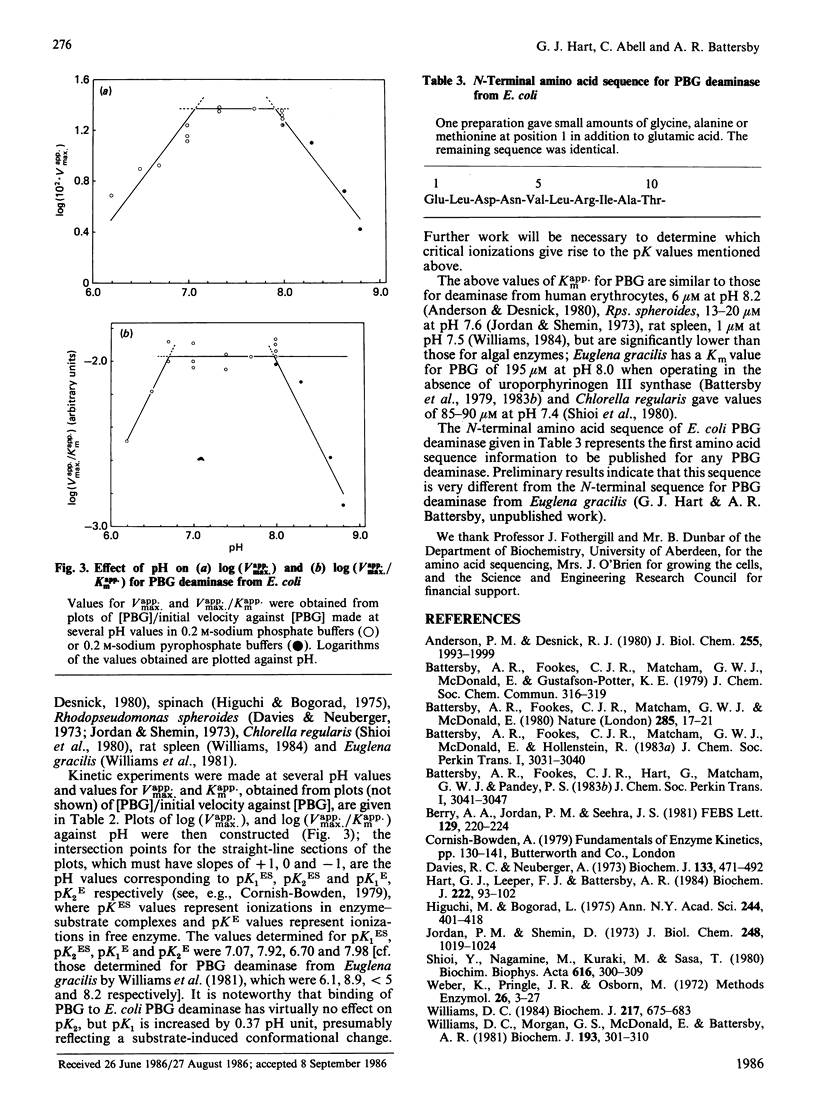
Abstract
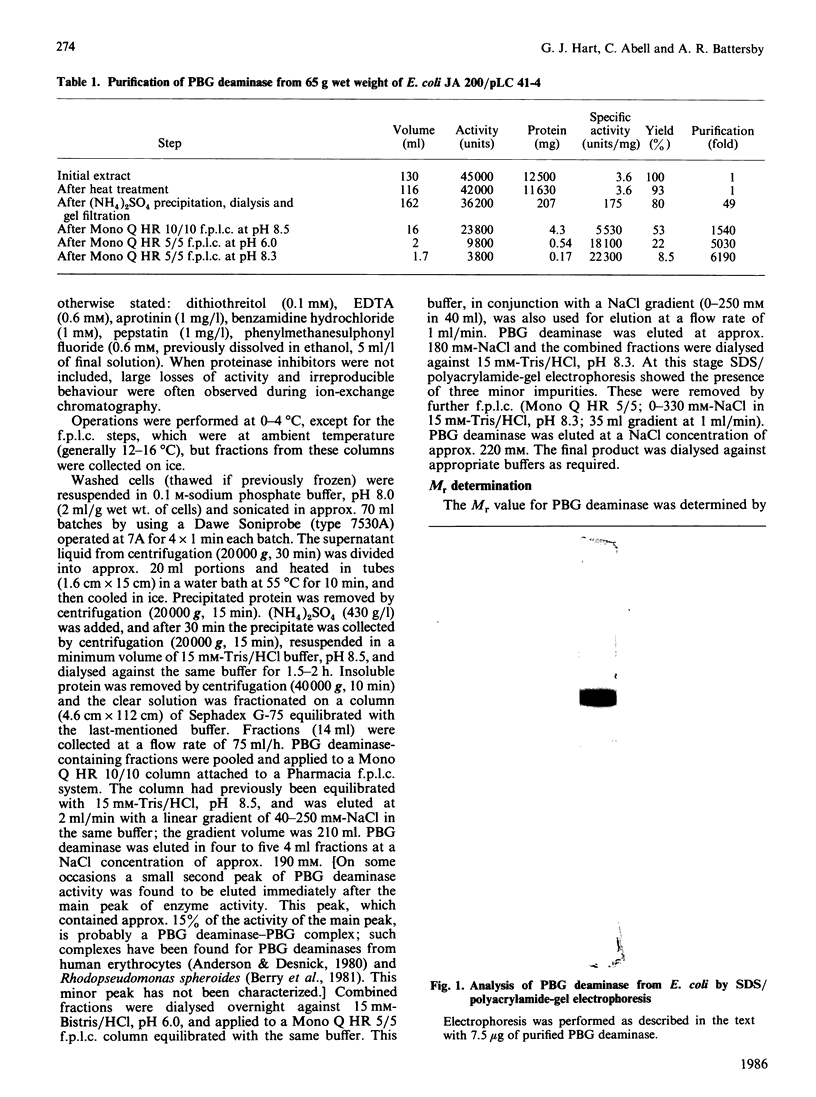
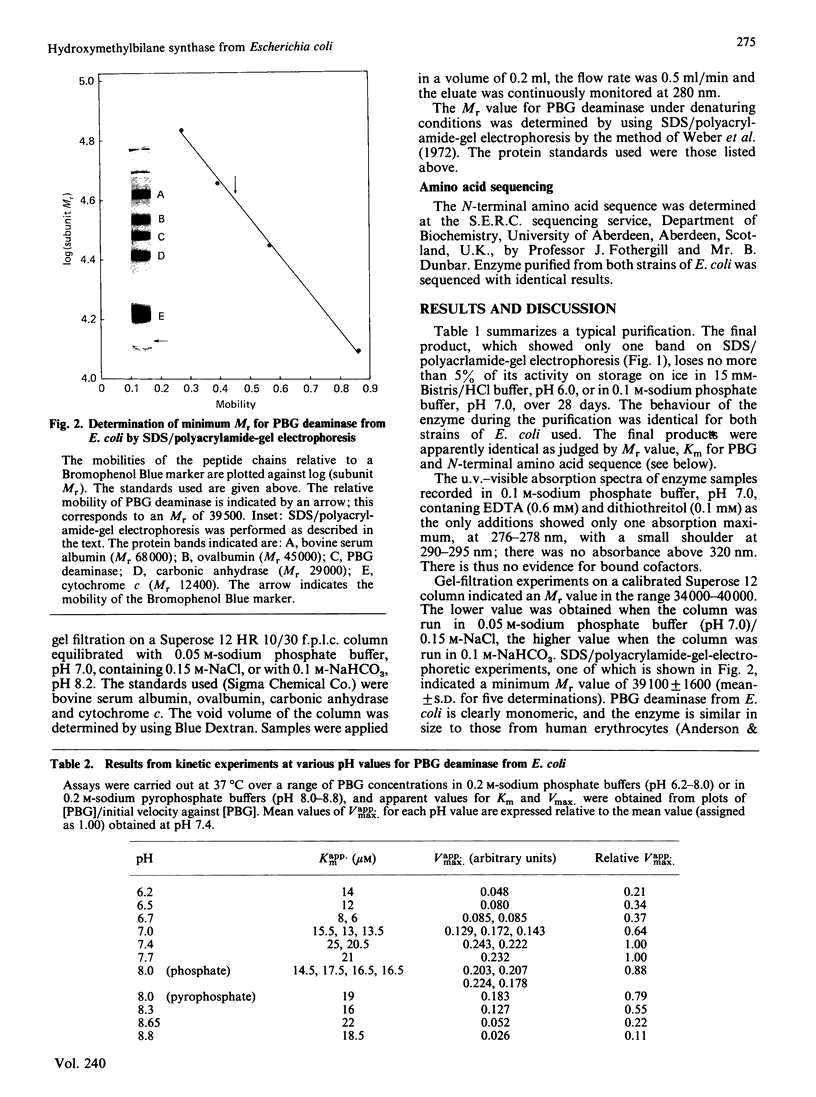
Hydroxymethylbilane synthase (porphobilinogen deaminase) was purified to apparent homogeneity from Escherichia coli. The enzyme is a monomer of Mr approx. 40,000. The Km for porphobilinogen and relative Vmax. values have been obtained at various pH values over the range 6.2-8.8, enabling pK values for ionizable groups important for activity to be determined. The N-terminal amino acid sequence is presented.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Purification and properties of uroporphyrinogen I synthase from human erythrocytes. Identification of stable enzyme-substrate intermediates. J Biol Chem. 1980 Mar 10;255(5):1993–1999. [PubMed] [Google Scholar]
- Battersby A. R., Fookes C. J., Matcham G. W., McDonald E. Biosynthesis of the pigments of life: formation of the macrocycle. Nature. 1980 May 1;285(5759):17–21. doi: 10.1038/285017a0. [DOI] [PubMed] [Google Scholar]
- Berry A., Jordan P. M., Seehra J. S. The isolation and characterization of catalytically competent porphobilinogen deaminase-intermediate complexes. FEBS Lett. 1981 Jul 6;129(2):220–224. doi: 10.1016/0014-5793(81)80169-x. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Neuberger A. Polypyrroles formed from porphobilinogen and amines by uroporphyrinogen synthetase of Rhodopseudomonas spheroides. Biochem J. 1973 Jul;133(3):471–492. doi: 10.1042/bj1330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. J., Leeper F. J., Battersby A. R. Modification of hydroxymethylbilane synthase (porphobilinogen deaminase) by pyridoxal 5'-phosphate. Demonstration of an essential lysine residue. Biochem J. 1984 Aug 15;222(1):93–102. doi: 10.1042/bj2220093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Bogorad L. The purification and properties of uroporphyrinogen I synthases and uroporphyrinogen III cosynthase. Interactions between the enzymes. Ann N Y Acad Sci. 1975 Apr 15;244:401–418. doi: 10.1111/j.1749-6632.1975.tb41545.x. [DOI] [PubMed] [Google Scholar]
- Jordan P. M., Shemin D. Purification and properties of uroporphyrinogen I synthetase from Rhodopseudomonas spheroides. J Biol Chem. 1973 Feb 10;248(3):1019–1024. [PubMed] [Google Scholar]
- Shioi Y., Nagamine M., Kuroki M., Sasa T. Purification by affinity chromatography and properties of uroporphyrinogen I synthetase from Chlorella regularis. Biochim Biophys Acta. 1980 Dec 4;616(2):300–309. doi: 10.1016/0005-2744(80)90147-3. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Williams D. C. Characterization of the multiple forms of hydroxymethylbilane synthase from rat spleen. Biochem J. 1984 Feb 1;217(3):675–683. doi: 10.1042/bj2170675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. C., Morgan G. S., McDonald E., Battersby A. R. Purification of porphobilinogen deaminase from Euglena gracilis and studies of its kinetics. Biochem J. 1981 Jan 1;193(1):301–310. doi: 10.1042/bj1930301. [DOI] [PMC free article] [PubMed] [Google Scholar]