Abstract
Over the last century, eosinophils have been regarded ambiguously either as ‘friends’ or ‘foes’. Recent developments have greatly enhanced our understanding of the role and function of eosinophils in health and disease. Pathogenic eosinophilic inflammation can lead to severe diseases in various organs, such as the gastrointestinal tract, airways, heart and skin. In a 2‐day focus workshop of the German Society for Allergology and Clinical Immunology (DGAKI), the state of the art was discussed and practical recommendations for diagnosis and treatment of eosinophilic diseases, with a particular focus on new biologics, such as anti‐interleukin 5 and anti‐interleukin 5R, were derived.
Keywords: anti‐interleukin 5, DNAzyme against GATA3; eosinophil, eosinophil diseases, hypereosinophilic syndrome
Abbreviations
- AEU
Allergic effector unit
- APRIL
A proliferation‐inducing ligand
- CCR
Chemokine receptor
- CRS
Chronic rhinosinusitis
- Dsg
Desmoglein
- ECP
Eosinophil cationic protein
- EET
Extracellular eosinophilic trap
- EGID
Eosinophilic gastrointestinal diseases
- Egpa
Eosinophilic granulomatosis and polyangiitis
- EoE
Eosinophilic esophagitis
- Eos
Eosinophils
- FDA
Food and Drug Administration
- GI
Gastro‐intestinal
- GM‐CSF
Granulocyte Monocyte colony‐stimulating factor
- HE
Hypereosinophilia
- HES
Hypereosinophilic syndrome
- IgA
Immunoglobulin A
- IL‐33
Interleukin 33
- JAK2
Janus kinase 2
- LPS
Lipopolysaccharide
- mAbs
Monoclonal antibodies
- MBP
Major basic protein
- MCs
Mast cells
- MLKL
Mixed lineage kinase‐like
- PDGFRA
Platelet‐Derived Growth Factor receptor alpha
- PPI
Proton pump inhibitors
- RIPK3
Receptor‐interacting protein kinase 3
- SEA‐SEE
Staphylococcal enterotoxin A‒E
- SIGLEC‐F
Sialic acid‐binding immunoglobulin‐type lectins
- SplD
Staphylococcus aureus‐derived protein
- Th
T helper cell
- TSLP
Thymic stromal lymphopoietin
- TSST‐1
Toxic shock syndrome toxin‐1
- WHO
World Health Organization
1. INTRODUCTION
Eosinophilic granulocytes are among the most distinctive cells in human blood, mainly due to the bright colour given by eosin, an aniline dye first used in 1877 by Paul Ehrlich 1 for the differentiation of white blood cells. In subsequent decades, there was marked controversy over whether these cells could be regarded as ‘good’ or ‘bad’, 2 and lately there has been marked progress and increasing interest in these cells. Therefore, the German Society of Allergology and Clinical Immunology (DGAKI) organized a ‘Focus’ workshop to reflect the state of the art in this field and discuss practical aspects of management of eosinophilic diseases. Here, we summarize the outcomes of this forum, focussing on current developments, and discuss clinical and experimental research of note in the field of eosinophil granulocytes and eosinophil‐related diseases.
2. BIOLOGY OF EOSINOPHILS
The pro‐inflammatory effects of eosinophils, exerted through release of toxic mediators, cytokines and other products and causing symptoms of allergic disorders (Figure 1), have generated much attention. However, eosinophils have many physiological functions. 3 , 4 Eosinophils are a major cell population of the gastrointestinal (GI) tract, and their homing to the lamina propria is independent of inflammation. Indeed, the first eosinophils appear in the GI tract already during foetal development, long before the gut is colonized by the microbiota. The development of normal‐sized Peyer´s patches in the GI tract requires eosinophils, and in their absence, IgA class switching is impaired. 5 Consequently, intestinal and serum IgA is significantly reduced in eosinophil‐deficient mice. Furthermore, eosinophils play a crucial role in immune homeostasis, as they control T cell responses to environmental antigens in mucosal tissues of the lung and the GI tract, 6 and prevent inflammatory reactions induced by infections with natural parasites, such as the bacterium Helicobacter pylori. 7 Eosinophils also have an essential role in long‐term immune protection. In the bone marrow, they provide the cytokine APRIL, which promotes plasma cell longevity. 8 Furthermore, eosinophils play an important role during puberty, 9 in the structural development of adipose tissue, and in tumour and transplant rejection. 2 Although hypereosinophilia in relation to tumours was first described more than 120 years ago, its role in tumours is still undefined. 10 Eosinophils release antitumorigenic (eg TNF‐α, cationic proteins and IL‐18) and protumorigenic molecules (eg pro‐angiogenetic factors) depending on the tumour microenvironment. 11 , 12 In several neoplasias (eg melanoma, gastric, colorectal, oral and prostate cancer), eosinophilia was related to a better outcome, whereas in others, such as Hodgkin's lymphoma, eosinophilia has been linked to poor prognosis. 12 , 13 A better understanding of the paradoxical role in cancer progression could help design therapeutic strategies.
FIGURE 1.
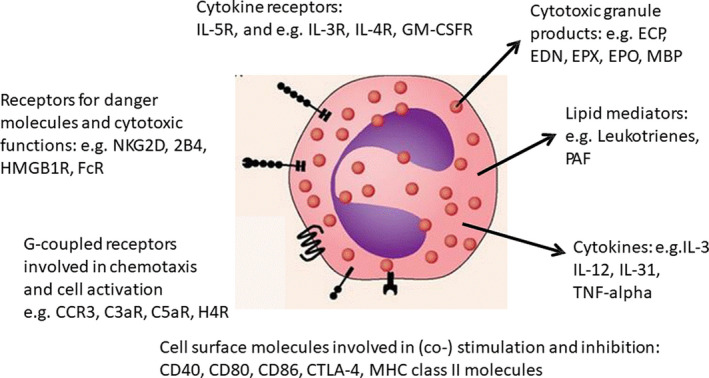
Examples of eosinophilic mediators and receptors involved in allergic inflammation
Eosinophils are components in adipose tissue infiltrates and promote glucose homeostasis. 14 Mesenchyme‐derived stromal cells in white adipose tissue activate resident type 2 innate lymphoid cells (ILC2) to release IL‐5. 15 IL‐5 activated eosinophils release IL‐4, which, together with ILC2 released IL‐13, induces the conversion of resident tissue macrophages to activated macrophages able to release norepinephrine‐stimulating adipose browning. 16 , 17 Eosinophil‐deficient mice developed weight gain and glucose intolerance whereas hypereosinophilic mice displayed neither weight gain and nor glucose intolerance when fed a high‐calorie diet. 14 However, recent data suggest that elevating adipose eosinophils in obese mice by IL‐5 did not rescue metabolic impairments. 18 Further research needs to clarify these findings. Transgenic mouse models have allowed new understanding of eosinophil functions. The IL‐4eGFP reporter mice were used to identify eosinophil‐committed precursor cells in the foetal liver where they express low levels of Siglec‐F, and no CCR3. 19 In adult mice, resting and activated eosinophils can be distinguished by expression of CD62L, Siglec‐F, Pir‐B and CD29 on the cell surface. 19
Corresponding functional studies have provided new insights into the physiology of eosinophils. Interestingly, analysis of eosinophil turnover by means of BrdU incorporation assays has revealed that helminth‐induced tissue eosinophilia was caused by recruitment of already existing eosinophils, rather than that of de novo‐generated eosinophils from the bone marrow. 20 Mice with eosinophil‐specific expression of the Cre recombinase have demonstrated an important role of the NF‐kappa B pathway for regulation of eosinophilia after helminth infection. 21 Furthermore, the alarmin IL‐33 can promote survival of eosinophils by autocrine GM‐CSF signalling. 22
Eosinophils are often found together with mast cells (MCs), not only in allergy conditions, but also in other diseases, such as mastocytosis, systemic lupus erythematosus, bullous pemphigoid (BP), scleroderma, chronic graft‐versus‐host disease, cancers, atherosclerosis and idiopathic hypereosinophilic syndrome (HES). A type of pro‐inflammatory cross‐talk, called ‘Allergic Effector Unit’, with CD48 and 2B4 at the core, results in increased eosinophil chemotaxis, survival, degranulation, cytokine production and MC survival (Figure 2). 23 , 24 , 25 , 26 , 27 , 28 This cross‐talk also involves inhibitory receptors, that is, CD300a and Siglec‐7 or Siglec 8, on MCs and eosinophils. 25 Specific monoclonal antibodies blocking CD48 and activating CD300a and Siglec‐7 or Siglec 8 could thus serve as potential drugs in future.
FIGURE 2.
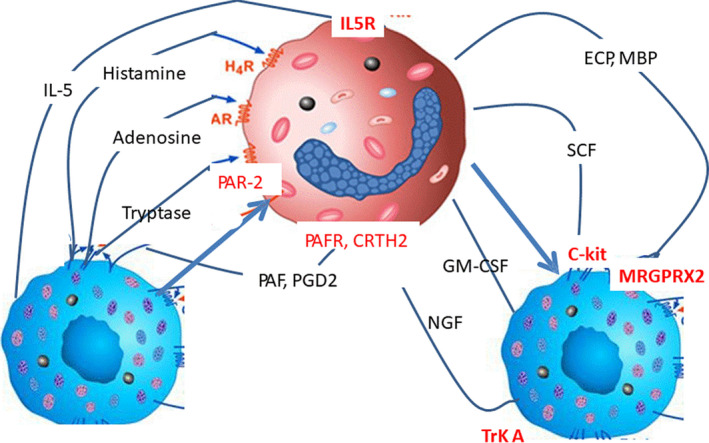
Examples of eosinophil‐mast cell interactions with effects on allergic inflammation
The activation of eosinophils results in several functional responses, including the release of granule proteins. However, control of the toxicity of these granule proteins within the eosinophil itself has not yet been identified. Recently, studies involving a combination of dye/immunostaining, immuno‐transmission electron microscopy and X‐ray micro‐crystallography have indicated that eosinophil major basic protein (MBP), a protein at the core of specific granules, is enclosed by a crystal lattice with amyloid‐like properties, enabling the inert storage of the otherwise toxic protein. 29 Following eosinophil activation, MBP is mobilized, unpacked and released as non‐toxic oligomers. Upon secretion, an aggregation process is initiated, resulting in the generation of toxic MBP that is able to exert its microbiocidal effects. Interestingly, larger extracellular non‐toxic MBP aggregates can be detected in eosinophilic tissues, which may explain how tissue destruction by MBP can be limited. These data indicate that the toxicity of eosinophil MBP is controlled by functional amyloid formation. 29
Granule proteins can also occur in the extracellular space, in association with so‐called ‘eosinophil extracellular traps’ (EETs). 30 Eosinophil activation initiates the formation of EETs, consisting of catapult‐like release of mitochondrial DNA and granule proteins that form structures able to bind and kill bacteria. Thymic stromal lymphopoietin (TSLP) and Staphylococcus aureus serve as strong stimuli for EET generation in the context of eosinophil adhesion. 31 Evidence that mitochondrial as well as nuclear DNA is released from eosinophils by an active process was obtained from live‐cell imaging analyses. 30 , 31 , 32 As a component of the innate immune response, EETs were detected in several allergic, infectious and autoimmune eosinophilic diseases, 31 including bronchial asthma, atopic dermatitis (AD), 33 , 34 eosinophilic esophagitis and chronic rhinosinusitis. 35 , 36
Recently, it has been proposed that eosinophil cytolysis is a manifestation of regulated necrosis, since the death process depends on receptor‐interacting protein kinase 3 (RIPK3) and mixed lineage kinase‐like (MLKL) protein. 37 Notably, previous pharmacological activation of autophagy inhibits eosinophil cytolysis. Thus, suppressing the RIPK3‐MLKL pathway and stimulating autophagy in eosinophils may represent new therapeutic strategies to prevent eosinophil‐mediated tissue damage.
3. EOSINOPHILIC DISEASES
Eosinophilic inflammation occurs in various diseases, 38 mostly allergic and parasitic diseases, but also in autoimmune diseases, in the course of malignancies (eg lymphoma), conditions induced by drugs (eg acetylsalicylic acid, cefoxitin, penicillin), and rare conditions, such as HES. 39
3.1. Eosinophilic skin diseases
Eosinophilic inflammation is a characteristic of many skin diseases of allergic, infectious, haematologic, autoimmune or vascular origin (Table 1). There are some particular conditions in which eosinophilic inflammation is idiopathic, such as episodic angioedema, 40 eosinophilic cellulitis (Well's syndrome), eosinophilic fasciitis, granuloma faciale, angiolymphoid hyperplasia with eosinophilia, 41 eosinophilic folliculitis (Ofuji syndrome), as well as in the context of hypereosinophilia (HE) or HES. 42 , 43 IgG4‐related diseases and drug reactions with eosinophilia and systemic symptoms (DRESS) are multisystem diseases with strong eosinophilic inflammation in the skin. 44 The first successful use of the anti‐IL‐5 monoclonal antibody mepolizumab was demonstrated in HES with skin involvement. 42 AD, a type 2 cytokine‐driven inflammatory skin disease, is associated with increased eosinophil numbers both in blood and skin, and with elevated serum and urine levels of eosinophilic secretion products. 45 A clinical trial investigated anti‐IL‐5, which target the major T‐cell‐derived eosinophilic growth and survival factor, for treatment of AD. 46 In this small proof‐of‐concept trial, the primary study endpoint of clear improvement of AD was not reached, but some individual parameters showed promise. A recently published placebo‐controlled study indicated the efficacy of mepolizumab in patients with moderate to severe AD, demonstrating the improvement of clinical scores in patients treated with subcutaneously administered mepolizumab. 47 Thus, future studies with anti‐IL‐5 or anti‐IL‐5R may yield additional positive results.
TABLE 1.
Diseases involving eosinophilic inflammation of the skin
| Type | Disease |
|---|---|
| Allergies | Eczema (atopic dermatitis) |
| Urticaria, angioedema | |
| Drug reactions (eg, DRESS) | |
| Infectious diseases | Helminth infestation |
| Onchocerciasis | |
| Autoimmune diseases | Bullous pemphigoid |
| Pemphigus | |
| Eosinophilic fasciitis | |
| Eosinophilia myalgia syndrome | |
| Vascular | Eosinophilic granulomatosis with polyangiitis (Churg‐Strauss syndrome) |
| Haematological Idiopathic | Hypereosinophilic syndromes (myeloid variant) |
| Eosinophilic cellulitis (Well's syndrome) | |
| Episodic angioedema with eosinophilia (Gleich syndrome) | |
| Granuloma faciale | |
| Angiolymphomatoid hyperplasia with eosinophilia | |
| Hypereosinophilic syndrome |
The role of eosinophils has been studied in bullous autoimmune dermatoses, such as pemphigus (involving auto‐antibodies against desmosomal proteins) and pemphigoid (involving auto‐antibodies against adhesion proteins). 48 In the pre‐bullous stage of pemphigus, eosinophils infiltrate the epidermis, known as eosinophilic spongiosis. Desmoglein‐induced secretion of IL‐4, ‐5 and ‐13‐positive CD4 + T cells may attract eosinophils via IL‐5. 48
In BP, which has a higher prevalence in the elderly population, blisters are filled with an eosinophil‐rich leukocyte infiltrate, with strongly enhanced activation of eosinophils, including those in the circulation. 49 Eosinophils can also release mitochondrial DNA together with granule proteins (EETs) in proximity to apoptotic keratinocytes in BP patients. 34 , 50 Moreover, IL‐5‐activated eosinophils potentially contribute to blister formation by directly causing dermal‐epidermal separation. 50 Eosinophils in BP also produce high levels of IL‐31, 51 which may be involved in the strong itching sensation. Studies with the anti‐IL‐5 antibody mepolizumab have demonstrated efficacy for the treatment of hypereosinophilic diseases. The primary endpoint was the improvement of clinical symptoms, decrease in the blood eosinophil count and reduction of the prednisone dose to 10 mg or less per day for 8 or more consecutive weeks. 52 Recently, a study indicated the efficacy of treatment with an anti‐eotaxin‐1 monoclonal antibody (bertilimumab) in BP and demonstrated an 81% decline in the subjects' BP Disease Area Index. The primary endpoint was safety, including the incidence of adverse effects. 53 , 54
3.2. Eosinophils in upper airway diseases
Chronic rhinosinusitis (CRS) is often considered an ‘eosinophilic’ upper airway inflammatory disease; however, there are several degrees of eosinophil involvement, recently described as non‐type 2, moderate type 2 and severe type 2 inflammation, ranging from CRS without to CRS with massive nasal polyps and comorbid asthma. 55 The risk for and the severity of type 2 inflammation in CRS can vary considerably depending on the patient's place of residence (eg Asia vs. Europe) and may furthermore increase over time at places with low incidence. 56 A possible role of Staphylococcus aureus has been discussed via release of enterotoxins SEA‐SEE and TSST‐1, or serine‐protease‐like proteins. 57 , 58 Serine‐protease‐like proteins can elicit IgE antibody responses in asthmatic patients and induce Th2 responses. 57 Repeated intratracheal applications of SplD induced allergic asthma in C57BL/6 J mice and led to IL‐33 and eotaxin production, eosinophilia, bronchial hyperreactivity and goblet cell hyperplasia. 58 Blocking IL‐33 activity with a soluble ST2 receptor consequently decreased lung eosinophils, innate lymphoid cells and T cells, as well as IL‐5 and IL‐13 production in lymph nodes, which resembled superantigen activities. 57 Furthermore, in nasal polyp tissue samples, EET formation was observed. 36 The understanding of eosinophil‐associated endotypes is likely to lead to new differentiated approaches in the management of CRS. 59 , 60
3.3. Eosinophils in lung diseases
In the clinical classification of eosinophilic lung disease, it is important to determine whether the disorder is limited to the lung or reflects a multisystemic disease. Lung‐limited eosinophilic diseases include eosinophilic pneumonia, 61 parasite infection or eosinophilic airway diseases, such as asthma or allergic broncho‐pulmonary aspergillosis. Classical multisystem eosinophilic diseases include eosinophilic granulomatosis and polyangiitis (eGPA), 62 , 63 drug reactions with eosinophilia and HES. Eosinophils are increased in atopic as well as in non‐atopic asthma. 40 In patients with asthma, treatment is guided by the absolute numbers of eosinophils in the blood, 64 particularly in patients with severe eosinophilic asthma, where treatment with either IL‐5 or IL‐5Rc antibodies results in marked improvement, 65 , 66 even in systemic steroid‐dependent patients. 67 , 68 Interestingly, in other lung diseases, such as eGPA, 69 anti‐IL‐5 treatment resulted in about 40% remission rates. 70 Clinical studies with IL‐5 blockade are warranted for eosinophilic pneumonia or allergic broncho‐pulmonary aspergillosis. 69 , 70
3.4. Eosinophilic gastrointestinal diseases
Eosinophilic esophagitis (EoE) has increased in prevalence both in adults and in children since its first description in the 90s. 71 , 72 Diagnostic criteria include oesophageal stenosis with dysphagia and symptoms not otherwise explained, together with tissue eosinophilia (>15 eosinophils per high‐power field). The factors responsible for EoE range from environmental factors, including food allergens and the microbiome, interacting with the oesophageal epithelium with consecutive release of atopy‐enhancing cytokines, to genetic factors such as gender and genetic variants. 73 A recent review revealed a delay of several years between symptom onset and diagnosis increasing the risk for fibrostenosis. 74 Moreover, relatively high recurrence rates and low rates of spontaneous recovery were described, which indicate that EoE is a disorder persisting from childhood to adulthood. 74
The majority of children with atopy have comorbid asthma, allergic rhinitis and/or atopic eczema (‘asthma of the oesophagus’). There is a broad overlap between eosinophilic esophagitis (EoE) and IgE‐mediated food allergies, particularly against cow's milk, beef, chicken, peanuts, chicken eggs, wheat and soy. 75 Therefore, a critical evaluation of the relevance of specific IgE levels or skin tests against food proteins is crucial in the diagnostic work‐up, in addition to endoscopy with biopsy. The response to first‐line therapy with proton pump inhibitors (PPI) defines the new subtypes of eosinophilic GI diseases, namely PPI‐unresponsive and often IgE‐associated EoE versus PPI‐responsive oesophageal eosinophilia. 76 However, it has been suggested that PPI responsiveness is not part of a diagnostic criteria but rather an effective treatment for some patients. 73 , 77 , 78 Less is known about eosinophilic inflammation of other parts of the GI tract, such as eosinophil gastroenteritis, which are often associated with symptoms of irritable bowel syndrome.
3.5. Hypereosinophilia and hypereosinophilic syndrome
Over the past two decades, understanding of HE and HE‐induced organ damage has increased substantially. 79 , 80 , 81 Neoplastic and reactive disorders underlying HE can be distinguished, and several proposals for the classification of HE‐related syndromes have been developed. 81 , 82 , 83 Certain myeloid neoplasms and various reactive conditions are frequently associated with HE, but HE may also be seen with no apparent underlying disease (idiopathic HE). 82 , 83 In myeloid neoplasms, eosinophils are considered to belong to the malignant clone, whereas in reactive conditions, eosinophilia is usually a reactive process triggered by eosinophilopoietic cytokines. HE‐related organ damage, also known as HES, is seen in patients with haematologic malignancies, but also in reactive conditions. 83 When no underlying disease is detected, the diagnosis is idiopathic HES. 83 In patients with clonal HE (with or without HES), several different molecular markers can be detected, such as fusion genes involving PDGFRA (eg FIP1L1‐PDGFRA), PDGFRB, FGFR1, ABL1 or JAK2 (eg PCM1‐JAK2). 83
4. MANAGEMENT AND NOVEL AND EXPERIMENTAL THERAPIES
Type‐2 inflammation is the predominant pattern in eosinophilic diseases. Eosinophils in blood and tissue serve as biomarkers. 68 , 84 , 85 , 86 , 87 Detection of granular protein deposits in tissues is helpful. A value exceeding 1.5 × 109/L that is unexplained by other diseases is regarded as HE, and when associated with organ damage or dysfunction in more than 2 organs is regarded as HES, or when associated with in one organ, as hypereosinophilic organ disease. 88 Conventional therapy for HE and HES consists of anti‐inflammatory treatment using systemic glucocorticoids, immunosuppressives and UVA‐1 phototherapy, 35 , 76 , 78 , 84 as well as by eradicating underlying conditions. Pharmacotherapy often does not lead to satisfactory results, due to toxicity and lack of efficacy.
Clonal HE and clonal HES are treated with drugs acting at specific molecular targets, for example, FIP1L1‐PDGFRA (the most sensitive target of imatinib). 39 , 83 , 89 , 90 , 91 For non‐responders or in those developing acute leukaemia, conventional chemotherapy and hematopoietic stem cell transplantation have to be considered, while in irreversible endomyocardial fibrosis and related heart failure, heart transplantation remains an option. 92 Biologics with specificity for eosinophils, targeting IL‐5 and IL‐5R, such as mepolizumab, reslizumab or benralizumab have shown promising effects. 42 , 52 , 93
Alternative approaches for targeting type‐2 inflammation consist of substances affecting eosinophil chemotaxis, such as anti‐eotaxin‐1 antibodies (bertilimumab), which have recently demonstrated promising results, 53 as well as substances that block production of the transcription factor GATA‐3, by means of a GATA‐3 mRNA‐specific antisense molecule, HgD40. HgD40 belongs to the class of DNAzymes, which are synthetic DNA molecules. HgD40 contains a catalytic domain through which it exerts its cleavage function once sequence‐specific domains bind to the GATA‐3 mRNA. Clinical efficacy of this treatment by inhalation has been shown following allergen provocation in mild and moderate asthmatic patients. 94 Clinical responses are associated with a significant reduction of eosinophils in the sputum. These observations have been now extended to a subgroup of chronic obstructive pulmonary disease patients, characterized by the presence of at least 2% sputum eosinophils. 95
5. CONCLUSIONS
Eosinophils are evolutionarily present from the early vertebrates. 96 They contribute to marked inflammation in tissues with both protective (eg against parasites) and pathogenic effects (eg hypereosinophilic conditions). In terms of general health effects, transgenic mouse models have shown that it is possible to live without eosinophils, but that the organism is better off having them. Eosinophilic diseases manifest in various organs, most notably in the skin, the GI tract, the airways, the heart, and with rare involvement of the nervous system and the vasculature. HES involves symptoms in various organs, with a fusion gene mutation or reactive IL‐5 up‐regulation. To manage hypereosinophilic conditions, history, physical examination, laboratory investigations, histology and imaging analyses are performed. Genetic analyses for mutations allow distinction between myeloid and lymphoid variants.
While systemic glucocorticoids remain the mainstay of treatment, particularly in the initial phase, prognosis has improved markedly through the introduction of targeted therapies implementing anti‐IL‐5 antibodies and kinase inhibitors. 43 , 97 Through a better understanding of eosinophil function in health and disease, and with the development of specific kinase inhibitors as well as targeted biologics, the era of precision medicine has now arrived in the field of eosinophilic diseases. Recent findings in transgenic mice and preliminary human studies targeting new receptors on eosinophils and blockade of transcription factors impart hope for further progress in the management of eosinophilic diseases.
Renz H, Bachert C, Berek C, et al. Physiology and pathology of eosinophils: Recent developments. Scand J Immunol. 2021;93:e13032. 10.1111/sji.13032
Funding information
Harald Renz was funded by the Universities Giessen Marburg Lung Center and the German Center for Lung Disease (DZL German Lung Center, no. 82DZL00502) for UGMLC. Claus Bachert was supported by grants from the European Commission’s Seventh Framework Program under grant agreement No. 260895 (PREDICTA), the Flemish Scientific Research Board (Research Foundation‐Flanders, projects 1841713N, G.039412N, G.067512N, and BOF14/GOA/019), and the Interuniversity Attraction Poles Program of the Belgian State Science Policy Office (No. IAP P7/30) (C.B.); The study was supported in part by the Austrian Science Fund (FWF) grant SFB F47‐B20 to Peter Valent. Eckard Hamelmann was funded by the German Ministry of Education and Research (BMBF: 01GL1742D CHAMP). David Voehringer was funded by the DFG grant Vo944/9‐1 to D.V. Ulrike Raap was funded by the DFG RA 1026 3‐1. Johannes Ring and Sabine Ploetz were supported by the Christine Kuehne Center for Allergy Research and Education (CK‐CARE), Davos, Switzerland. Thomas Werfel was funded by Hanover Medical University, by the DFG cluster of excellence RESIST and by DFG grant WeXXX. Open Access funding enabled and organized by Projekt DEAL.WOA Institution: PHILIPPS‐UNIVERSITAET MARBURGBlended DEAL: Projekt DEAL.
DATA AVAILABILITY STATEMENT
n/a
REFERENCES
- 1. Ehrlich P. Beiträge zur Kenntnis der Anilinfärbungen und ihre Verwendung in der mikroskopischen Technik. Arch Mikroskop Anat. 1877;263‐277. [Google Scholar]
- 2. Chusid MJE. Friends or Foes? The journal of allergy and clinical immunology. practice. 2018;6:1439‐1444. [DOI] [PubMed] [Google Scholar]
- 3. Berek C. Eosinophils can more than kill. J Exp Med. 2018;215:1967‐1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gleich GJ, Klion AD, Lee JJ, Weller PF. The consequences of not having eosinophils. Allergy. 2013;68:829‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Chu T, Beller A, Rausch S, et al. Eosinophils promote generation and maintenance of immunoglobulin‐A‐expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40:582‐593. [DOI] [PubMed] [Google Scholar]
- 6. Wen T, Rothenberg ME. The Regulatory Function of Eosinophils. Microbiology spectr. 2016;4(5). 10.1128/microbiolspec.MCHD-0020-2015. (ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnold IC, Artola‐Borán M, Tallón de Lara P, et al. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med. 2018;215:2055‐2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Chu T, Fröhlich A, Steinhauser G, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151‐159. [DOI] [PubMed] [Google Scholar]
- 9. Gouon‐Evans V, Pollard JW. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology. 2001;142:4515‐4521. [DOI] [PubMed] [Google Scholar]
- 10. Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer immunol immunother. 2019;68:823‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591‐5596. [DOI] [PubMed] [Google Scholar]
- 12. Varricchi G, Galdiero MR, Loffredo S, et al. Eosinophils: The unsung heroes in cancer? Oncoimmunology. 2018;7:e1393134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reichman H, Itan M, Rozenberg P, et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol Res. 2019;7:388‐400. [DOI] [PubMed] [Google Scholar]
- 14. Wu D, Molofsky AB, Liang H‐E, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahlakõiv T, Flamar A‐L, Johnston LK, et al. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin‐33. Sci Immunol. 2019;4(35):eaax0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon H‐U, Yousefi S, Germic N, et al. The Cellular Functions of Eosinophils: Collegium Internationale Allergologicum (CIA) Update 2020. Int Arch Allergy Immunol. 2020;181:11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolus WR, Peterson KR, Hubler MJ, Kennedy AJ, Gruen ML, Hasty AH. Elevating adipose eosinophils in obese mice to physiologically normal levels does not rescue metabolic impairments. Mol Metab. 2018;8:86‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voehringer D, van Rooijen N, Locksley RM. Eosinophils develop in distinct stages and are recruited to peripheral sites by alternatively activated macrophages. J Leukoc Biol. 2007;81:1434‐1444. [DOI] [PubMed] [Google Scholar]
- 20. Ohnmacht C, Pullner A, van Rooijen N, Voehringer D. Analysis of eosinophil turnover in vivo reveals their active recruitment to and prolonged survival in the peritoneal cavity. J Immunol. 1950;2007(179):4766‐4774. [DOI] [PubMed] [Google Scholar]
- 21. Schwartz C, Willebrand R, Huber S, et al. Eosinophil‐specific deletion of IκBα in mice reveals a critical role of NF‐κB‐induced Bcl‐xL for inhibition of apoptosis. Blood. 2015;125:3896‐3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Willebrand R, Voehringer D. IL‐33‐Induced cytokine secretion and survival of mouse eosinophils is promoted by autocrine GM‐CSF. PLoS One. 2016;11:e0163751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elishmereni M, Alenius HT, Bradding P, et al. Physical interactions between mast cells and eosinophils. A novel mechanism enhancing eosinophil survival in vitro. Allergy. 2011;66:376‐385. [DOI] [PubMed] [Google Scholar]
- 24. Elishmereni M, Bachelet I, Nissim Ben‐Efraim AH, Mankuta D, Levi‐Schaffer F. Interacting mast cells and eosinophils acquire an enhanced activation state in vitro. Allergy. 2013;68:171‐179. [DOI] [PubMed] [Google Scholar]
- 25. Gangwar RS, Landolina N, Arpinati L, Levi‐Schaffer F. Mast cell and eosinophil surface receptors as targets for anti‐allergic therapy. Pharmacol Ther. 2017;170:37‐63. [DOI] [PubMed] [Google Scholar]
- 26. Rocha‐de‐Souza CM, Berent‐Maoz B, Mankuta D, Moses AE, Levi‐Schaffer F. Human mast cell activation by Staphylococcus aureus. Interleukin‐8 and tumor necrosis factor alpha release and the role of Toll‐like receptor 2 and CD48 molecules. Infect Immun. 2008;76:4489‐4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gangwar RS, Levi‐Schaffer F. sCD48 is anti‐inflammatory in Staphylococcus aureus enterotoxin B‐induced eosinophilic inflammation. Allergy. 2016;71:829‐839. [DOI] [PubMed] [Google Scholar]
- 28. O'Sullivan JA, Wei Y, Carroll DJ, et al. Frontline Science. Characterization of a novel mouse strain expressing human Siglec‐8 only on eosinophils. J Leukoc Biol. 2018;104:11‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soragni A, Yousefi S, Stoeckle C, et al. Toxicity of eosinophil MBP is repressed by intracellular crystallization and promoted by extracellular aggregation. Mol Cell. 2015;57:1011‐1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yousefi S, Gold JA, Andina N, et al. Catapult‐like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949‐953. [DOI] [PubMed] [Google Scholar]
- 31. Morshed M, Yousefi S, Stöckle C, Simon H‐U, Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy. 2012;67:1127‐1137. [DOI] [PubMed] [Google Scholar]
- 32. Ueki S, Melo RCN, Ghiran I, Spencer LA, Dvorak AM, Weller PF. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion‐competent eosinophil granules in humans. Blood. 2013;121:2074‐2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dworski R, Simon H‐U, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simon D, Hoesli S, Roth N, Staedler S, Yousefi S, Simon H‐U. Eosinophil extracellular DNA traps in skin diseases. J Allergy Clin Immunol. 2011;127:194‐199. [DOI] [PubMed] [Google Scholar]
- 35. Simon D, Radonjic‐Hösli S, Straumann A, Yousefi S, Simon H‐U. Active eosinophilic esophagitis is characterized by epithelial barrier defects and eosinophil extracellular trap formation. Allergy. 2015;70:443‐452. [DOI] [PubMed] [Google Scholar]
- 36. Gevaert E, Zhang N, Krysko O, et al. Extracellular eosinophilic traps in association with Staphylococcus aureus at the site of epithelial barrier defects in patients with severe airway inflammation. J Allergy Clin Immunol. 2017;139:1849‐1860.e6. [DOI] [PubMed] [Google Scholar]
- 37. Radonjic‐Hoesli S, Wang X, de Graauw E, et al. Adhesion‐induced eosinophil cytolysis requires the receptor‐interacting protein kinase 3 (RIPK3)‐mixed lineage kinase‐like (MLKL) signaling pathway, which is counterregulated by autophagy. J Allergy Clin Immunol. 2017;140:1632‐1642. [DOI] [PubMed] [Google Scholar]
- 38. Williams KW, Ware J, Abiodun A, Holland‐Thomas NC, Khoury P, Klion AD. Hypereosinophilia in children and adults. A retrospective comparison. J Allergy and Clin Immunol Pract. 2016;4:941‐947.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ogbogu PU, Bochner BS, Butterfield JH, et al. Hypereosinophilic syndrome. A multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319‐1325.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gleich GJ. The pathology of asthma. With emphasis on the role of the eosinophil. N Engl Reg Allergy Proc. 1986;7:421‐424. [DOI] [PubMed] [Google Scholar]
- 41. Rongioletti F, Cecchi F, Pastorino C, Scaparro M. Successful management of refractory angiolymphoid hyperplasia with eosinophilia with thalidomide. J Eur Acad Dermatol Venereol. 2016;30:527‐529. [DOI] [PubMed] [Google Scholar]
- 42. Plötz S‐G, Simon H‐U, Darsow U, et al. Use of an anti‐interleukin‐5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med. 2003;349:2334‐2339. [DOI] [PubMed] [Google Scholar]
- 43. Plötz SG, Hüttig B, Aigner B, et al. Clinical overview of cutaneous features in hypereosinophilic syndrome. Curr Allergy Asthma Rep. 2012;12:85‐98. [DOI] [PubMed] [Google Scholar]
- 44. Leiferman KM, Peters MS. Eosinophil‐Related Disease and the Skin. The journal of allergy and clinical immunology. practice. 2018;6:1462‐1482.e6. [DOI] [PubMed] [Google Scholar]
- 45. Werfel T, Allam J‐P, Biedermann T, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:336‐349. [DOI] [PubMed] [Google Scholar]
- 46. Oldhoff JM, Darsow U, Werfel T, et al. Anti‐IL‐5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60:693‐696. [DOI] [PubMed] [Google Scholar]
- 47. Kang EG, Narayana PK, Pouliquen IJ, Lopez MC, Ferreira‐Cornwell MC, Getsy JA. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy. 2020;75:950‐953. [DOI] [PubMed] [Google Scholar]
- 48. Pollmann R, Schmidt T, Eming R, Hertl MP. A Comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clin Rev Allergy Immunol. 2018;54:1‐25. [DOI] [PubMed] [Google Scholar]
- 49. Engmann J, Rüdrich U, Behrens G, et al. Increased activity and apoptosis of eosinophils in blister fluids, skin and peripheral blood of patients with bullous pemphigoid. Acta dermato‐venereologica. 2017;97:464‐471. [DOI] [PubMed] [Google Scholar]
- 50. de Graauw E, Sitaru C, Horn M, et al. Evidence for a role of eosinophils in blister formation in bullous pemphigoid. Allergy. 2017;72:1105‐1113. [DOI] [PubMed] [Google Scholar]
- 51. Rüdrich U, Gehring M, Papakonstantinou E, et al. Eosinophils are a Major Source of Interleukin‐31 in Bullous Pemphigoid. Acta dermato‐venereologica. 2018;766‐771. [DOI] [PubMed] [Google Scholar]
- 52. Rothenberg ME, Klion AD, Roufosse FE, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215‐1228. [DOI] [PubMed] [Google Scholar]
- 53. Izumi K, Bieber K, Ludwig RJ. Current clinical trials in pemphigus and pemphigoid. Front Immunol. 2019;10:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Simon D, Borradori L, Simon H‐U. Eosinophils as putative therapeutic targets in bullous pemphigoid. Exp Dermatol. 2017;26:1187‐1192. [DOI] [PubMed] [Google Scholar]
- 55. Tomassen P, Vandeplas G, van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137:1449‐1456.e4. [DOI] [PubMed] [Google Scholar]
- 56. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis. A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344‐1353. [DOI] [PubMed] [Google Scholar]
- 57. Stentzel S, Teufelberger A, Nordengrün M, et al. Staphylococcal serine protease‐like proteins are pacemakers of allergic airway reactions to Staphylococcus aureus. J Allergy Clin Immunol. 2017;139:492‐500.e8. [DOI] [PubMed] [Google Scholar]
- 58. Teufelberger AR, Nordengrün M, Braun H, et al. The IL‐33/ST2 axis is crucial in type 2 airway responses induced by Staphylococcus aureus‐derived serine protease‐like protein D. J Allergy Clin Immunol. 2018;141:549‐559.e7. [DOI] [PubMed] [Google Scholar]
- 59. Bachert C, Sousa AR, Lund VJ, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab. Randomized trial. J Allergy Clin Immunol. 2017;140:1024‐1031.e14. [DOI] [PubMed] [Google Scholar]
- 60. Bachert C, Zhang N, Hellings PW, Bousquet J. Endotype‐driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1543‐1551. [DOI] [PubMed] [Google Scholar]
- 61. Allen J, Wert ME. Eosinophilic pneumonias. J Allergy Clin Immunol Pract. 2018;6:1455‐1461. [DOI] [PubMed] [Google Scholar]
- 62. Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol. 1951;27:277‐301. [PMC free article] [PubMed] [Google Scholar]
- 63. Wu EY, Hernandez ML, Jennette JC, Falk RJ. Eosinophilic granulomatosis with polyangiitis clinical pathology conference and review. J Allergy Clin Immunol Pract. 2018;6:1496‐1504. [DOI] [PubMed] [Google Scholar]
- 64. Ortega H, Katz L, Gunsoy N, Keene O, Yancey S. Blood eosinophil counts predict treatment response in patients with severe eosinophilic asthma. J Allergy Clin Immunol. 2015;136:825‐826. [DOI] [PubMed] [Google Scholar]
- 65. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA). A randomised, double‐blind, placebo‐controlled phase 3 trial, (vol. 388). Lancet (London, England): 2016:2128‐2141. [DOI] [PubMed] [Google Scholar]
- 66. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198‐1207. [DOI] [PubMed] [Google Scholar]
- 67. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189‐1197. [DOI] [PubMed] [Google Scholar]
- 68. Nair P, Wenzel S, Rabe KF, et al. Oral Glucocorticoid‐Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med. 2017;376:2448‐2458. [DOI] [PubMed] [Google Scholar]
- 69. Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid‐sparing treatment option in patients with Churg‐Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336‐1343. [DOI] [PubMed] [Google Scholar]
- 70. Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or Placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376:1921‐1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Straumann AE. Eosinophilic esophagitis: emerging therapies and future perspectives. Gastroenterol Clin North Am. 2014;43:385‐394. [DOI] [PubMed] [Google Scholar]
- 72. Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154:319‐332:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. O'Shea KM, Aceves SS, Dellon ES, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology. 2018;154:333‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shaheen NJ, Mukkada V, Eichinger CS, Schofield H, Todorova L, Falk GW. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus. 2018;31. 10.1093/dote/doy015. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hill DA, Dudley JW, Spergel JM. The prevalence of eosinophilic esophagitis in pediatric patients with IgE‐mediated food allergy. J Allergy Clin Immunol Pract. 2017;5:369‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ruffner MA, Spergel JM. Eosinophilic esophagitis in children. Curr Allergy Asthma Rep. 2017;17:54. [DOI] [PubMed] [Google Scholar]
- 77. Molina‐Infante J, Bredenoord AJ, Cheng E, et al. Proton pump inhibitor‐responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut. 2016;65:524‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lucendo AJ, Molina‐Infante J, Arias Á, et al. Guidelines on eosinophilic esophagitis: evidence‐based statements and recommendations for diagnosis and management in children and adults. United European gastroenterology J. 2017;5:335‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gleich GJ. Mechanisms of eosinophil‐associated inflammation. J Allergy Clin Immunol. 2000;105:651‐663. [DOI] [PubMed] [Google Scholar]
- 80. Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils Biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709‐50. [DOI] [PubMed] [Google Scholar]
- 81. Tefferi A, Patnaik MM, Pardanani AE. Secondary, clonal and idiopathic. Br J Haematol. 2006;133:468‐492. [DOI] [PubMed] [Google Scholar]
- 82. Simon D, Simon H‐U. Eosinophilic disorders. J Allergy Clin Immunol. 2007;119:1291‐300; quiz 1301‐2. [DOI] [PubMed] [Google Scholar]
- 83. Valent P, Klion AD, Horny H‐P, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607‐612.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wagener AH, de Nijs SB, Lutter R, et al. External validation of blood eosinophils, FE(NO) and serum periostin as surrogates for sputum eosinophils in asthma. Thorax. 2015;70:115‐120. [DOI] [PubMed] [Google Scholar]
- 86. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils. A biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:523‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brightling CE, Bleecker ER, Panettieri RA, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia. A randomised, double‐blind, placebo‐controlled, phase 2a study. The Lancet. Respir Med. 2014;2:891‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Khoury P, Bochner BS. Consultation for elevated blood eosinophils. Clinical presentations, high value diagnostic tests, and treatment options. J Allergy Clin Immunol Pract. 2018;6:1446‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201‐1214. [DOI] [PubMed] [Google Scholar]
- 90. Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009;23:157‐165. [DOI] [PubMed] [Google Scholar]
- 91. Radonjic‐Hoesli S, Valent P, Klion AD, Wechsler ME, Simon H‐U. Novel targeted therapies for eosinophil‐associated diseases and allergy. Annu Rev Pharmacol Toxicol. 2015;55:633‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fassnacht F, Roumier M, Fouret P, et al. Successful Heart Transplantation for unreversible endomyocardial fibrosis related to FIP1L1‐PDGFRA chronic eosinophilic leukemia. Transplantation. 2015;99:e176‐e177. [DOI] [PubMed] [Google Scholar]
- 93. Kuang FL, Legrand F, Makiya M, et al. Benralizumab for PDGFRA‐negative Hypereosinophilic syndrome. N Engl J Med. 2019;380:1336‐1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Krug N, Hohlfeld JM, Kirsten A‐M, et al. Allergen‐induced asthmatic responses modified by a GATA3‐specific DNAzyme. N Engl J Med. 2015;372:1987‐1995. [DOI] [PubMed] [Google Scholar]
- 95. Greulich T, Hohlfeld JM, Neuser P, et al. A GATA3‐specific DNAzyme attenuates sputum eosinophilia in eosinophilic COPD patients. A feasibility randomized clinical trial. Respir Res. 2018;19(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rosenberg HF, Dyer KD, Foster PSE. Changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Klion ADE. A pragmatic approach to diagnosis and treatment. Hematology Am Soc Hematol Educ Program. 2015;2015;92‐97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
n/a


