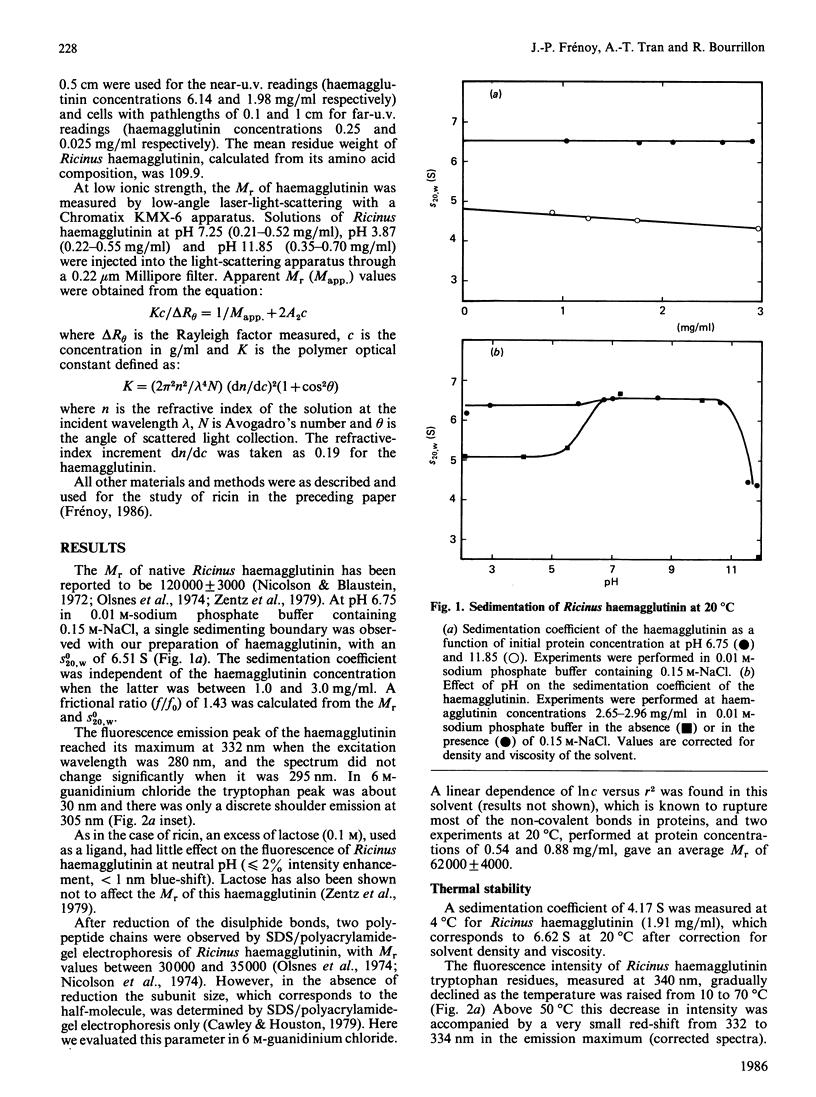
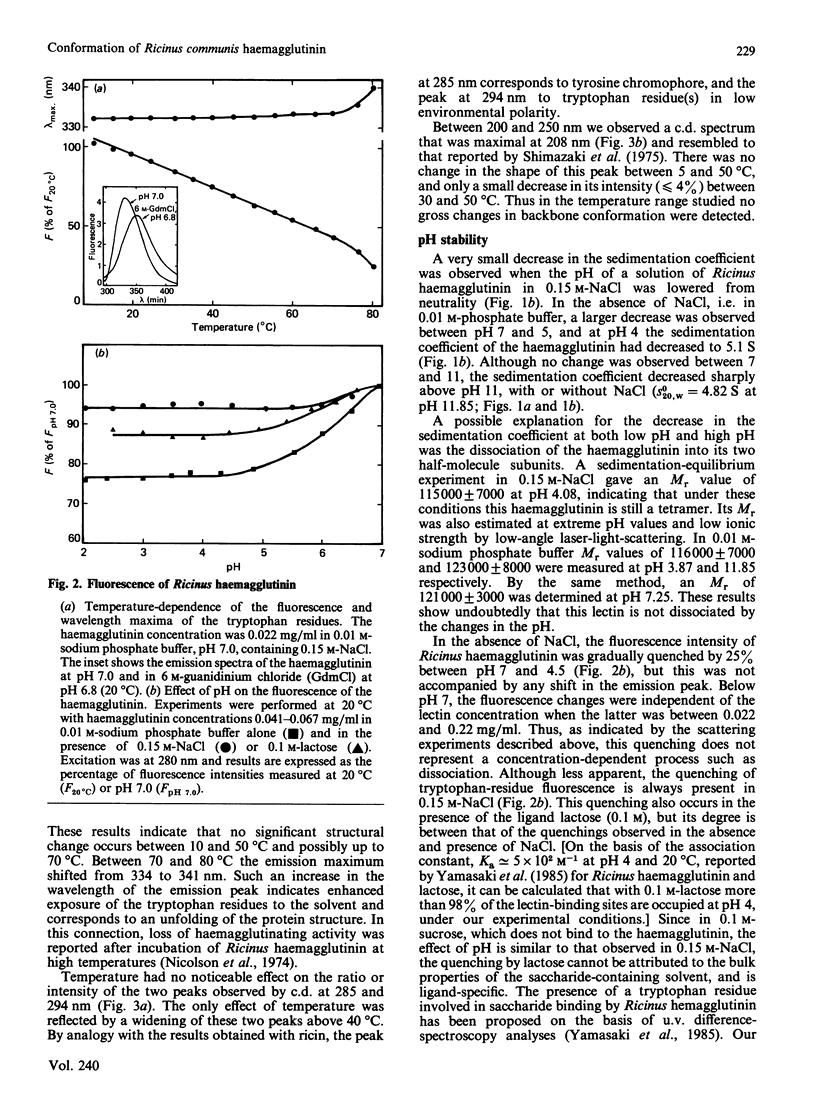
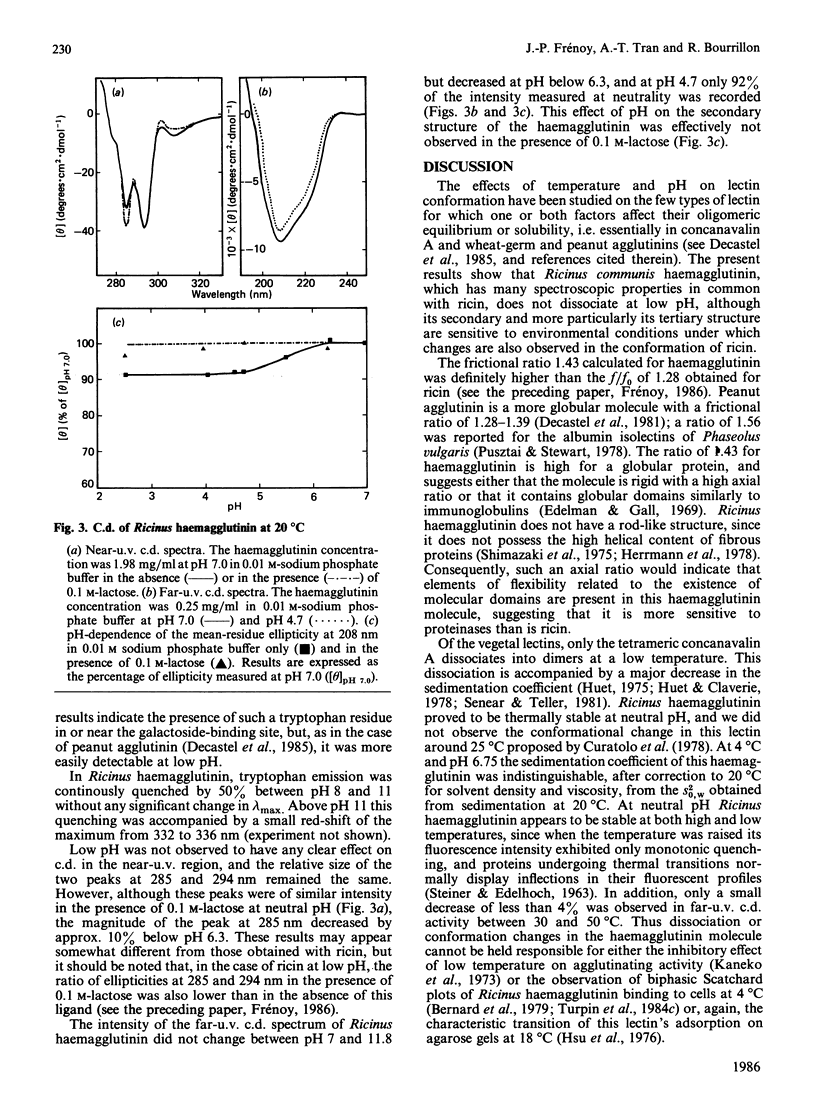

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J. U., Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979 Oct 10;254(19):9795–9799. [PubMed] [Google Scholar]
- Bernard B., Aubery M., Bourrillon R. Changes in the sensitivity of chick fibroblasts to Ricinus lectin (RCA I) toxicity in relation to the stage of embryo development. Biochem J. 1979 Jul 15;182(1):33–38. doi: 10.1042/bj1820033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewitt M. G., Chung L. A., London E. Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry. 1985 Sep 24;24(20):5458–5464. doi: 10.1021/bi00341a027. [DOI] [PubMed] [Google Scholar]
- Butterworth A. G., Lord J. M. Ricin and Ricinus communis agglutinin subunits are all derived from a single-size polypeptide precursor. Eur J Biochem. 1983 Dec 1;137(1-2):57–65. doi: 10.1111/j.1432-1033.1983.tb07795.x. [DOI] [PubMed] [Google Scholar]
- Cawley D. B., Hedblom M. L., Houston L. L. Homology between ricin and Ricinus communis agglutinin: amino terminal sequence analysis and protein synthesis inhibition studies. Arch Biochem Biophys. 1978 Oct;190(2):744–755. doi: 10.1016/0003-9861(78)90335-1. [DOI] [PubMed] [Google Scholar]
- Cawley D. B., Houston L. L. Effect of sulfhydryl reagents and protease inhibitors on sodium dodecyl sulfate-heat induced dissociation of Ricinus communis agglutinin. Biochim Biophys Acta. 1979 Nov 23;581(1):51–62. doi: 10.1016/0005-2795(79)90220-4. [DOI] [PubMed] [Google Scholar]
- Curatolo W. Temperature dependence of Ricinus communis agglutinin activity. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1340–1345. doi: 10.1016/0006-291x(82)91260-8. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Yau A. O., Small D. M., Sears B. Lectin-induced agglutination of phospholipid/glycolipid vesicles. Biochemistry. 1978 Dec 26;17(26):5740–5744. doi: 10.1021/bi00619a022. [DOI] [PubMed] [Google Scholar]
- Debray H., Decout D., Strecker G., Spik G., Montreuil J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem. 1981 Jun;117(1):41–55. doi: 10.1111/j.1432-1033.1981.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Decastel M., Bourrillon R., Frénoy J. P. Cryoinsolubility of peanut agglutinin. Effect of saccharides and neutral salts. J Biol Chem. 1981 Sep 10;256(17):9003–9008. [PubMed] [Google Scholar]
- Decastel M., De Boeck H., Goussault Y., De Bruyne C. K., Loontiens F. G., Frénoy J. P. Effect of pH on oligomeric equilibrium and saccharide-binding properties of peanut agglutinin. Arch Biochem Biophys. 1985 Aug 1;240(2):811–819. doi: 10.1016/0003-9861(85)90090-6. [DOI] [PubMed] [Google Scholar]
- Dodeur M., Aubery M., Bourrillon R. Toxic effect of Ricinus lectin on hepatoma cells in relation to enzyme modification of the cell surface. Biochim Biophys Acta. 1980 Mar 20;628(3):303–313. doi: 10.1016/0304-4165(80)90379-7. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Hamlin L. M., Miller R. L. The macromolecular properties of peanut agglutinin. Arch Biochem Biophys. 1978 Oct;190(2):693–698. doi: 10.1016/0003-9861(78)90328-4. [DOI] [PubMed] [Google Scholar]
- Frénoy J. P. Effect of physical environment on the conformation of ricin. Influence of low pH. Biochem J. 1986 Nov 15;240(1):221–226. doi: 10.1042/bj2400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits T. T., Jacobs R., Nag K. The effects of salts and ureas on the subunit dissociation of concanavalin A. Biochim Biophys Acta. 1983 Jan 12;742(1):142–154. doi: 10.1016/0167-4838(83)90370-9. [DOI] [PubMed] [Google Scholar]
- Hsu H. W., Davis D. S., Wei C. H., Yang W. K. Temperature effects on affinity chromatography of two lectins from the seeds of Ricinus communis. Anal Biochem. 1976 Jun;73(2):513–521. doi: 10.1016/0003-2697(76)90201-3. [DOI] [PubMed] [Google Scholar]
- Huet M., Claverie J. M. Sedimentation studies of the reversible dimer-tetramer transition kinetics of concanavalin A. Biochemistry. 1978 Jan 24;17(2):236–241. doi: 10.1021/bi00595a007. [DOI] [PubMed] [Google Scholar]
- Huet M. Factors affecting the molecular structure and the agglutinating ability of concanavalin A and other lectins. Eur J Biochem. 1975 Nov 15;59(2):627–632. doi: 10.1111/j.1432-1033.1975.tb02491.x. [DOI] [PubMed] [Google Scholar]
- Kaneko I., Satoh H., Ukita T. Effect of metabolic inhibitors on the agglutination of tumor cells by concanavalin A and Ricinus communis agglutinin. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1087–1094. doi: 10.1016/0006-291x(73)91518-0. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. Partial specific volumes and interactions with solvent components of proteins in guanidine hydrochloride. Biochemistry. 1974 Jan 15;13(2):257–265. doi: 10.1021/bi00699a005. [DOI] [PubMed] [Google Scholar]
- Lord J. M. Synthesis and intracellular transport of lectin and storage protein precursors in endosperm from castor bean. Eur J Biochem. 1985 Jan 15;146(2):403–409. doi: 10.1111/j.1432-1033.1985.tb08666.x. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Sene C., Obrenovitch A., Roche A. C., Delmotte F., Boschetti E. Properties of succinylated wheat-germ agglutinin. Eur J Biochem. 1979 Jul;98(1):39–45. doi: 10.1111/j.1432-1033.1979.tb13157.x. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J., Etzler M. E. Characterization of two plant lectins from Ricinus communis and their quantitative interaction with a murine lymphoma. Biochemistry. 1974 Jan 1;13(1):196–204. doi: 10.1021/bi00698a029. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Podder S. K., Surolia A., Bachhawat B. K. On the specificity of carbohydrate-lectin recognition. The interaction of a lectin from Ricinus communis beans with simple saccharides and concanavalin A. Eur J Biochem. 1974 May 2;44(1):151–160. doi: 10.1111/j.1432-1033.1974.tb03468.x. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Stewart J. C. Isolectins of Phaseolus vulgaris. Physicochemical studies. Biochim Biophys Acta. 1978 Sep 26;536(1):38–49. doi: 10.1016/0005-2795(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Etzler M. E. Subunit structure of wheat germ agglutinin. Biochem Biophys Res Commun. 1974 Jul 10;59(1):414–419. doi: 10.1016/s0006-291x(74)80222-6. [DOI] [PubMed] [Google Scholar]
- STEINER R. F., EDELHOCH H. The ultraviolet fluorescence of proteins. I. The influence of pH and temperature. Biochim Biophys Acta. 1963 May 21;66:341–355. doi: 10.1016/0006-3002(63)91203-4. [DOI] [PubMed] [Google Scholar]
- Senear D. F., Teller D. C. Thermodynamics of concanavalin A dimer-tetramer self-association: sedimentation equilibrium studies. Biochemistry. 1981 May 26;20(11):3076–3083. doi: 10.1021/bi00514a014. [DOI] [PubMed] [Google Scholar]
- Shimazaki K., Walborg E. F., Jr, Neri G., Jirgensons B. Circular dichroism and saccharide-induced conformational transitions of lectins from Ricinus communis. Arch Biochem Biophys. 1975 Aug;169(2):731–736. doi: 10.1016/0003-9861(75)90218-0. [DOI] [PubMed] [Google Scholar]
- Turpin E., Frénoy J. P., Goussault Y. Nature of the interaction between Ricinus communis agglutinin and blood cells. FEBS Lett. 1984 Sep 17;175(1):82–86. doi: 10.1016/0014-5793(84)80574-8. [DOI] [PubMed] [Google Scholar]
- Turpin E., Goussault Y., Lis H., Sharon N. Nature of the receptor sites for galactosyl-specific lectins on human lymphocytes. Exp Cell Res. 1984 Jun;152(2):486–492. doi: 10.1016/0014-4827(84)90650-5. [DOI] [PubMed] [Google Scholar]
- Turpin E., Wantyghem J., Beaudry P., Néel D., Goussault Y. Modification of biological activities of Ricinus communis agglutinin by cross-linking with formaldehyde. Can J Biochem Cell Biol. 1984 Apr;62(4):203–208. doi: 10.1139/o84-029. [DOI] [PubMed] [Google Scholar]
- Yamasaki N., Absar N., Funatsu G. Ultraviolet difference spectroscopic analysis of the saccharide-binding properties of Ricinus communis agglutinin. Biochim Biophys Acta. 1985 Apr 5;828(2):155–161. doi: 10.1016/0167-4838(85)90052-4. [DOI] [PubMed] [Google Scholar]
- Zentz C., Frenoy J. P., Bourrillon R. Interaction entre l'hémagglutinine de ricin et ses ligands, galactose et lactose. Etude par microcalorimétrie et dialyse à l'équilibre. Biochimie. 1979;61(1):1–6. doi: 10.1016/s0300-9084(79)80307-7. [DOI] [PubMed] [Google Scholar]


