Abstract
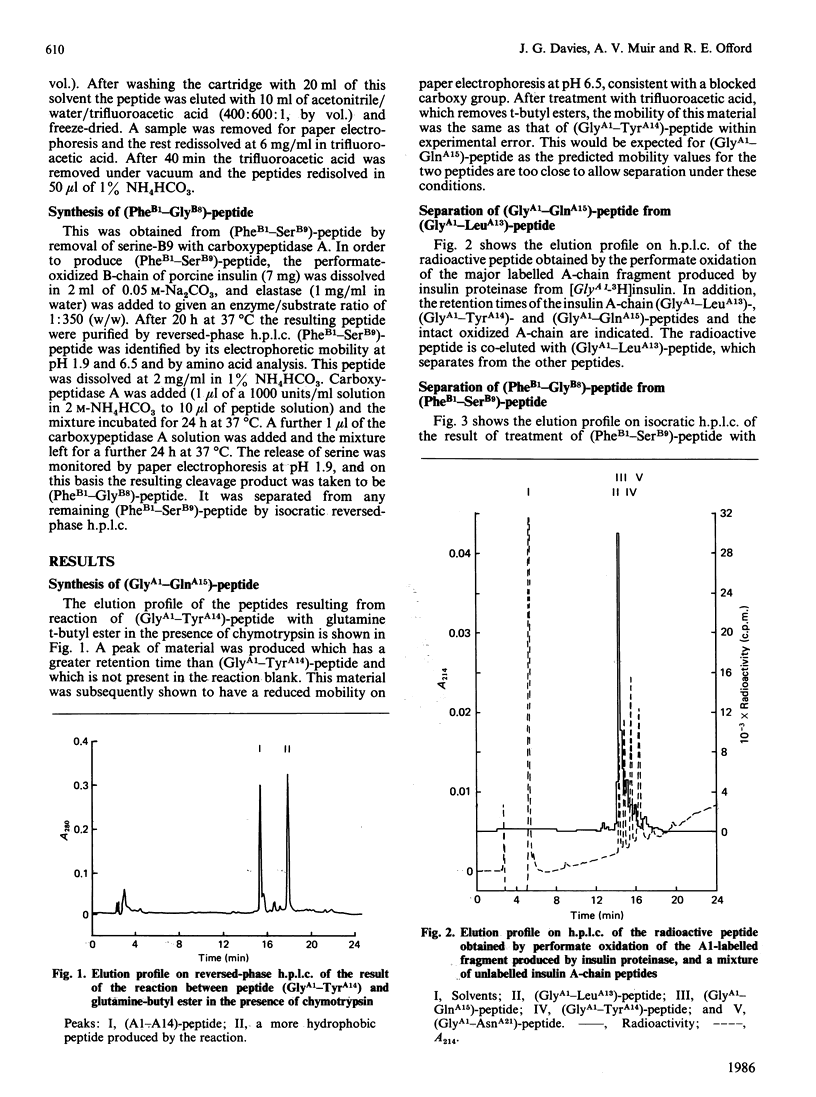
In a previous study [Muir, Offord & Davies (1986) Biochem. J. 237, 631-637] the chromatographic and electrophoretic behaviour of a major labelled fragment in the degradation of tritiated insulins by insulin proteinase were used to locate the probable sites of cleavage which had produced this fragment. In order to define these cleavage sites more precisely, authentic markers for the fragments which would be produced by cleavages at, or adjacent to, the most likely sites have now been synthesized. These markers were compared with labelled fragments of the A- and B-chains of insulin produced by insulin proteinase. The results, together with those of our previous study, show that in order to produce the observed major labelled fragment, the enzyme must have cleaved the insulin A-chain between leucine-A13 and tyrosine-A14 and the insulin B-chain between serine-B9 and histidine-B10. In addition, a minor component was observed in the labelled B-chain fragment which corresponded to a cleavage either between histidine-B10 and leucine-B11 or between leucine-B11 and valine-B12.
Full text
PDF
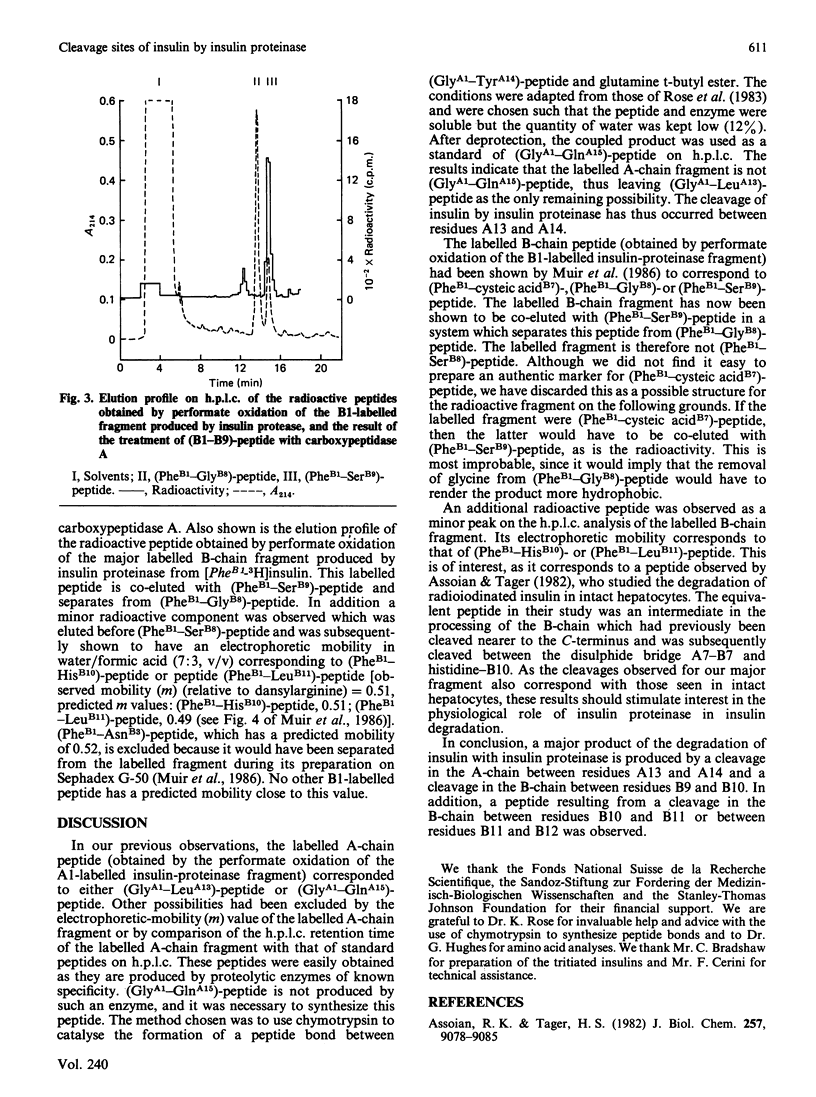

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Tager H. S. Peptide intermediates in the cellular metabolism of insulin. J Biol Chem. 1982 Aug 10;257(15):9078–9085. [PubMed] [Google Scholar]
- Davies J. G., Offord R. E. The preparation of tritiated insulin specifically labelled by semisynthesis at glycine-A1. Biochem J. 1985 Oct 15;231(2):389–392. doi: 10.1042/bj2310389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C., Heinemann M. A., Kitabchi A. E. Purification of insulin-specific protease by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3698–3702. doi: 10.1073/pnas.69.12.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C., Kitabchi A. E. Insulin metabolism and degradation. Endocr Rev. 1981 Spring;2(2):210–233. doi: 10.1210/edrv-2-2-210. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Offord R. E. The preparation of a semisynthetic tritiated insulin with a specific radioactivity of up to 20 Curies per millimole. Biochem J. 1975 Nov;151(2):219–225. doi: 10.1042/bj1510219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A., Offord R. E., Davies J. G. The identification of a major product of the degradation of insulin by 'insulin proteinase' (EC 3.4.22.11). Biochem J. 1986 Aug 1;237(3):631–637. doi: 10.1042/bj2370631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Rose K., De Pury H., Offord R. E. Rapid preparation of human insulin and insulin analogues in high yield by enzyme-assisted semi-synthesis. Biochem J. 1983 Jun 1;211(3):671–676. doi: 10.1042/bj2110671. [DOI] [PMC free article] [PubMed] [Google Scholar]


