Abstract
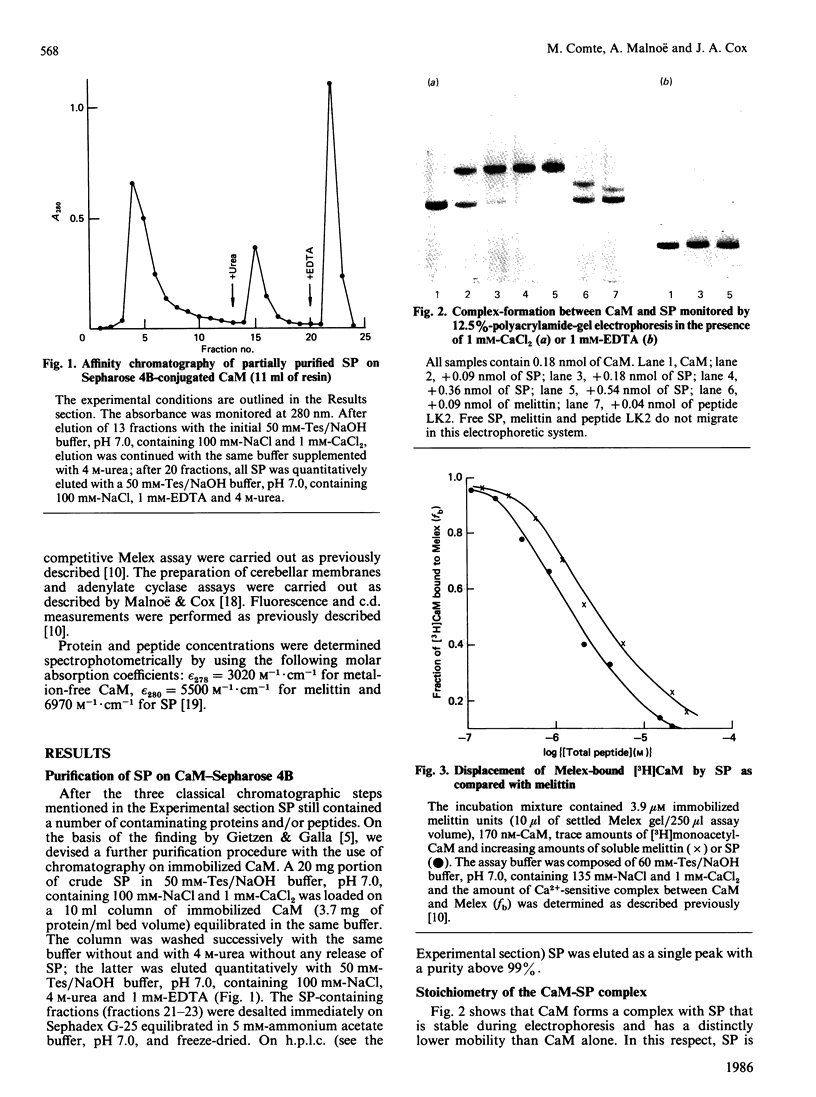
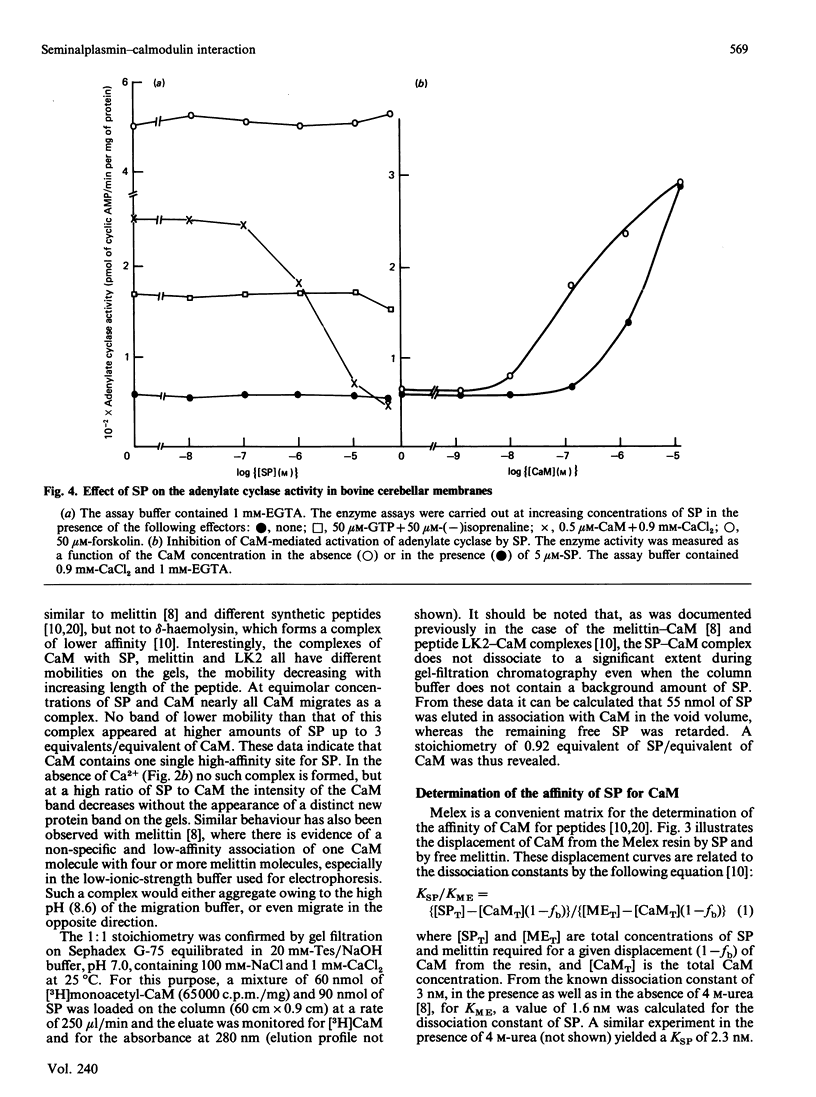
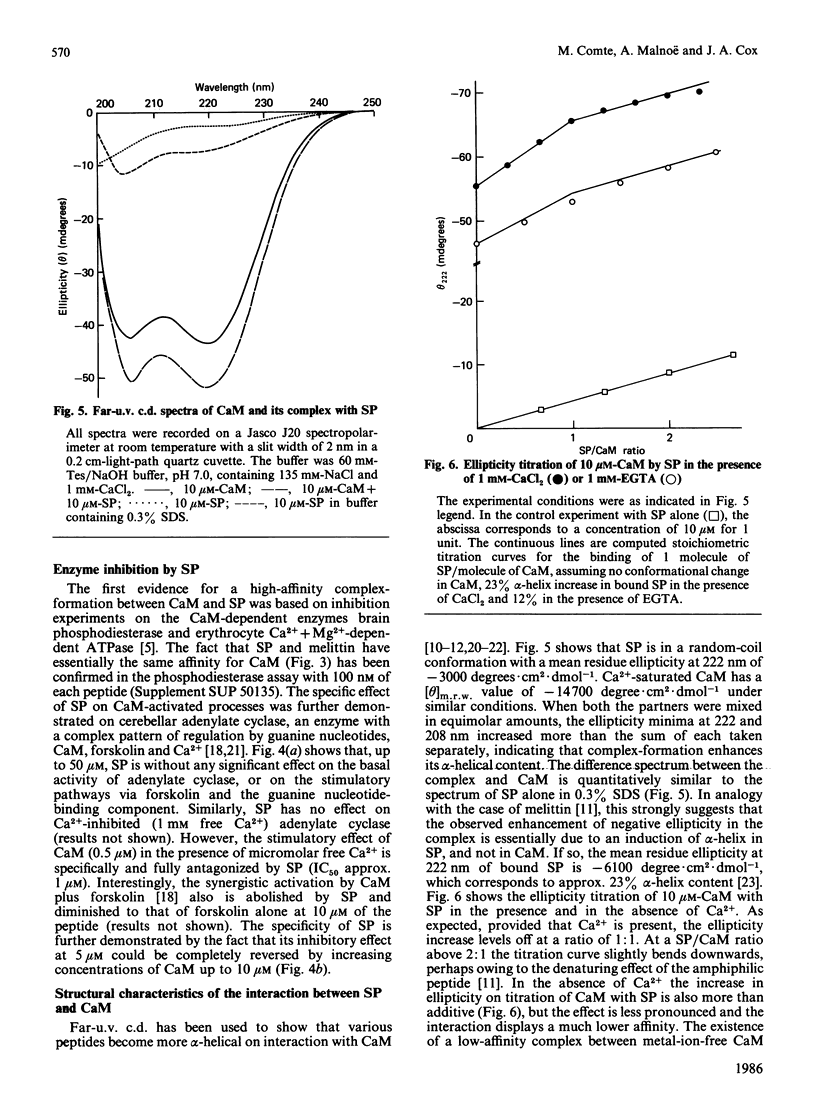
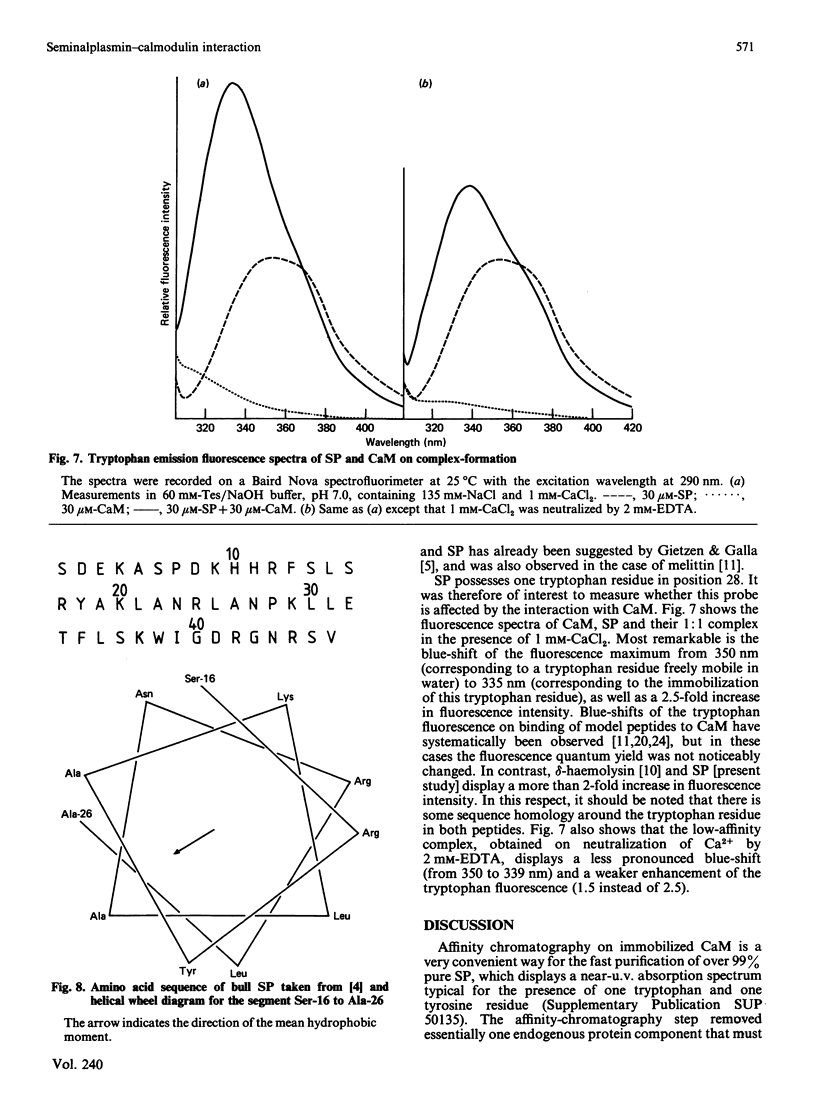
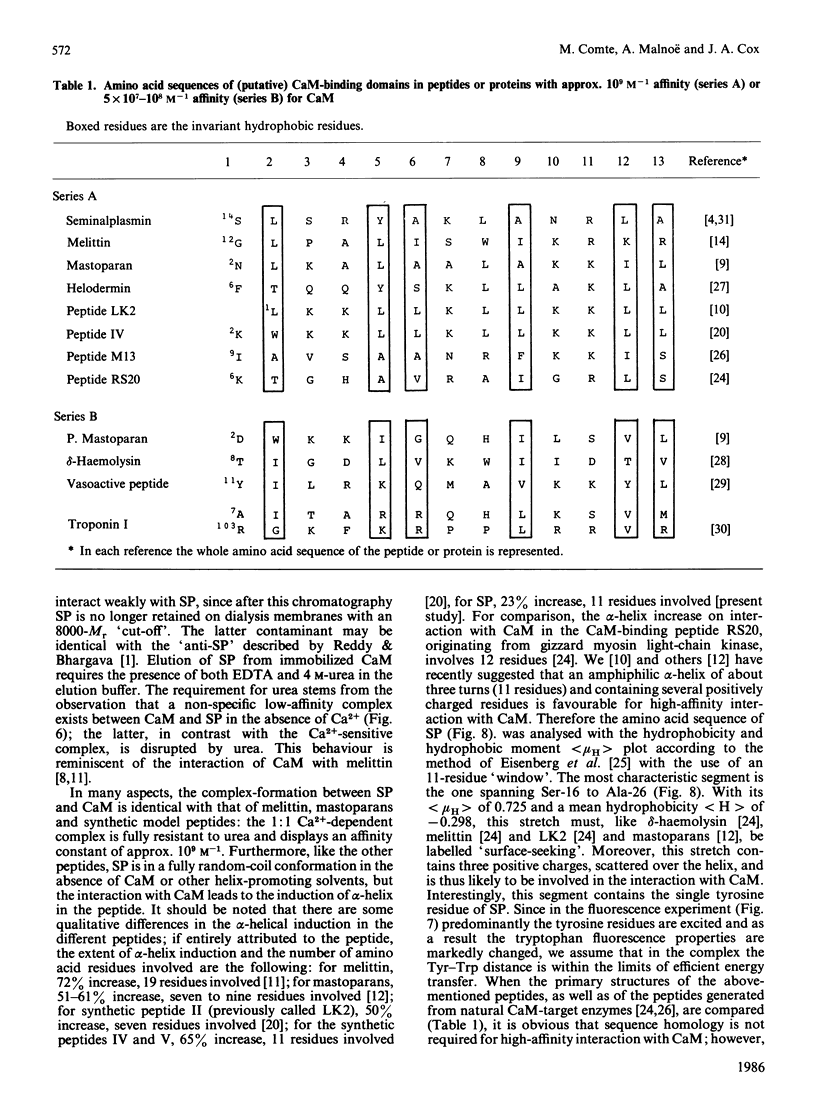
Bull seminalplasmin antagonizes with high potency and selectivity the activating effect of calmodulin on target enzymes [Gietzen & Galla (1985) Biochem. J. 230, 277-280]. In the present paper we establish that seminalplasmin forms a 1:1, Ca2+-dependent and urea-resistant complex with calmodulin. The dissociation constant equals 1.6 nM. In the absence of Ca2+ a low-affinity complex is formed that is disrupted by 4 M-urea. On the basis of these properties, a fast affinity purification of seminalplasmin was developed. The high specificity of seminalplasmin as a calmodulin antagonist was demonstrated for the multipathway-regulated adenylate cyclase of bovine cerebellum. Far-u.v. c.d. properties are consistent with a random form of seminalplasmin in aqueous solution; 23% alpha-helix is induced on interaction with calmodulin. The fluorescence properties of the single tryptophan residue of seminalplasmin are markedly changed on formation of the complex. These studies allowed us to locate tentatively the peptide segment that interacts with calmodulin, and to ascertain the structural homology between seminalplasmin and other calmodulin-binding peptides. Additional material, showing the inhibition of calmodulin-mediated activation of bovine brain phosphodiesterase by melittin and seminalplasmin and also the near-u.v. spectrum of affinity-purified seminalplasmin, has been deposited as supplement SUP 50135 (4 pages) at the British Library Lending Division, Boston Spa, Wetherby, West Yorkshire LS23 7BQ, U.K., from whom copies may be obtained on the terms indicated in Biochem. J. (1986) 233, 5.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Comte M., Maulet Y., Cox J. A. Ca2+-dependent high-affinity complex formation between calmodulin and melittin. Biochem J. 1983 Jan 1;209(1):269–272. doi: 10.1042/bj2090269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Fitton J. E., DeGrado W. F. The interaction of calmodulin with amphiphilic peptides. J Biol Chem. 1985 Feb 25;260(4):2527–2534. [PubMed] [Google Scholar]
- Cox J. A. Sequential events in calmodulin on binding with calcium and interaction with target enzymes. Fed Proc. 1984 Dec;43(15):3000–3004. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DeGrado W. F., Prendergast F. G., Wolfe H. R., Jr, Cox J. A. The design, synthesis, and characterization of tight-binding inhibitors of calmodulin. J Cell Biochem. 1985;29(2):83–93. doi: 10.1002/jcb.240290204. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fitton J. E., Dell A., Shaw W. V. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 1980 Jun 30;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Giedroc D. P., Keravis T. M., Staros J. V., Ling N., Wells J. N., Puett D. Functional properties of covalent beta-endorphin peptide/calmodulin complexes. Chlorpromazine binding and phosphodiesterase activation. Biochemistry. 1985 Feb 26;24(5):1203–1211. doi: 10.1021/bi00326a023. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Galla H. J. Seminalplasmin. An endogenous calmodulin antagonist. Biochem J. 1985 Aug 15;230(1):277–280. doi: 10.1042/bj2300277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Blumenthal D. K., Wemmer D. E., Krebs E. G. Interaction of calmodulin and a calmodulin-binding peptide from myosin light chain kinase: major spectral changes in both occur as the result of complex formation. Biochemistry. 1985 Dec 31;24(27):8152–8157. doi: 10.1021/bi00348a047. [DOI] [PubMed] [Google Scholar]
- Kohn J., Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun. 1982 Aug;107(3):878–884. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Agustin J. S., Kruggel W., Lardy H. A. The structure of caltrin, the calcium-transport inhibitor of bovine seminal plasma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6490–6491. doi: 10.1073/pnas.82.19.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas T. J., Burgess W. H., Prendergast F. G., Lau W., Watterson D. M. Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry. 1986 Mar 25;25(6):1458–1464. doi: 10.1021/bi00354a041. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of hormones and neuropeptides by calmodulin. Biochemistry. 1983 Apr 12;22(8):1995–2001. doi: 10.1021/bi00277a040. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Demonstration of a fluorometrically distinguishable intermediate in calcium binding by calmodulin-mastoparan complexes. Biochem Biophys Res Commun. 1986 Mar 28;135(3):1050–1057. doi: 10.1016/0006-291x(86)91034-x. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. High affinity binding of the mastoparans by calmodulin. Biochem Biophys Res Commun. 1983 Jul 18;114(1):50–56. doi: 10.1016/0006-291x(83)91592-9. [DOI] [PubMed] [Google Scholar]
- Malnoë A., Cox J. A. Relationship among calmodulin-, forskolin-, and guanine nucleotide-dependent adenylate cyclase activities in cerebellar membranes: studies by limited proteolysis. J Neurochem. 1985 Oct;45(4):1163–1171. doi: 10.1111/j.1471-4159.1985.tb05537.x. [DOI] [PubMed] [Google Scholar]
- Manalan A. S., Klee C. B. Calmodulin. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:227–278. [PubMed] [Google Scholar]
- Maulet Y., Cox J. A. Structural changes in melittin and calmodulin upon complex formation and their modulation by calcium. Biochemistry. 1983 Nov 22;22(24):5680–5686. doi: 10.1021/bi00293a035. [DOI] [PubMed] [Google Scholar]
- McDowell L., Sanyal G., Prendergast F. G. Probable role of amphiphilicity in the binding of mastoparan to calmodulin. Biochemistry. 1985 Jun 4;24(12):2979–2984. doi: 10.1021/bi00333a026. [DOI] [PubMed] [Google Scholar]
- Reddy E. S., Bhargava P. M. Seminalplasmin--an antimicrobial protein from bovine seminal plasma which acts in E. coli by specific inhibition of rRNA synthesis. Nature. 1979 Jun 21;279(5715):725–728. doi: 10.1038/279725a0. [DOI] [PubMed] [Google Scholar]
- Shivaji S. Binding of seminalplasmin to the plasma and acrosomal membranes of bovine spermatozoa. Fluorescence studies on the changes in the lipid-phase fluidity. FEBS Lett. 1986 Feb 17;196(2):255–258. doi: 10.1016/0014-5793(86)80258-7. [DOI] [PubMed] [Google Scholar]
- Sitaram N., Kumari V. K., Bhargava P. M. Seminalplasmin and caltrin are the same protein. FEBS Lett. 1986 Jun 9;201(2):233–236. doi: 10.1016/0014-5793(86)80615-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Takahashi S., Mitsui Y., Itoh S., Iitaka Y., Kasai H., Okuyama T. X-ray crystallographic and chromatographic characterization of the crystals of Ca2+-calmodulin complexed with bee venom melittin. J Mol Biol. 1985 Dec 5;186(3):675–677. doi: 10.1016/0022-2836(85)90141-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Grand R. J. Comparison of amino acid sequence of troponin I from different striated muscles. Nature. 1978 Jan 5;271(5640):31–35. doi: 10.1038/271031a0. [DOI] [PubMed] [Google Scholar]



