Abstract
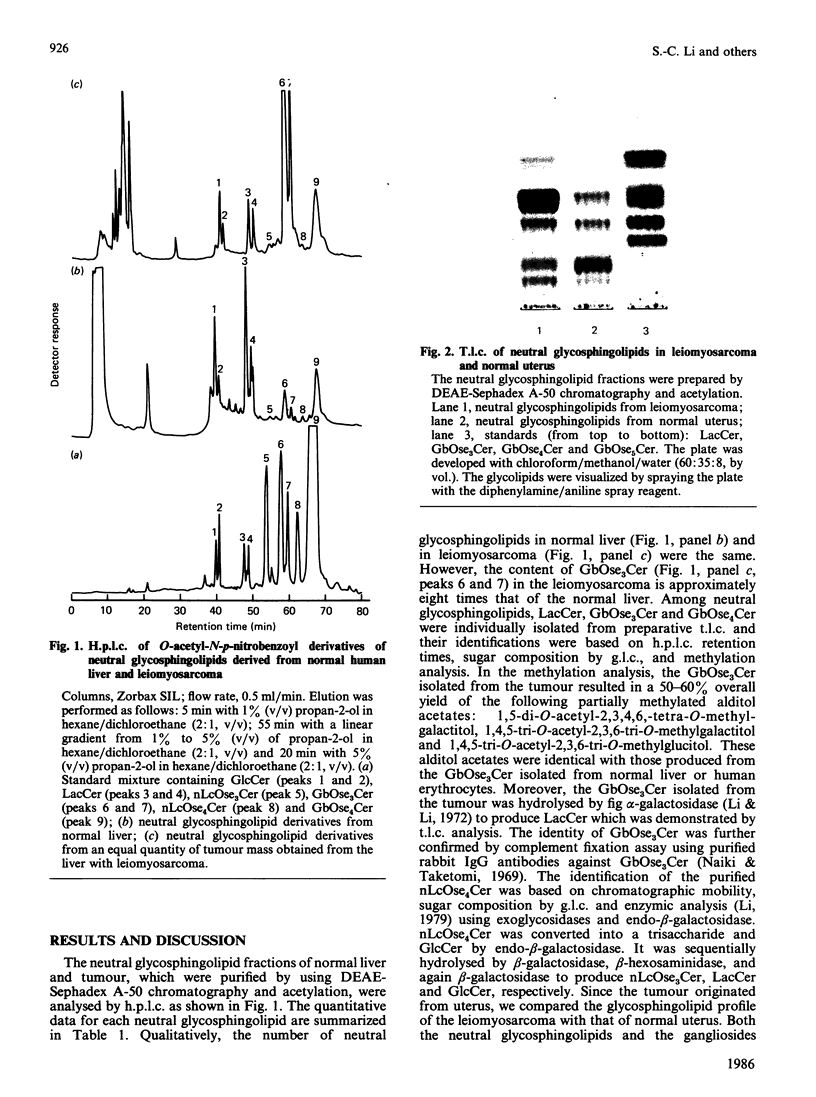
Analysis of the glycosphingolipid composition in one case of uterine leiomyosarcoma metastasized to the liver showed an accumulation of globotriaosylceramide as compared with normal liver and uterus from which the tumour originated. The structure and the amount of glycosphingolipids were established by using specific glycosidases, permethylation analysis and h.p.l.c. The reason for the accumulation of globotriaosylceramide in leiomyosarcoma remains to be answered.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Chang N. C., Yu R. K. High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal Biochem. 1978 Sep;89(2):437–450. doi: 10.1016/0003-2697(78)90373-1. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycolipids of tumor cell membrane. Adv Cancer Res. 1973;18:265–315. doi: 10.1016/s0065-230x(08)60755-1. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Structures and organization of cell surface glycolipids dependency on cell growth and malignant transformation. Biochim Biophys Acta. 1975 Mar 20;417(1):55–89. doi: 10.1016/0304-419x(75)90008-6. [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Chakravarty S. K., Roy S. K., Roy A. K. DEAE-silica gel and DEAE-controlled porous glass as ion exchangers for isolation of glycolipids. J Chromatogr. 1979 Feb 11;170(1):65–72. doi: 10.1016/s0021-9673(00)84238-7. [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Roy S. K. A rapid and quantitative method for the isolation of gangliosides and neutral glycosphingolipids by DEAE-silica gel chromatography. J Lipid Res. 1978 Mar;19(3):390–395. [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K., Eng L. F. Gangliosides of human myelin: sialosylgalactosylceramide (G7) as a major component. J Neurochem. 1973 Oct;21(4):829–839. doi: 10.1111/j.1471-4159.1973.tb07527.x. [DOI] [PubMed] [Google Scholar]
- Naiki M., Kamimura H., Taketomi T., Ichikawa R. Chemical, physico-chemical and morphological properties of Forssman hapten purified from caprine erythrocytes. Jpn J Exp Med. 1972 Jun;42(3):205–219. [PubMed] [Google Scholar]
- Naiki M., Taketomi T. Chemical and immunochemical properties of glycolipids from pig spleen. Jpn J Exp Med. 1969 Dec;39(6):549–571. [PubMed] [Google Scholar]
- Nudelman E., Kannagi R., Hakomori S., Parsons M., Lipinski M., Wiels J., Fellous M., Tursz T. A glycolipid antigen associated with Burkitt lymphoma defined by a monoclonal antibody. Science. 1983 Apr 29;220(4596):509–511. doi: 10.1126/science.6836295. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Suzuki A., Kundu S. K., Marcus D. M. An improved technique for separation of neutral glycosphingolipids by high-performance liquid chromatography. J Lipid Res. 1980 May;21(4):473–477. [PubMed] [Google Scholar]
- Svennerholm L., Månsson J. E., Li Y. T. Isolation and structural determination of a novel ganglioside, a disialosylpentahexosylceramide from human brain. J Biol Chem. 1973 Jan 25;248(2):740–742. [PubMed] [Google Scholar]
- Wiels J., Holmes E. H., Cochran N., Tursz T., Hakomori S. Enzymatic and organizational difference in expression of a Burkitt lymphoma-associated antigen (globotriaosylceramide) in Burkitt lymphoma and lymphoblastoid cell lines. J Biol Chem. 1984 Dec 10;259(23):14783–14787. [PubMed] [Google Scholar]
- Yogeeswaran G. Cell surface glycolipids and glycoproteins in malignant transformation. Adv Cancer Res. 1983;38:289–350. doi: 10.1016/s0065-230x(08)60191-8. [DOI] [PubMed] [Google Scholar]