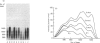Abstract
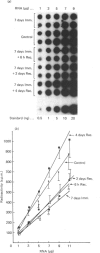
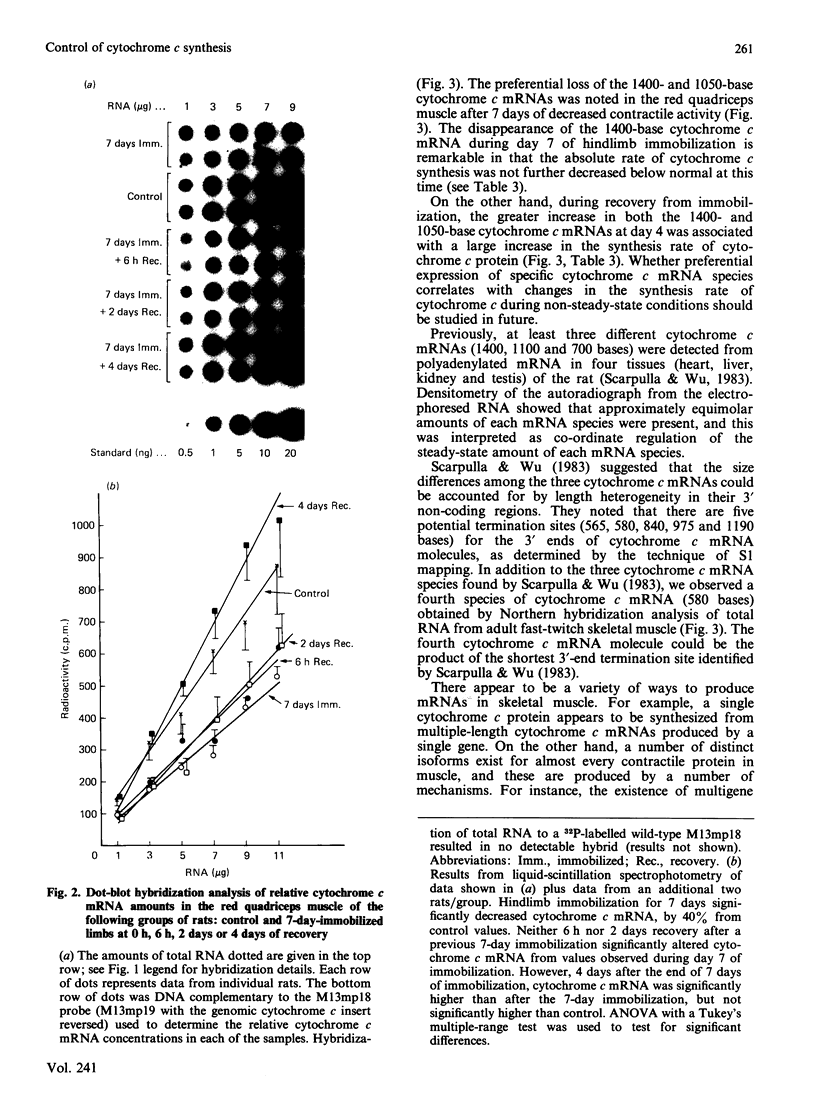
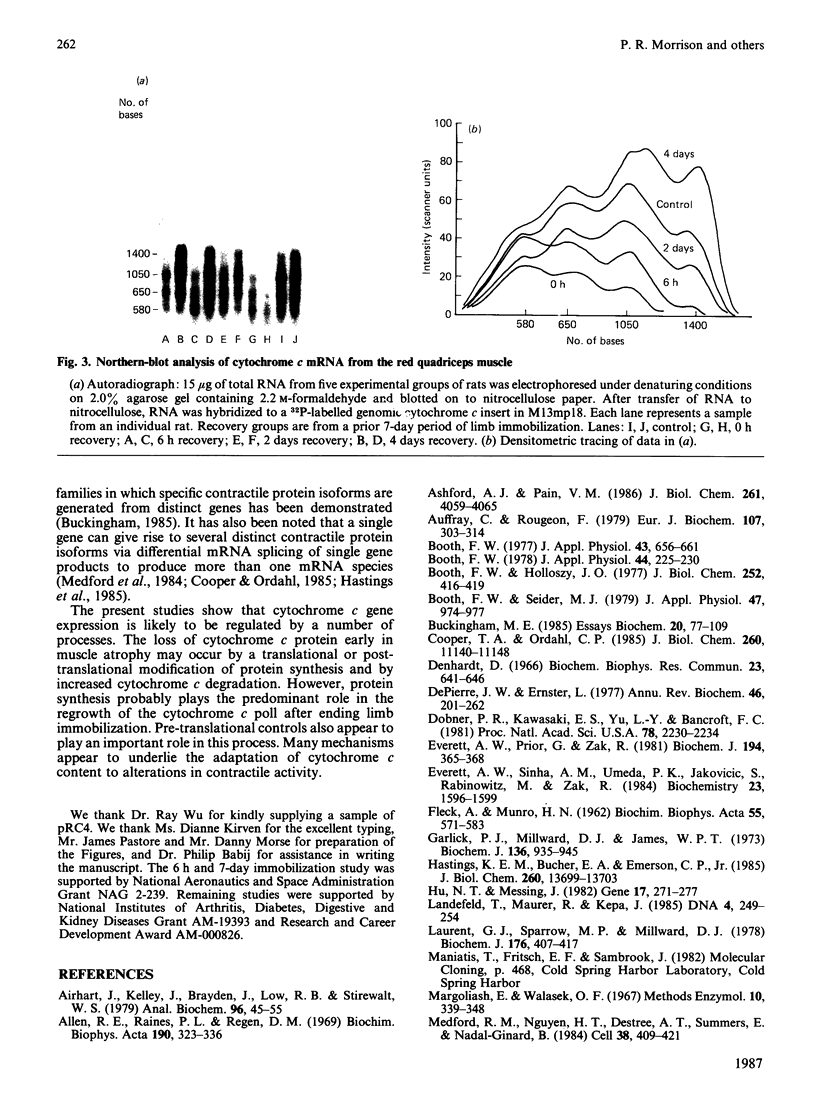
It is known that immobilization of the rat hindlimb by plaster casting leads to muscle atrophy and loss of muscle protein. In the present study, immobilization of the rat hindlimb for 6 h resulted in a significant 27% decrease in the absolute rate of cytochrome c synthesis in the red quadriceps muscle, without any change in the relative amount of cytochrome c mRNA. Cytochrome c mRNA in normal red quadriceps muscle was observed to be of four different lengths (1400, 1050, 650 and 580 bases). After 7 days of immobilization, the absolute rate of cytochrome c synthesis remained depressed and cytochrome c mRNA decreased by 40%; each of the cytochrome c mRNAs decreased, with a preferential disappearance of the 1050- and 1400-base lengths. Immobilization was ended on day 7, and the atrophied muscle was allowed to recover. At day 4 of recovery, the absolute rate of cytochrome c synthesis was 92% higher and the amount of cytochrome c mRNA had returned to control values. The abundances of the 1050- and 1400-base cytochrome c mRNAs had increased more than the shorter cytochrome c mRNAs, so that they were higher than control values. It appears that acute decreases in contractile activity of the red quadriceps muscle alter cytochrome c synthesis rates via translational or post-translational mechanisms, whereas chronic periods of modified contractile activity alter its synthesis rate via pre-translational mechanisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Kelley J., Brayden J. E., Low R. B., Stirewalt W. S. An ultramicro method of amino acid analysis: application to studies of protein metabolism in cultured cells. Anal Biochem. 1979 Jul 1;96(1):45–55. doi: 10.1016/0003-2697(79)90552-9. [DOI] [PubMed] [Google Scholar]
- Allen R. E., Raines P. L., Regen D. M. Regulatory significance of transfer RNA charging levels. I. Measurements of charging levels in livers of chow-fed rats, fasting rats, and rats fed balanced or imbalanced mixtures of amino acids. Biochim Biophys Acta. 1969 Oct 22;190(2):323–336. doi: 10.1016/0005-2787(69)90083-5. [DOI] [PubMed] [Google Scholar]
- Ashford A. J., Pain V. M. Effect of diabetes on the rates of synthesis and degradation of ribosomes in rat muscle and liver in vivo. J Biol Chem. 1986 Mar 25;261(9):4059–4065. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Booth F. W., Holloszy J. O. Cytochrome c turnover in rat skeletal muscles. J Biol Chem. 1977 Jan 25;252(2):416–419. [PubMed] [Google Scholar]
- Booth F. W. Regrowth of atrophied skeletal muscle in adult rats after ending immobilization. J Appl Physiol Respir Environ Exerc Physiol. 1978 Feb;44(2):225–230. doi: 10.1152/jappl.1978.44.2.225. [DOI] [PubMed] [Google Scholar]
- Booth F. W., Seider M. J. Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol Respir Environ Exerc Physiol. 1979 Nov;47(5):974–977. doi: 10.1152/jappl.1979.47.5.974. [DOI] [PubMed] [Google Scholar]
- Booth F. W. Time course of muscular atrophy during immobilization of hindlimbs in rats. J Appl Physiol Respir Environ Exerc Physiol. 1977 Oct;43(4):656–661. doi: 10.1152/jappl.1977.43.4.656. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E. Actin and myosin multigene families: their expression during the formation of skeletal muscle. Essays Biochem. 1985;20:77–109. [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985 Sep 15;260(20):11140–11148. [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Kawasaki E. S., Yu L. Y., Bancroft F. C. Thyroid or glucocorticoid hormone induces pre-growth-hormone mRNA and its probable nuclear precursor in rat pituitary cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2230–2234. doi: 10.1073/pnas.78.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A. W., Prior G., Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J. 1981 Jan 15;194(1):365–368. doi: 10.1042/bj1940365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett A. W., Sinha A. M., Umeda P. K., Jakovcic S., Rabinowitz M., Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry. 1984 Apr 10;23(8):1596–1599. doi: 10.1021/bi00303a002. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., Millward D. J., James W. P. The diurnal response of muscle and liver protein synthesis in vivo in meal-fed rats. Biochem J. 1973 Dec;136(4):935–945. doi: 10.1042/bj1360935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K. E., Bucher E. A., Emerson C. P., Jr Generation of troponin T isoforms by alternative RNA splicing in avian skeletal muscle. Conserved and divergent features in birds and mammals. J Biol Chem. 1985 Nov 5;260(25):13699–13703. [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Landefeld T., Maurer R., Kepa J. Luteinizing hormone beta-subunit mRNA amounts increase during the preovulatory surge of luteinizing hormone in the ewe: the highest levels are observed at the completion of the peak. DNA. 1985 Jun;4(3):249–254. doi: 10.1089/dna.1985.4.249. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Destree A. T., Summers E., Nadal-Ginard B. A novel mechanism of alternative RNA splicing for the developmentally regulated generation of troponin T isoforms from a single gene. Cell. 1984 Sep;38(2):409–421. doi: 10.1016/0092-8674(84)90496-3. [DOI] [PubMed] [Google Scholar]
- Rannels D. E., Wartell S. A., Watkins C. A. The measurement of protein synthesis in biological systems. Life Sci. 1982 May 17;30(20):1679–1690. doi: 10.1016/0024-3205(82)90300-9. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Agne K. M., Wu R. Isolation and structure of a rat cytochrome c gene. J Biol Chem. 1981 Jun 25;256(12):6480–6486. [PubMed] [Google Scholar]
- Scarpulla R. C., Wu R. Nonallelic members of the cytochrome c multigene family of the rat may arise through different messenger RNAs. Cell. 1983 Feb;32(2):473–482. doi: 10.1016/0092-8674(83)90467-1. [DOI] [PubMed] [Google Scholar]
- Tucker K. R., Seider M. J., Booth F. W. Protein synthesis rates in atrophied gastrocnemius muscles after limb immobilization. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jul;51(1):73–77. doi: 10.1152/jappl.1981.51.1.73. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C., Stephen J. M. The effect of low protein diets on the turn-over rates of serums, liver and muscle proteins in the rat, measured by continuous infusion of L-[14C]lysine. Clin Sci. 1968 Oct;35(2):287–305. [PubMed] [Google Scholar]
- Watson P. A., Stein J. P., Booth F. W. Changes in actin synthesis and alpha-actin-mRNA content in rat muscle during immobilization. Am J Physiol. 1984 Jul;247(1 Pt 1):C39–C44. doi: 10.1152/ajpcell.1984.247.1.C39. [DOI] [PubMed] [Google Scholar]
- Williams J. N., Jr, Thorp S. L. Re-evaluation of cytochrome c concentrations in rat organs using a new method for cytochrome c. Biochim Biophys Acta. 1969 Sep 16;189(1):25–28. doi: 10.1016/0005-2728(69)90220-5. [DOI] [PubMed] [Google Scholar]