Summary
Background
Tuberculosis (TB) remains a significant cause of mortality globally, yet first-line treatment has hardly changed for fifty years. The dose of rifampicin, the most important drug in this regimen, has been historically based on pragmatic cost- and risk-benefit considerations. Evidence suggests the current recommended dose (8–12 mg/kg) may not maximise the potential benefits of this drug. We sought to evaluate the efficacy and safety of higher doses of rifampicin in adults with presumed drug-susceptible TB.
Methods
In this systematic review we searched MEDLINE, EMBASE, CENTRAL and Global Health databases for randomised controlled trials up to 31 July 2024 of adults with presumed drug-susceptible TB receiving first-line treatment with an intervention of rifampicin doses higher than currently recommended. Meta-analyses were performed using random effects models where background regimens were the same. Risk ratio was used as the measure for treatment effect. Outcomes of interest related to efficacy and safety.
Findings
Of the 5441 total records identified by our searches, nineteen studies (6332 patients, 31.0% female) were eligible for the systematic review and twelve (3763 patients, 31.0% female) for meta-analysis. Rifampicin doses varied from 8 to 35 mg/kg and implementation of the intervention varied between trials. There was no evidence for increased efficacy with higher doses of rifampicin, however the majority of trials investigated minimally increased doses (up to 20 mg/kg). At higher doses (>20 mg/kg), there may be evidence of increased risk of drug-induced liver injury, albeit with no consistent dose–response relationship.
Interpretation
Evidence on the efficacy of higher doses of rifampicin in the first-line regimen for TB remains incomplete. While higher doses appear generally safe, the risk of drug-induced liver injury may be increased above doses of 20 mg/kg. Larger clinical trials reporting definitive outcomes are needed to determine whether dosing up to 40 mg/kg could safely improve treatment outcomes or reduce duration of first-line therapy.
Funding
WHO, Wellcome Trust.
Keywords: High dose rifampicin, Optimisation of rifampicin, Tuberculosis, Systematic review, Meta-analysis
Research in context.
Evidence before this study
Despite rifampicin being the most important drug in the first-line regimen for drug-susceptible TB, the pharmacological basis of this fundamental role is not fully understood. Rifampicin demonstrates high inter-person and inter-study variability in pharmacokinetic studies, however there is evidence that higher doses result in higher exposure in plasma and/or cerebrospinal fluid. Studies also suggest that doses up to 40 mg/kg may be tolerable in humans and the current recommended dose (8–12 mg/kg) may not maximise the potential benefits of this drug. Two previous systematic reviews investigated increased doses of rifampicin, both focusing exclusively on pulmonary TB and reporting the main outcome as the intermediate endpoint of culture conversion. One of these reviews described some longer-term outcomes, finding no difference in mortality or moderate to severe liver toxicity between standard and higher dose groups. This is an updated version of a WHO commissioned review published in the WHO operational handbook on tuberculosis (2022 update).
Added value of this study
To our knowledge, this is the first systematic review and meta-analysis reporting on the efficacy and safety of higher dose rifampicin in adults with any type of presumed drug-susceptible TB. The review focused on key long-term outcomes and study settings were diverse in terms of both geographical location and TB incidence. Many countries with the highest TB rates were represented, with the notable exception of China. Despite diverse designs and reported outcomes in the eighteen included trials, meaningful data synthesis was possible for key objectives. Higher than currently recommended doses of rifampicin appear to be safe and may be associated with reduced rates of treatment failure, relapse and all-cause mortality in pulmonary and meningeal TB.
Implications of all the available evidence
Larger clinical trials reporting definitive outcomes are needed to determine whether rifampicin doses up to 40 mg/kg could safely improve treatment outcomes or reduce duration of first-line therapy. The limited data does not permit identification of any important subgroups (e.g., those with HIV-associated TB or diabetes) that might benefit from this intervention. Drug–drug interaction studies are essential to understand the robustness of antiretroviral therapy and facilitate enrolment of more people with HIV-associated TB into future trials. Ongoing and planned Phase III randomised controlled trials of high-dose rifampicin in pulmonary, meningeal and disseminated TB will increase certainty of evidence at the higher doses examined in this review.
Introduction
Tuberculosis (TB) remains a pressing public health issue, with 1.3 million deaths worldwide and incident cases increasing to 10.6 million in 2022.1 While 85% of people successfully complete first-line treatment comprising rifampicin (RIF), isoniazid, pyrazinamide and ethambutol, this regimen requires six months to achieve durable cure, representing a significant burden for people with TB and their carers.
Of first-line treatment, RIF is the cornerstone. Mono-resistance to RIF is independently associated with the highest risk of treatment failure (RR 5.5)2 and duration of RIF administration is a key determinant of risk of relapse.3 This fundamental role is not fully understood but may be due to activity against non-replicating, antibiotic-tolerant Mycobacterium tuberculosis organisms4,5 and accumulation in pulmonary TB lesions.6
Mycobacterial killing is believed to be driven by the parameter area under the curve of RIF plasma concentration divided by Minimum Inhibitory Concentration of infecting organism (AUC/MIC). However, plasma concentrations of RIF exhibit high inter-person and inter-study variability.7 AUC/MIC values achieved on current doses of RIF vary more than three-fold8 and are on average much lower than those predicted to be optimal in preclinical systems, especially in the cerebrospinal space.9 This evidence suggests increased dosing of RIF could plausibly increase rates of treatment success. Whether these potential benefits can be realised in long-term outcomes and be achieved without additional toxicity has not been established.
RIF has traditionally been administered at a dose of 10 (range 8–12) mg/kg, based on pragmatic cost- and risk-benefit considerations. Perceptions of immune-mediated side effects and of drug-induced liver injury (DILI) may have limited exploration of higher dose levels.10 More recently, renewed study of dose and concentration-response relationships has caused re-evaluation of dosing schedules. In pre-clinical models, humanised levels of pharmacokinetic (PK) exposure do not maximise elimination of bacilli.11 Early phase trials have shown evidence of incremental dose–response beyond the current dose range,12 confirmed in two recent Phase IIB trials of pulmonary disease.8,13 Modestly higher doses of RIF have also been evaluated in extrapulmonary disease, particularly meningeal TB.14,15 Two phase III trials of higher dose RIF in pulmonary TB have recently been reported.16,17 Studies have not to date reported increased risk of serious adverse events (SAEs) such as hepatotoxicity at higher doses. Souleymane et al.18 recently stopped recruitment to the intervention arm (30 mg/kg RIF and high-dose isoniazid) of the TRIDORE RCT owing to significantly higher rates of adverse drug reactions in this arm. Given the addition of high-dose isoniazid, these AEs cannot be solely attributed to RIF. Phase II and III trials aiming to optimise higher dosing of RIF in pulmonary TB, HIV-associated TB and in tuberculous meningitis are ongoing (Appendix 1).
Maximising the efficacy of the first-line regimen is key to improving long-term outcomes of TB treatment and ensuring robustness against variability in adherence, PK, pharmacogenetics and resistance emergence. Intensification of treatment could also be important for those with severe or disseminated disease and higher RIF doses could ultimately reduce the duration of first-line treatment, which is amongst the objectives of a number of included studies.
Our primary objective is to assess the efficacy and safety of doses of RIF higher than those currently recommended by the World Health Organisation (WHO) when used as part of a combination regimen for treating adults with presumed drug-susceptible TB.
Methods
This systematic review and meta-analysis was reported in line with the PRISMA statement.19 This review was not registered.
Search strategy and selection criteria
We used the same search strategy as in our previous review,20 searching for eligible trials in MEDLINE (OVID), EMBASE (OVID), CENTRAL (Cochrane central register of controlled trials), WHO International Clinical Trials Registry and Clinicaltrials.gov, with no limits for language, date of publication or publication status (Appendix 2). Searches were run up to 31 July 2024 by a Cochrane information specialist (VL). Reference lists of retrieved reports were examined for unidentified studies. Active investigators in the field were contacted to provide information on any unidentified, ongoing or planned trials.
Inclusion criteria were randomised controlled trials of adults with presumed drug-sensitive TB (pulmonary, extrapulmonary, disseminated) on first-line treatment. Adults were defined as aged 18 years or over, or treated as adults in participating centres. Where it was not possible to differentiate data of participants under 18 years, the younger minority were included.
Assessed interventions were TB treatment regimens of any duration containing RIF at doses higher than recommended in current WHO guidelines (8–12 mg/kg). The comparator was treatment regimens containing RIF at recommended doses. Inclusion for meta-analysis required the same background regimen alongside RIF. Randomised concentration-controlled trials were not within the scope of this review. Although not recommended by WHO guidance, intravenous administration of RIF was permissible. The dose metric used was actual weight-adjusted dose in mg/kg. Where not specified, this was determined using weight data provided. Where weight-banded dosing regimens were used, average target weight-adjusted dose stated in the report of manufacturer’s summary of product characteristics was accepted or, if not specified, the average of weight-adjusted doses computed using the midpoint of each band.
Search results were uploaded to the Covidence interface21 and de-duplicated. Two reviewers independently screened titles and abstracts against inclusion and exclusion criteria. Two authors (KAH, HHT) independently screened full-text study reports. Reasons for non-inclusion were documented, with disagreements resolved by discussion, or the assistance of a third author (GD).
Data analysis
A data extraction form was designed, piloted and optimised. Two authors (KAH, HHT) independently extracted data. Where appropriate, multiple reports on the same study were collated. Data extraction completion was verified by two authors (KAH, HHT, LB, PEN). Data extraction included details on source, methods, participants, microbiological methods, pharmacology, interventions and outcomes (Appendix 3).
Primary outcomes were treatment success, treatment failure, relapse, death and adverse events. Secondary outcomes were SAEs, drug-specific adverse events of interest and disease-specific efficacy outcomes of interest. Definitions of outcomes can be found in appendices (Appendix 4). Summary data were extracted, where available, to enable intention to treat (ITT) and per protocol (PP) analyses.
Extracted data was imported into R version 4.3.322 for analysis. Meta-analysis was performed with random effects models.23 Risk ratio (RR) was used as the measure of effect. Analysis of the primary outcomes was on an ITT basis. For studies where more than one intervention arm was included, each arm was compared separately to the comparator to avoid splitting the control group. Stratified forest plots were used to present data. Corresponding 95% confidence intervals and p-values were computed with a significance level of 0.05. Heterogeneity was assessed by inspecting forest plots and, for meta-analysis, using the Iˆ2 statistic, with a value of 50% taken to indicate significant statistical heterogeneity.
Two authors independently assessed the methodological quality of each study using the Cochrane risk of bias tool.24 A threshold of 90% was set for adequate follow-up of randomised participants. We attempted to contact study authors if information was unspecified or unclear. Funnel and Galbraith plots were inspected for evidence of publication bias. Summary of findings tables were constructed to present certainty of evidence ratings for effect estimates for each outcome along with relative and absolute measures of effect using the GRADE approach.25
Role of the funding source
The funding source had no role in study design, data collection, analysis, interpretation or writing of the report.
Results
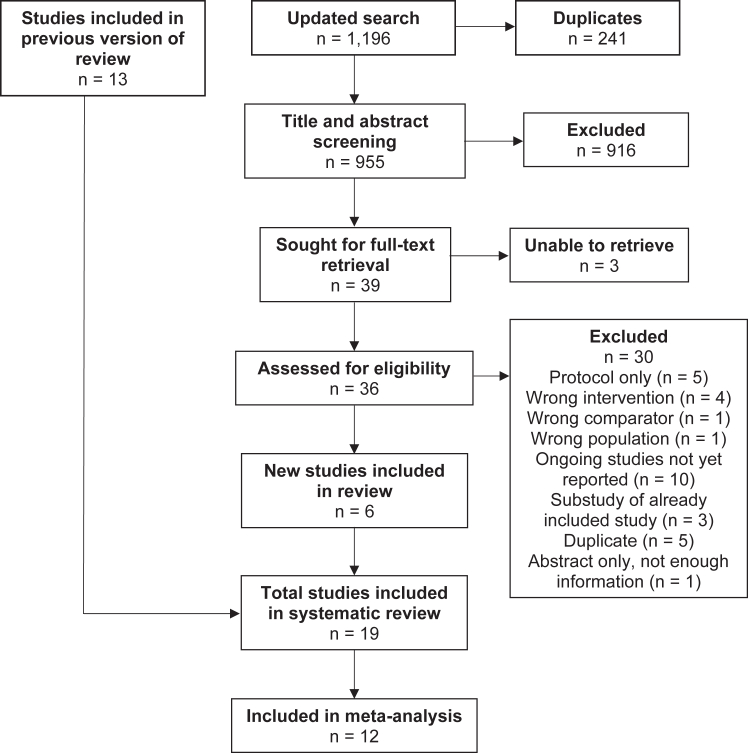
Searches identified 1196 records, reduced to 955 after deduplication, and to 39 after title and abstract screening. After full-text review, a further six studies were included (Fig. 1).
Fig. 1.
PRISMA diagram. Table of excluded studies in Appendix 5.
Eligible studies were published between 1979 and 2024 and included 6332 participants recruited from inpatient and outpatient settings in Africa,13,16,17,26, 27, 28, 29, 30, 31, 32 Asia,14, 15, 16, 17,31,33, 34, 35, 36, 37 South America8,17,31 and North America38(Table 1). All except one32 were in full paper format. Most (n = 14) included participants of 18 years or above, but some included participants of 14 years or above,38 15 years or above14,34,35 and 17 years or above.33
Table 1.
Characteristics of included studies.
| Setting | Comparator RIF dose (mg/kg) | Intervention RIF dose (mg/kg); other intervention | Participants, total (n = female) | Controls | Type of TB | TB diagnosis | Length of RIF intervention | HIV status (positive, n (%)) | Included in meta-analysis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aarnoutse et al. (2017)29 | Inpatients, outpatients; Tanzania | 10 | 15, 20 | 150 (15) | 50 | Pulmonary | ZN stain (confirmed with AccuProbe, LJ or MGIT) | 2/12 | 15 (10%), unclear if all tested | Yes |
| Atwine et al. (2020)30 | Unclear; Uganda | 10 | 20; different doses of efavirenz | 98 (27) | 33 | Pulmonary | GeneXpert MTB/RIF | 2/52 | 98 (100%), inclusion criterion | Yes |
| Boeree et al. (2017)13 | Inpatients, outpatients; Tanzania, South Africa | 10 | 35, 20 + SQ109, 20+moxifloxacin, 10 + SQ109 | 365 (107) | 123 | Pulmonary | ZN stain, GeneXpert MTB/RIF | 12/52 | 24 (6.6%), all tested | Yes; arms with comparable background regimens only |
| Cresswell et al. (2021)27 | Inpatients; Uganda | 10 | 35, 20 (IV) then 35 | 61 (27) | 21 | Meningeal | Clinical diagnosis; CSF glucose to plasma <50% or CSF glucose <65 mg/dl or positive CSF AFB smear or positive GeneXpert MTB/RIF or Ultra | 8/52 | 56 (91.8), all tested | Yes |
| Davis et al. (2023)26 | Inpatients; South Africa | 10 | 35+linezolid, 35+linezolid + aspirin | 52 (22) | 20 | Meningeal | Definite, probable or possible TBM | 56/7 | 52 (100%), | No; different background regimens between control and intervention arms |
| Dian et al. (2018)34 | Inpatients; Indonesia | 10 | 20, 30 | 60 (28) | 20 | Meningeal | Clinical diagnosis; CSF/blood glucose ratio <0.5 | 1/12 | 6 (10%), all tested | Yes |
| Heemskerk et al. (2016)15 | Inpatients; Vietnam | 10 | 15+levofloxacin | 817 (257) | 409 | Meningeal | Clinical diagnosis; 5/7 symptoms, nuchal rigidity, CSF abnormal | 2/12 | 349 (42.7%), all tested | No; different background regimen between control and intervention arms |
| Jindani et al. (2016)31 | ?outpatients; Bolivia, Nepal, Uganda | 10 | 15, 20 | 300 (95) | 100 | Pulmonary | 2x sputum ZN stain | 16/52 | 0 (0%), all tested | Yes |
| Jindani et al. (2023)17 | Outpatients; Uganda, Guinea, Peru, Nepal, Botswana, Pakistan | 10 | ∼23 (1200 mg), ∼35 (1800 mg) | 672 (154) | 224 | Pulmonary | GeneXpert MTB/RIF | 4/12 | 0 (0%), exclusion criterion | Yes |
| Kannabiran et al. (2024)37 | Unclear; India | 10 | 25, 35 | 333 (96) | 109 | Pulmonary | GeneXpert MTB/RIF, LJ, MGIT | 8/52 | 0 (0%), exclusion criterion | Yes |
| Long et al. (1979)38 | Inpatients, outpatients; USA | ∼8 (450 mg) | ∼11 (600 mg), ∼13 (750 mg) | 822 (176) | 167 | Pulmonary | AFB on sputum microscopy; CXR suggestive | 20/52 | Not documented | No; not comparable with any other study and provided data inconsistent |
| Maug et al. (2020)35 | Outpatients; Bangladesh | 10 | 20 | 701 (187) | 348 | Pulmonary | Smear positive | 6/12 | Not tested, known HIV excluded | Yes |
| Merle et al. (2016)32 | Unclear; Benin, Guinea, Senegal | 10 | 15; ART initiation at 2/52 or 8/52 | 778 (339) | 262 | Unclear; sputum samples taken, assume pulmonary | Bacteriologically confirmed TB | 2/12 | 778 (100%), inclusion criterion | Yes |
| Paton et al. (2023)16 | Outpatients; Indonesia, Philippines, Thailand, Uganda, India | 10 | 35 (reduced to 20 during trial) + linezolid, 35 (reduced to 20 during trial) + clofazimine; rifapentine + linezolid + levofloxacin; bedaquilline + linezolid | 675 (254) | 181 | Pulmonary | GeneXpert MTB/RIF; symptoms of TB or CXR suggestive | 2/12 | 0 (0%), originally exclusion criterion | No; different background regimens between control and intervention arms |
| Ruslami et al. (2007)36 | Outpatients; Indonesia | 10 | 13 | 50 (24) | 25 | Pulmonary | Microscopy; CXR suggestive | 6/12 | At least 1 (0.5%), but unclear; all tested | No; not comparable with any other study |
| Ruslami et al. (2013)14 | Inpatients; Indonesia | 10 | 13 (IV); moxifloxacin two doses; ethambutol | 60 (27) | 12 | Meningeal | Definite, probable or possible TBM | 14/7 | 7 (11.7%), all tested | No; different background regimen between control and intervention arms |
| Sekaggya-Wiltshire et al. (2022)28 | Outpatients; Uganda | 10+dolutegravir, 10+efavirenz | 35+dolutegravir, 35+efavirenz, | 128 (47) | 67 | Pulmonary | GeneXpert MTB/RIF, urine LAM, sputum culture | 8/52 | (128), all tested | Yes |
| Velasquez et al. (2018)8 | Outpatients; Peru | 10 | 15, 20 | 180 (66) | 60 | Pulmonary | Smear positive | 8/52 | 5 (2.8%), all tested | Yes |
| Yunivita et al. (2016)33 | Inpatients; Indonesia | 13 (IV) | 17, 20 | 30 (12) | 10 | Meningeal | Definite, probable or possible TBM | 14/7 | 6 (20%), all tested | No; not comparable with any other study |
Studies predominantly recruited participants with pulmonary (n = 13) or meningeal (n = 6) TB. HIV infection was documented in all but one38 study. Three studies recruited only people living with HIV.26,30,32 One study did not test all participants, but excluded known HIV positive individuals.35 Across other trials the range of HIV coinfection varied from 0 to 99%. Where HIV was reported, 27.7% (1525/5510) of participants were living with HIV.
All but two reports presented doses of RIF in mg/kg format. For Long 197938 and Jindani 2023,17 mg/kg values were estimated using weight band data provided. Evaluated doses varied from 8 mg/kg to 35 mg/kg. Seventeen trials compared the standard 10 mg/kg dose to higher doses. One used 13 mg/kg given intravenously (IV)33 as the lower dose. Another compared oral 10 mg/kg to IV 13 mg/kg.14 Data from oral versus IV doses were not included in meta-analysis but reported separately where appropriate. Treatment was directly observed for the whole intervention period in twelve studies. Length of follow-up varied considerably from two to ninety-six weeks. Length of intervention varied from two weeks to six months. Data from the Jindani 202317 estimated 23 mg/kg arm and the Kannabiran 202437 25 mg/kg arm were pooled for meta-analysis as variability of dosing within weight bands between both studies approximately equates to 22–27 mg/kg.
Ten studies provided treatment success data.8,13,17,28, 29, 30, 31, 32,35,38 Numerous trial-specific definitions of treatment success were used by investigators (Appendix 6). There was a high certainty of no evidence for higher rates of treatment success at RIF doses of 15 mg/kg (RR 1.00 [0.97–1.03], RD −1.0% [−5.5% to 3.5%], 4 trials, 916 participants) and 20 mg/kg (RR 1.01 [0.97–1.06], RD 2.9% [−1.1% to 7.0%], 6 trials, 1302 participants) and a modest reduction at 23 mg/kg (RR 0.96 [0.91–1.03], RD −3.2% [−8.9% to 2.4%], 1 trial, 373 participants) and 35 mg/kg (RR 0.97 [0.90–1.04], RD −6.1% [−13.8% to −1.6%], 2 trials, 316 participants) (Appendix 7; analyses 1–3). Jindani 2023 evaluated 35 mg/kg at a shorter duration of four months and did not conclude non-inferiority.17 Similar results were obtained for per protocol analyses (Appendix 6; analysis 4). Long 197938 used distinct dose levels from other included studies, thus data was not combined; the trial found no statistically significant difference between estimated 10 mg/kg and 13 mg/kg doses.
Nine studies provided treatment failure data8,17,28, 29, 30,32,35,36,38 although there was a lack of consistency of definitions used by investigators (Appendix 6). Four studies17,28,35,36 defined treatment failure in line with our definition, based on sputum smear positivity from month five onwards. Two studies8,32 did not specify a definition of treatment failure but, as these are recent studies of patients with pulmonary TB, are assumed to have used the WHO definition. The evidence suggests RIF doses of 15 mg/kg may reduce treatment failure (RR 0.71 [0.27–1.88], RD −1.0% [−3.6% to 1.6%], 2 trials, 616 participants), doses of 20 mg/kg may result in no difference (RR 1.01 [0.29–3.61], RD 0.5% [−1.9% to 2.8%], 2 trials, 821 participants) and doses of 23 mg/kg may increase treatment failure (RR 4.52 [0.99–20.66], RD 3.8% [0.3%–7.2%], 1 trial, 375 participants) (Appendix 7; analysis 5).
Five trials reported relapse rates.8,17,32,35,38 The minimum follow-up time for these studies was one year. RIF doses of 15 mg/kg (RR 0.79 [0.25–2.45], RD −0.7% [−2.8% to 1.5%], 2 trials, 617 participants) and 20 mg/kg (RR 0.93 [0.07–12.72], RD −0.0% [−1.2% to 1.2%], 2 trials, 821 participants) may reduce risk of relapse (Appendix 7; analysis 6). Jindani 202317 provided relapse data for mITT population with very low numbers of events leading to wide confidence intervals (Appendix 7; analysis 7).
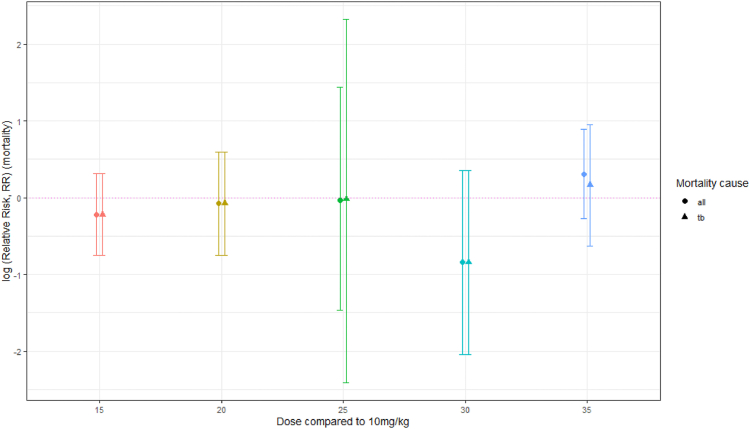
All but one study33 provided mortality data. The evidence suggests RIF doses of 15 mg/kg (RR 0.80 [0.47–1.37], RD −1.1% [−4.0% to 1.8%], 4 trials, 916 participants) and 20 mg/kg (RR 0.93 [0.47–1.81], RD −0.4% [−2.2% to 1.3%], 7 trials, 1342 participants) probably reduce all-cause mortality. Similar results were obtained for PP analysis. Eight studies compared doses higher than 20 mg/kg with control; six had the same background regimens allowing for data synthesis. Evidence across these studies is uncertain owing to small numbers of participants or events (<5%; 32 events, 837 participants). Doses of 25 mg/kg (RR 0.97 [0.23–4.22], RD 0.3% [−2.1% to 2.6%], 2 trials, 780 participants) and 30 mg/kg (RR 0.43 [0.13–1.43], RD −20.0% [−46.1% to 6.1%], 1 trial, 40 participants) may reduce all-cause mortality compared to control. Doses of 35 mg/kg may increase all-cause mortality although the evidence is uncertain (RR 1.35 [0.76–2.43], RD 1.4% [−0.8% to 3.7%], 5 trials, 1139 participants) (Appendix 7; analyses 8–11). No clear dose–response relationship is evident (Fig. 2).
Fig. 2.
Summary plot of mortality RR per comparison group for all-cause versus TB mortality (log (RR and 95% confidence intervals), dotted horizontal line RR = log (1)).
Velasquez 20188 was the only study with no participant mortality. Sekaggya-Wiltshire 2023,28 Jindani 202317 and Kannabiran 202437 specified causes of death that were not TB-related. Assuming all other deaths were TB-related, analysis at 25 mg/kg (RR 0.98 [0.09–10.17], RD 0.6% [−1.4% to 2.6%], 2 trials, 780 participants) suggests TB-related mortality may reduce, albeit with wide confidence intervals owing to low numbers of events, but at 35 mg/kg (RR 1.18 [0.53–2.60], RD 0.1% [−1.7% to 1.9%], 5 trials, 1139 participants) there may be increased TB-related mortality (Fig. 2; Appendix 7, analysis 12).
Adverse event reporting was consistent across all studies, albeit with great variability in data presentation. Several studies reported only hepatotoxicity-related adverse events. No studies reported statistically significant difference in adverse events between groups. Studies were not comparable by meta-analysis owing to background regimen variability.
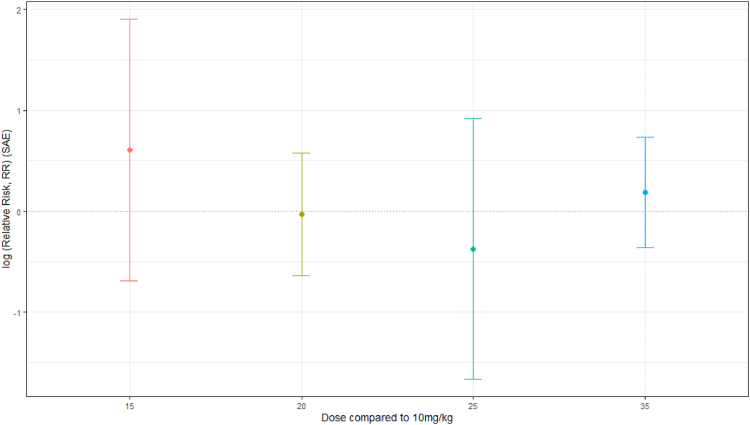
Ten studies provided SAE data.8,13,16,17,27, 28, 29, 30, 31,35,37 For RIF doses of 20 mg/kg (RR 0.97 [0.53–1.78], RD −0.9% [−3.3% to 1.5%], 6 trials, 1302 participants) and 25 mg/kg (RR 0.69 [0.19–2.51], RD −0.6% [−2.5% to 1.2%], 2 trials, 780 participants) the evidence suggests there may be a decrease in SAEs, while for doses of 15 mg/kg (RR 1.83 [0.50–6.69], RD 1.4% [−1.3% to 4.2%], 3 trials, 417 participants) and 35 mg/kg (RR 1.21 [0.70–2.09], RD 0.8% [−1.8% to 3.3%], 5 trials, 1139 participants) higher doses may increase SAEs, but the evidence is very uncertain and there is no clear dose response (Fig. 3; Appendix 7, analyses 13–14).
Fig. 3.
Summary plot of SAE RR per comparison group (log (RR and 95% confidence intervals), dotted horizontal line RR = log (1)).
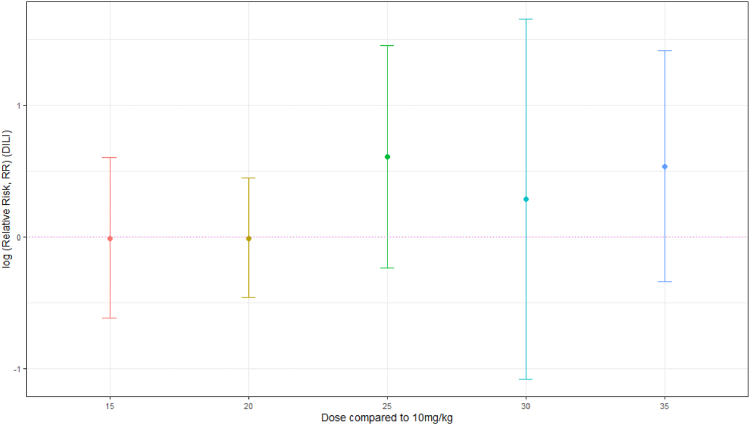
All but one study38 reported DILI. However, the majority of authors did not specify whether they felt DILI was due to RIF or other potentially hepatotoxic concomitant medications. The evidence suggests that there is little or no difference in risk of DILI at RIF doses of 15 mg/kg (RR 0.99 [0.54–1.83], RD 1.1% [−2.9% to 5.0%], 4 trials, 589 participants) or 20 mg/kg (RR 0.99 [0.63–1.56], RD 0.0% [−2.3% to 2.3%], 7 trials, 1342 participants). Data synthesis suggests, with uncertainty, that doses of 25 mg/kg (RR 1.84 [0.79–4.27], RD 2.0% [−0.7% to 4.8%], 2 trials, 780 participants), 30 mg/kg (RR 1.33 [0.34–5.21], RD 5.0% [−18.5% to 28.5%], 1 trial, 40 participants) and 35 mg/kg (RR 1.70 [0.71–4.10], RD 3.3% [0.7%–5.9%], 5 trials, 1139 participants) may increase risk of DILI, although no clear dose–response relationship is apparent (Fig. 4; Appendix 7, analyses 15–17).
Fig. 4.
Summary plot of drug-induced liver injury RR per comparison group (log (RR and 95% confidence intervals), dotted horizontal line RR = log (1)).
Five studies reported treatment success and/or mortality data but were not appropriate to be included in meta-analysis or pooled estimation owing to variance in background regimens, with the exception of Boeree 2017,13 where some intervention arms were included in meta-analysis where background regimens were comparable. RR of reported outcomes in these studies are in keeping with cumulative estimates from studies included in meta-analyses, including for studies where fluoroquinolones were an additional intervention in the context of higher RIF doses (Appendix 8).
Reporting of other secondary outcomes can be found in the appendices (Appendix 9).
Risk of bias for efficacy and safety outcomes were separated and tables generated (Appendix 10).39 Risk of bias for both efficacy and safety results was generally unclear to high, predominantly owing to the open-label nature of many included trials. Detail on bias interpretation can be found in Appendix 10.
Discussion
This review addresses whether higher doses of RIF than currently recommended could safely improve outcomes of first-line TB treatment in adults. Despite diverse designs and reported outcomes in the 19 included trials, meaningful data synthesis was possible. Doses studied ranged from 8 to 35 mg/kg with some variation of companion regimens. Most included trials were Phase IIB or small Phase III superiority trials with no difference in duration of arms. RIFASHORT17 was the only included non-inferiority trial, a relatively small trial that had a shorter duration of intervention, which may have potentially biased against the intervention in a pooled analysis. With the notable exception of Long 1979,38 trials were published within the last sixteen years. Studies reporting the highest doses of 30 mg/kg34 and 35 mg/kg13,16,17,26, 27, 28,37 were published within the last seven years.
RIF doses above 10 mg/kg were not associated with higher rates of treatment success. Higher doses did not appear to be associated with any change in rate of treatment failure or relapse, but reported numbers of events were low. Efficacy results >20 mg/kg were dominated by RIFASHORT,17 as such there is minimal evidence on whether treatment success would improve at a duration of six months, but there is a low certainty of evidence that the intervention at four months has worse outcomes. There is some evidence that doses of RIF higher than 10 mg/kg may reduce all-cause mortality, but there is substantial uncertainty and dose–response relationships were inconsistent. Furthermore, most data derive from trials using doses up to 20 mg/kg. Trials with shorter follow-up periods may have missed later mortality.
During first-line TB therapy, hepatotoxicity may occur at an incidence of 1% or more and has been a rationale for caution against RIF dose escalation.10 In this review, inconsistent safety reporting limited data synthesis and interpretation of adverse events was complicated by diversity of background regimens. There was no clear evidence to suggest higher rates of SAEs with higher doses of RIF (Fig. 3). However, the data suggest, with uncertainty, that doses of RIF higher than 20 mg/kg may be associated with increased risk of DILI, albeit with no evident dose–response relationship between 25 and 35 mg/kg (Fig. 4). It has been suggested that RIF-induced DILI could be an idiosyncratic reaction.40
Study settings were diverse. Many countries with the highest TB rates were represented, with the notable exception of China. Trials focussed on pulmonary and meningeal TB. Disseminated or other forms of extrapulmonary TB were not represented. People living with HIV were relatively under-represented. Trial investigators frequently imposed restrictions on use of antiretroviral therapy to avoid drug–drug interactions. Most trials were conducted in settings where HIV was uncommon, excluded people with HIV, or allowed enrolment only if baseline CD4 count was >200 cells/mmˆ3. Diabetes did not form part of the rationale of any study and was rarely reported on, despite being associated with poorer outcomes.41 These restrictions limit the immediate applicability of the evidence in settings with high HIV incidence or for people with diabetes.
The review focused on programmatically important outcomes. As many trials were smaller Phase II studies, the outcomes of interest were frequently not reported or below the Optimal Information Size. This was reflected in broad confidence intervals, typically including substantial benefit and harm. However, heterogeneity as measured by the Iˆ2 statistic was uniformly low across almost all analyses. Direct comparison was not possible between a number of studies owing to varying background regimens, as investigators frequently aimed to trial a novel regimen with potentially increased potency rather than focusing on defining RIF dose–response (Appendix 8). There is not currently adequate evidence on higher doses of RIF with key companion drugs such as fluoroquinolones. Further evidence on these interventions with a range of durations would be desirable.
Certainty of evidence has been summarised using the GRADE approach in a Summary of Findings table (Appendix 11). Problems with risk of bias were identified, particularly regarding safety outcomes (Appendix 10). This mainly resulted from open-label methodology; although authors did not judge blinding to be feasible, predominantly due to the likelihood of increased discolouration of bodily fluids with higher doses of RIF, the risk of performance and detection bias remains. To limit bias in the review process, measures outlined in the Cochrane group were followed.24 No publication bias was detected for key outcomes (Appendix 12).
Several early phase studies claimed evidence of improved efficacy at higher doses on the basis of investigator-defined endpoints, typically measures of culture conversion at eight or twelve weeks of therapy. Compared to 10 mg/kg, Boeree 201713 concluded that a RIF dose of 35 mg/kg reduced time to culture conversion, Jindani 201631 reported an increase in culture conversion at 20 mg/kg (p = 0.09), Kannabiran 202437 reported increased culture conversion at 25 mg/kg (p < 0.001), Sekaggya-Wiltshire 202228 and Kannabiran 202437 both reported increases in culture conversion at 35 mg/kg (p = 0.063 and p < 0.001 respectively) and Velasquez 20188 demonstrated dose–response at 15 and 20 mg/kg based on statistical modelling of sputum colony counts. However, whether improvements in culture status at early timepoints translate consistently into better long-term outcomes is unclear.
The scope of this review was restricted to regimens as similar as possible to the current recommended first-line regimen, as such studies of intermittent high-dose RIF regimens were excluded. Such regimens have typically been designed to deliver a cumulative weekly dose similar to daily regimens and, as PK-PD interactions between dose size and interval for RIF are not completely characterised, these studies may have been difficult to interpret.
Some included trials were either primarily PK studies or had nested PK studies. All but one of these33 reported higher doses of RIF consistently resulted in higher exposure in plasma and/or cerebrospinal fluid.29,34,36,37 PK studies not included in this review report that higher PK exposures may be tolerable in humans at doses up to 40 mg/kg, suggesting scope for future trials at higher doses.40 We note that studies of newer rifamycins routinely include comparatively higher doses than are typically used in rifampicin studies (e.g., study 3142).
Two previously published systematic reviews investigating high dose RIF were identified. Both focused solely on pulmonary TB.43,44 Steingart 2011 reported higher doses of RIF resulted in improved culture conversion rates, advocating for clinical trials to confirm efficacy and tolerability.44 Onorato 2021 reported that higher doses of RIF were associated with increased rate of sputum culture conversion, particularly at doses of 20 mg/kg or above. No difference was detected in mortality between treatment groups and similar rates of hepatotoxicity were observed.43 Although these reviews focused more on intermediate bacterial endpoints than longer-term outcomes such as treatment success, findings agree with the conclusions of our review, particularly with regard to safety outcomes.
Four systematic reviews investigating RIF PK were identified. Two reported that patients often had subtherapeutic drug concentrations of RIF.7,45 Two more recently reported that low concentrations of RIF appear to be related to poor treatment outcome.46,47 These reviews strengthen the rationale for higher RIF doses in individualised therapy and suggest the possibility that globally higher doses might reduce the risk that an important subgroup could fall below target PK-PD thresholds.
The main limitations of this systematic review and meta-analysis are the small numbers of studies in some dosing brackets, the variability across definitions of treatment success and the risk of bias. In addition, funnel plots should ideally be utilised when there are at least ten studies. In the absence of a better tool, we used funnel plots to provide an estimation of publication bias.
In conclusion, we did not find evidence that RIF doses higher than the recommended 10 mg/kg were associated with higher rates of treatment success in pulmonary and meningeal TB, although they may be associated with reduced rates of treatment failure, relapse and all-cause mortality. No important differences were found in safety outcomes and tolerability, with the exception of DILI, where doses >20 mg/kg may be associated with increased risk. The evidence for these outcomes is uncertain and there was no consistent pattern of dose–response. This review does not therefore provide direct support for increasing RIF dosing for all patients in routine practice, but suggests that increased dosing appears safe. The limited data does not permit identification of any important subgroups that might benefit from the intervention. Larger clinical trials reporting definitive outcomes are needed to determine whether RIF doses up to 40 mg/kg could safely improve treatment outcomes or reduce duration of first-line therapy. Results of ongoing and planned Phase III RCTs of high-dose RIF in pulmonary, meningeal and disseminated TB will increase certainty of evidence at the higher doses examined in this review.
Contributors
GD and KAH were responsible for conceptualisation of this work. GD, KAH, VL and SN developed methodology. KAH and HHT screened articles. KAH and HHT extracted data; KAH, HHT, LB and PEN reviewed extracted results. Statistical analysis was undertaken by KAH with support from SN. Original manuscript was prepared by KAH with support from GD. All authors had full access to data and reviewed final manuscript, sharing responsibility for the decision to submit for publication.
Data sharing statement
Data collected for this study and R code will be made available to others on request by email to the corresponding author.
Declaration of interests
GD was supported by a consultancy contract from WHO for the initial published version of this review and chaired the Data Safety Monitoring Board for RIFASHORT and the Trial Steering Committee for TRUNCATE-TB. All other authors declare no competing interests.
Acknowledgements
KAH is funded by Wellcome Clinical PhD Fellowship (University of Liverpool block award grant number 203919/Z/16/Z). PEN is funded by the South African Medical Research Council Institutional Researcher Programme under the Research Capacity Development scholarship (project 57029). We would like to acknowledge the WHO Global TB Programme who commissioned, funded and provided input for the original review.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102857.
Appendix ASupplementary data
References
- 1.Global tuberculosis report 2023. World Health Organization; Geneva: 2023. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 2.Espinal M.A., Kim S.J., Suarez P.H., et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283(19):2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 3.Khan F.A., Minion J., Al-Motairi A., Benedetti A., Harries A.D., Menzies D. An updated systematic review and meta-analysis on the treatment of active tuberculosis in patients with HIV infection. Clin Infect Dis. 2012;55(8):1154–1163. doi: 10.1093/cid/cis630. [DOI] [PubMed] [Google Scholar]
- 4.Dickinson J.M., Mitchison D.A. Bactericidal activity in vitro and in the Guinea-pig of isoniazid, rifampicin and ethambutol. Tubercle. 1976;57(4):251–258. doi: 10.1016/s0041-3879(76)80002-5. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y., Liu A., Menendez M.C., et al. HspX knock-out in Mycobacterium tuberculosis leads to shorter antibiotic treatment and lower relapse rate in a mouse model – a potential novel therapeutic target. Tuberculosis. 2015;95(1):31–36. doi: 10.1016/j.tube.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Prideaux B., Via L.E., Zimmerman M.D., et al. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med. 2015;21(10):1223–1227. doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stott K.E., Pertinez H., Sturkenboom M.G.G., et al. Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: a systematic review and meta-analysis. J Antimicrob Chemother. 2018;73(9):2305–2313. doi: 10.1093/jac/dky152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velasquez G.E., Brooks M.B., Coit J.M., et al. Efficacy and safety of high-dose rifampin in pulmonary tuberculosis. A randomised controlled trial. Am J Respir Crit Care Med. 2018;198(5):657–666. doi: 10.1164/rccm.201712-2524OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding J., Thuy Thuong Thuong N., Pham T.V., et al. Pharmacokinetics and pharmacodynamics of intensive antituberculosis treatment of tuberculous meningitis. Clin Pharmacol Ther. 2020;107(4):1023–1033. doi: 10.1002/cpt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lienhardt C., Nunn A., Chaisson R., et al. Advances in clinical trial design: weaving tomorrow's TB treatments. PLoS Med. 2020;17(2) doi: 10.1371/journal.pmed.1003059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaram R., Gaonkar S., Kaur P., et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47(7):2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boeree M.J., Diacon A.H., Dawson R., et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med. 2015;191(9):1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 13.Boeree M.J., Heinrich N., Aarnoutse R., et al. High-dose rifampin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis. 2017;17(1):39–49. doi: 10.1016/S1473-3099(16)30274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruslami R., Ganiem A.R., Dian S., et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13(1):27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 15.Heemskerk A.D., Bang N.D., Mai N.T., et al. Intensified antituberculous therapy in adults with tuberculous meningitis. N Engl J Med. 2016;374(2):124–134. doi: 10.1056/NEJMoa1507062. [DOI] [PubMed] [Google Scholar]
- 16.Paton N.I., Cousins C., Suresh C., et al. Treatment strategy for rifampin-susceptible tuberculosis. N Engl J Med. 2023;388(10):873–887. doi: 10.1056/NEJMoa2212537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jindani A., Atwine D., Grint D., et al. Four-month-high-dose rifampicin regimens for pulmonary tuberculosis. NEJM Evid. 2023;2(9) doi: 10.1056/EVIDoa2300054. [DOI] [PubMed] [Google Scholar]
- 18.Souleymane M.B., Kadri S., Piubello A., et al. High rate of adverse drug reactions with a novel tuberculosis re-treatment regimen combining triple doses of both isoniazid and rifampicin. Int J Infect Dis. 2023;133:78–81. doi: 10.1016/j.ijid.2023.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organisation, Haigh K., Twabi H., et al. World Health Organisation; 2022. WHO operational handbook on tuberculosis: module 4: treatment: drug-susceptible tuberculosis treatment: web annex 2: optimisation of dosage of the first-line medicines rifampicin, isoniazid, ethambutol and pyrazinamide in treatment of drug-susceptible tuberculosis: summary of evidence from four systematic reviews.https://iris.who.int/handle/10665/354539 License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 21.Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org Available at:
- 22.R Core Team . R foundation for statistical computing; Vienna, Austria: 2024. R: a language and environment for statistical computing.https://www.R-project.org Available at: [Google Scholar]
- 23.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P.T., Altman D.G., Gotzsche P.C., et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt G.H., Oxman A.D., Vist G.E., et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis A.G., Wasserman S., Stek C., et al. A phase 2A trial of the safety and tolerability of increased dose rifampicin and adjunctive linezolid, with or without aspirin, for human immunodeficiency virus-associated tuberculous meningitis: the LASER-TBM trial. Clin Infect Dis. 2023;76(8):1412–1422. doi: 10.1093/cid/ciac932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cresswell F.V., Meya D.B., Kagimu E., et al. High-dose oral and intravenous rifampicin for the treatment or tuberculous meningitis in predominantly human immunodeficiency virus (HIV)-positive Ugandan adults: a phase II open-label randomised controlled trial. Clin Infect Dis. 2021;73(5):876–884. doi: 10.1093/cid/ciab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekaggya-Wiltshire C., Nabisere R., Musaazi J., et al. Decreased dolutegravir and efavirenz concentrations with preserved virological suppression in patients with tuberculosis and human immunodeficiency virus receiving high-dose rifampicin. Clin Infect Dis. 2023;76(3):e910–e919. doi: 10.1093/cid/ciac585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aarnoutse R.E., Kibiki G.S., Reither K., et al. Pharmacokinetics, tolerability, and bacteriological response of rifampin administered at 600, 900, and 1200 milligrams daily in patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 2017;61(11) doi: 10.1128/AAC.01054-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atwine A., Baudin E., Gele T., et al. Effect of high-dose rifampicin on efavirenz pharmacokinetics: drug-drug interaction randomized trial. J Antimicrob Chemother. 2020;75(5):1250–1258. doi: 10.1093/jac/dkz557. [DOI] [PubMed] [Google Scholar]
- 31.Jindani A., Borgulya G., Westermann de Patino I., et al. A randomised phase II trial to evaluate the toxicity of high-dose rifampicin to treat pulmonary tuberculosis. Int J Tuberc Lung Dis. 2016;20(6):832–838. doi: 10.5588/ijtld.15.0577. [DOI] [PubMed] [Google Scholar]
- 32.Merle C.S., Floyd S., Ndiaye A., et al. 21st international AIDS conference: July 2016; Durban, South Africa. 2016. High-dose rifampicin tuberculosis treatment regimen to reduce 12-month mortality of TB/HIV co-infected patients: the RAFA trial results [abstract] [Google Scholar]
- 33.Yunivita V., Dian S., Ganiem A.R., et al. Pharmacokinetics and safety/tolerability of higher oral and intravenous doses of rifampicin in adult tuberculous meningitis patients. Int J Antimicrob Agents. 2016;48(4):415–421. doi: 10.1016/j.ijantimicag.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Dian A., Yunivita V., Ganiem A.R., et al. Double-blind, randomised, placebo-controlled phase II dose-finding study to evaluate high-dose rifampin for tuberculous meningitis. Antimicrob Agents Chemother. 2018;62(12):e01014–e01018. doi: 10.1128/AAC.01014-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maug A.K.J., Hossain M.A., Gumusboga M., et al. First-line tuberculosis treatment with double-dose rifampicin is well tolerated. Int J Tuberc Lung Dis. 2020;24(5):499–505. doi: 10.5588/ijtld.19.0063. [DOI] [PubMed] [Google Scholar]
- 36.Ruslami R., Nijland H.M.J., Alisjahbana B., Parwati I., van Crevel R., Aarnoutse R.E. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob Agents Chemother. 2007;51(7):2546–2551. doi: 10.1128/AAC.01550-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannabiran B.P., Palaniappan N.A., Manoharan T., et al. Safety and efficacy of 25 mg/kg and 35 mg/kg vs 10 mg/kg rifampicin in pulmonary TB: a phase IIb randomized controlled trial. Open Forum Infect Dis. 2024;11(3):ofae034. doi: 10.1093/ofid/ofae034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Long M.W., Snider Jr DE., Farer L.S. U.S. public health service cooperative trial of three rifampin-isoniazid regimens in treatment of pulmonary tuberculosis. Am Rev Respir Dis. 1979;119(6):879–894. doi: 10.1164/arrd.1979.119.6.879. [DOI] [PubMed] [Google Scholar]
- 39.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 40.Te Brake L.H.M., de Jager V., Narunsky K., et al. Increased bactericidal activity but dose-limiting intolerability at 50mg.kg-1 rifampicin. Eur Respir J. 2021;58(1) doi: 10.1183/13993003.00955-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker M.A., Harries A.D., Jeon C.Y., et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dorman S.E., Nahid P., Kurbatova E.V., et al. Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med. 2021;384(18):1705–1718. doi: 10.1056/NEJMoa2033400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onorato L., Gentile V., Russo A., et al. Standard versus high dose rifampicin in the treatment of pulmonary tuberculosis: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(6):830–837. doi: 10.1016/j.cmi.2021.03.031. [DOI] [PubMed] [Google Scholar]
- 44.Steingart K.R., Jotblad S., Robsky K., et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2011;15(3):305–316. [PubMed] [Google Scholar]
- 45.Mota L., Al-Efraij K., Campbell J.R., Cook V.J., Marra F., Johnston J. Therapeutic drug monitoring in anti-tuberculous treatment: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2016;20(6):819–826. doi: 10.5588/ijtld.15.0803. [DOI] [PubMed] [Google Scholar]
- 46.Perumal R., Naidoo K., Naidoo A., et al. A systematic review and meta-analysis or first-line tuberculosis drug concentrations and treatment outcomes. Int J Tuberc Lung Dis. 2020;24(1):48–64. doi: 10.5588/ijtld.19.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sileshi T., Tadesse E., Makonnen E., Aklillu E. The impact of first-line anti-tubercular drugs’ pharmacokinetics on treatment outcome: a systematic review. Clin Pharmacol. 2021;13:1–12. doi: 10.2147/CPAA.S289714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.