Abstract
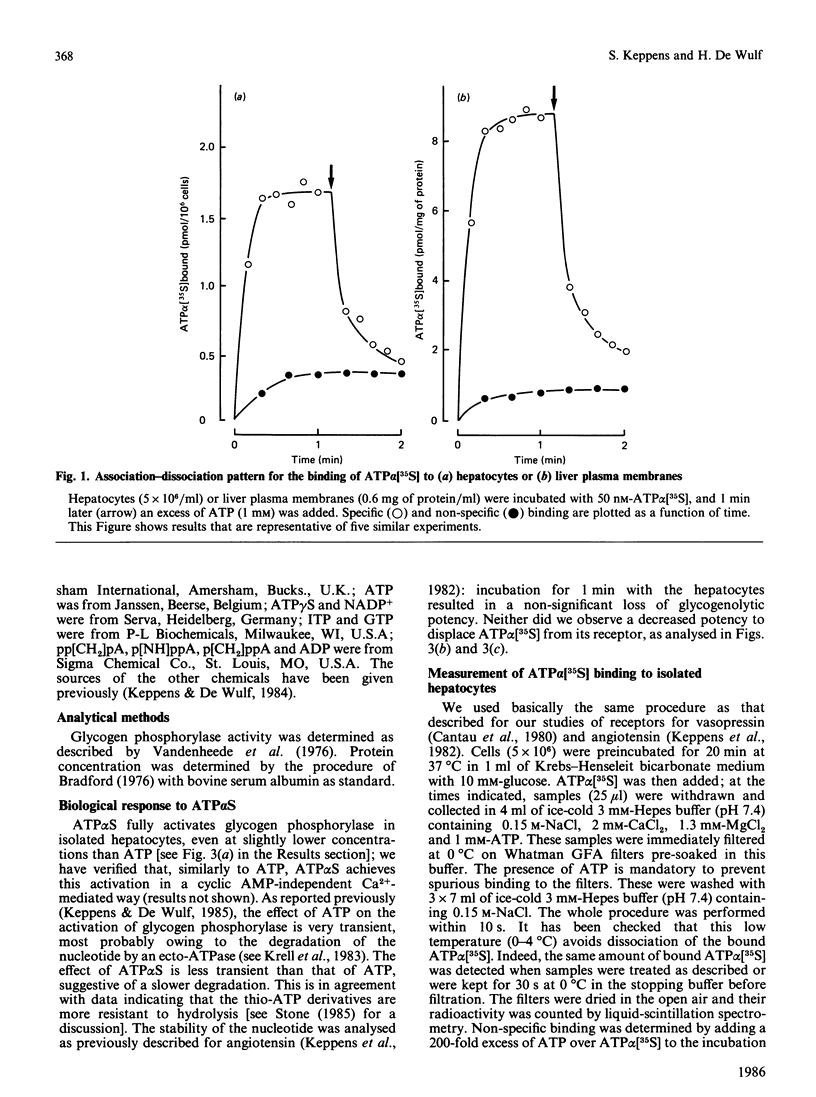
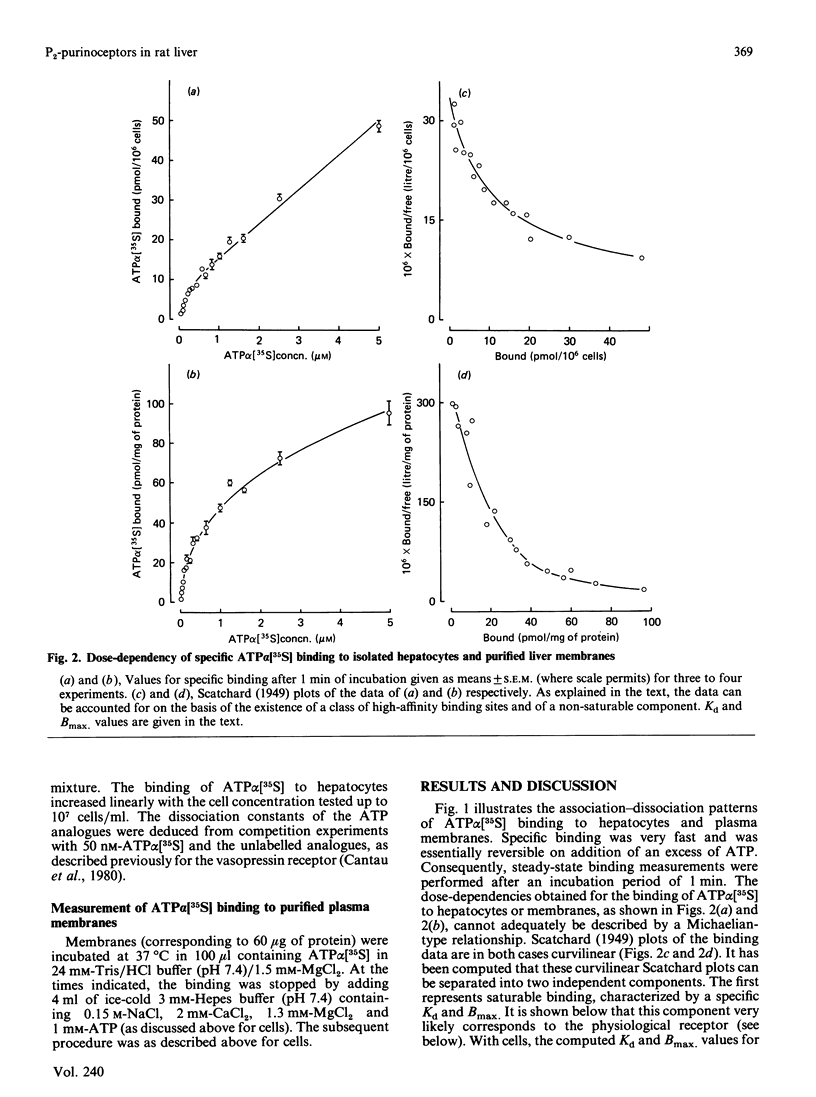
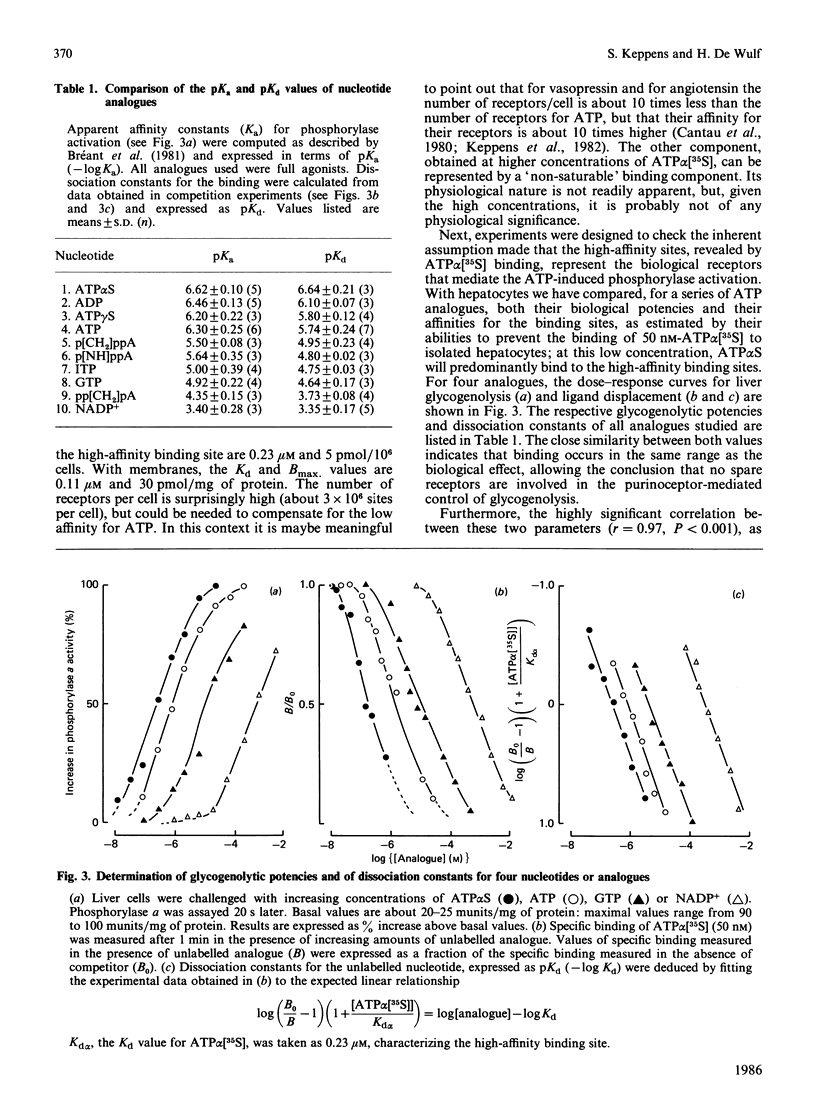
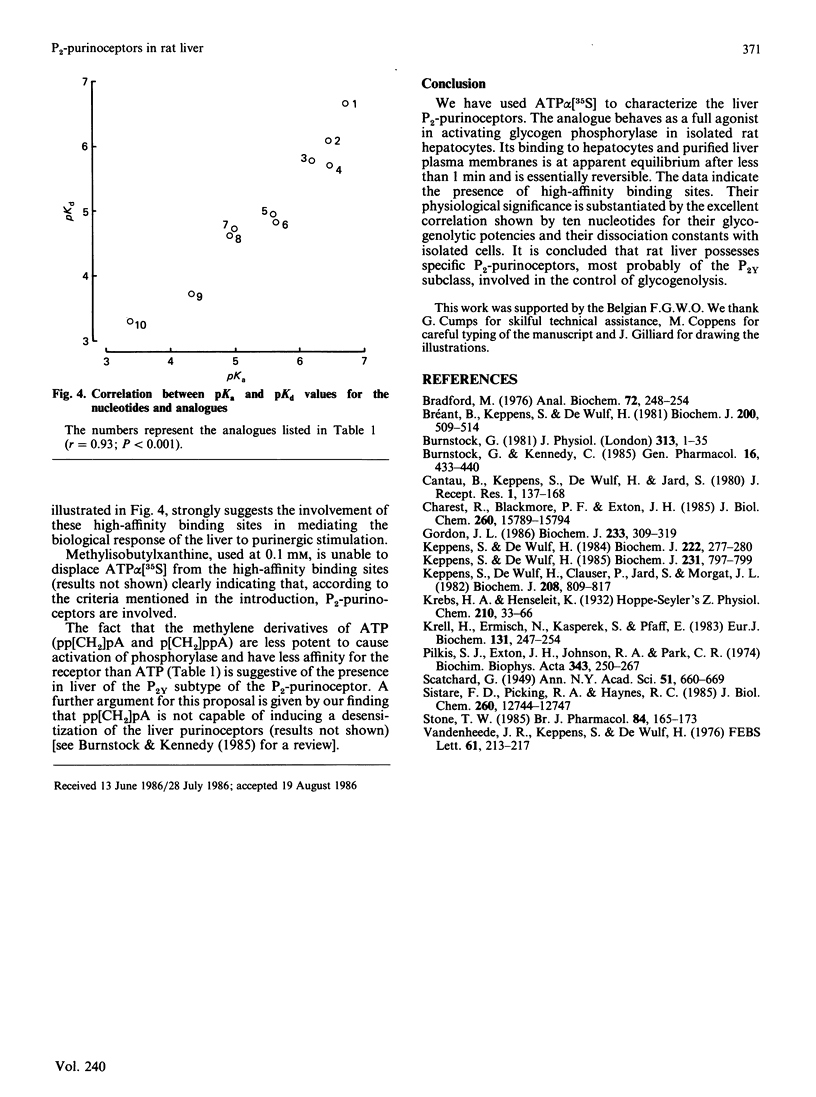
Evidence has been presented for the existence in rat liver of P2-purinoceptors which are involved in the control of glycogenolysis. Isolated rat hepatocytes and purified liver plasma membranes have been used to study the binding of the ATP analogue adenosine 5'-[alpha- [35S]thio]triphosphate (ATP alpha [35S]) to these postulated P2-purinoceptors. The nucleotide analogue behaves as a full agonist for the activation of glycogen phosphorylase in isolated hepatocytes, 0.3 microM being required for half-maximal activation. Specific binding of ATP alpha [35S] to hepatocytes and plasma membranes occurs within 1 min and is essentially reversible. The analysis of the dose-dependency at equilibrium indicates the presence of binding sites with Kd of 0.23 microM with hepatocytes and Kd of 0.11 microM with plasma membranes. The relative affinities of 10 nucleotide analogues were deduced from competition experiments for ATP alpha [35S] binding to hepatocytes, and these correlated highly with their biological activity (activation of glycogen phosphorylase in hepatocytes). For all the agonists, binding occurs in the same concentration range as the biological effect. These data clearly suggest that the detected binding sites correspond to the physiological P2-purinoceptors involved in the regulation of liver glycogenolysis. The rank order of potency of some ATP analogues suggests that liver possesses the P2Y-subclass of P2-purinoceptors.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bréant B., Keppens S., De Wulf H. Heterologous desensitization of the cyclic AMP-independent glycogenolytic response in rat liver cells. Biochem J. 1981 Dec 15;200(3):509–514. doi: 10.1042/bj2000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H., Clauser P., Jard S., Morgat J. L. The liver angiotensin receptor involved in the activation of glycogen phosphorylase. Biochem J. 1982 Dec 15;208(3):809–817. doi: 10.1042/bj2080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. P2-purinergic control of liver glycogenolysis. Biochem J. 1985 Nov 1;231(3):797–799. doi: 10.1042/bj2310797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Vasopressin and angiotensin control the activity of liver phosphodiesterase. Biochem J. 1984 Aug 15;222(1):277–280. doi: 10.1042/bj2220277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell H., Ermisch N., Kasperek S., Pfaff E. On the mechanisms of ATP-induced and succinate-induced redistribution of cations in isolated rat liver cells. Eur J Biochem. 1983 Mar 15;131(2):247–254. doi: 10.1111/j.1432-1033.1983.tb07256.x. [DOI] [PubMed] [Google Scholar]
- Pilkis S. J., Exton J. H., Johnson R. A., Park C. R. Effects of glucagon on cyclic AMP and carbohydrate metabolism in livers from diabetic rats. Biochim Biophys Acta. 1974 Mar 20;343(1):250–267. doi: 10.1016/0304-4165(74)90258-x. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Stone T. W. The activity of phosphorothioate analogues of ATP in various smooth muscle systems. Br J Pharmacol. 1985 Jan;84(1):165–173. [PMC free article] [PubMed] [Google Scholar]
- Vandenheede J. R., Keppens S., De Wulf H. The activation of liver phosphorylase b kinase by glucagon. FEBS Lett. 1976 Jan 15;61(2):213–217. doi: 10.1016/0014-5793(76)81040-x. [DOI] [PubMed] [Google Scholar]


