Abstract
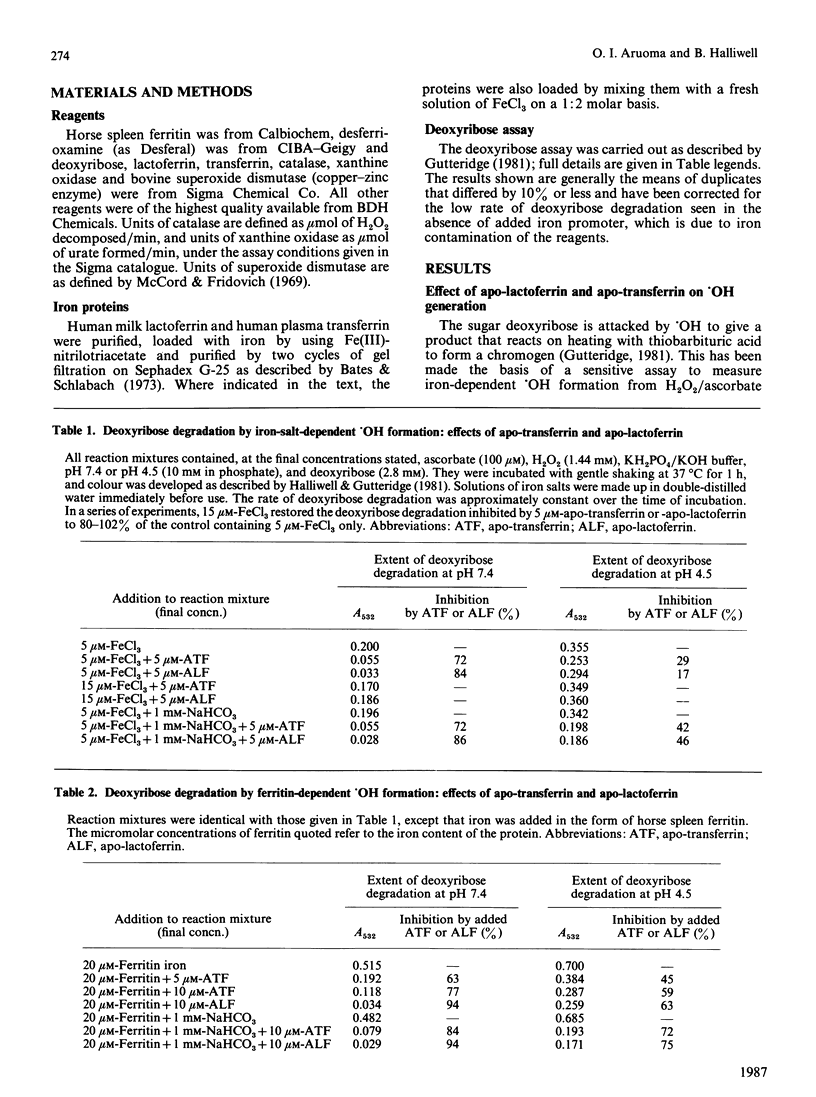
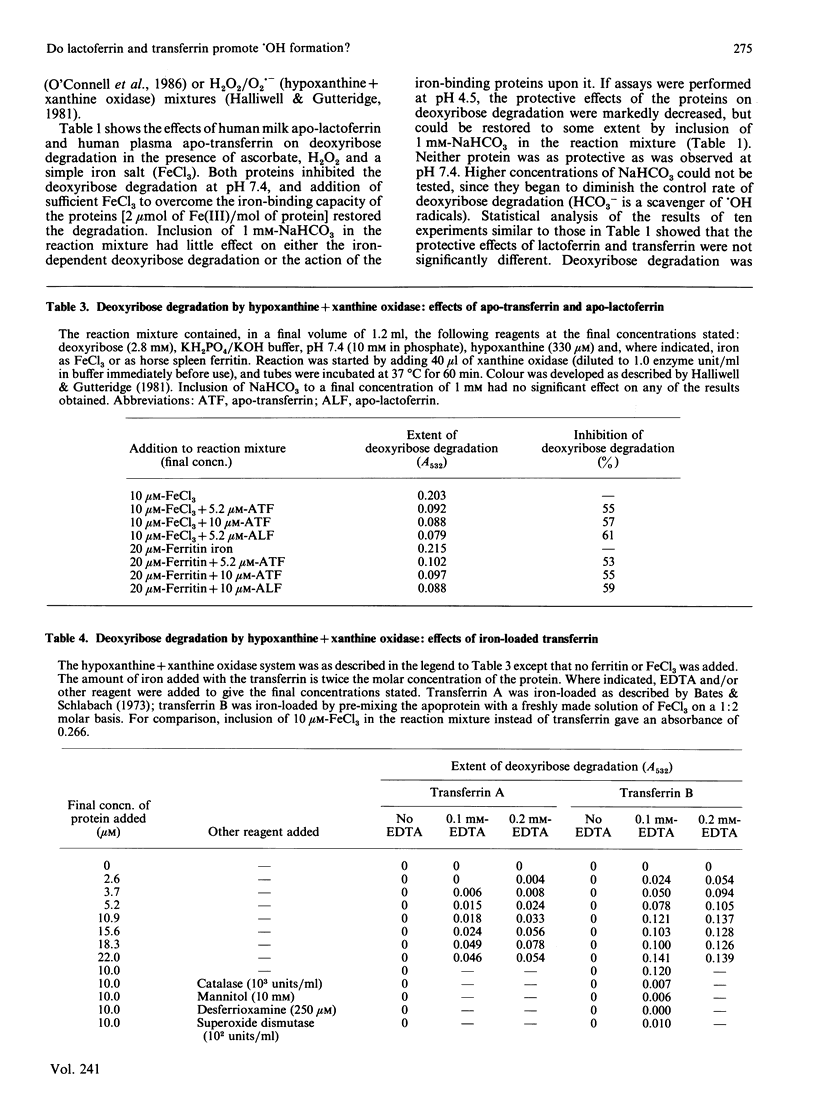
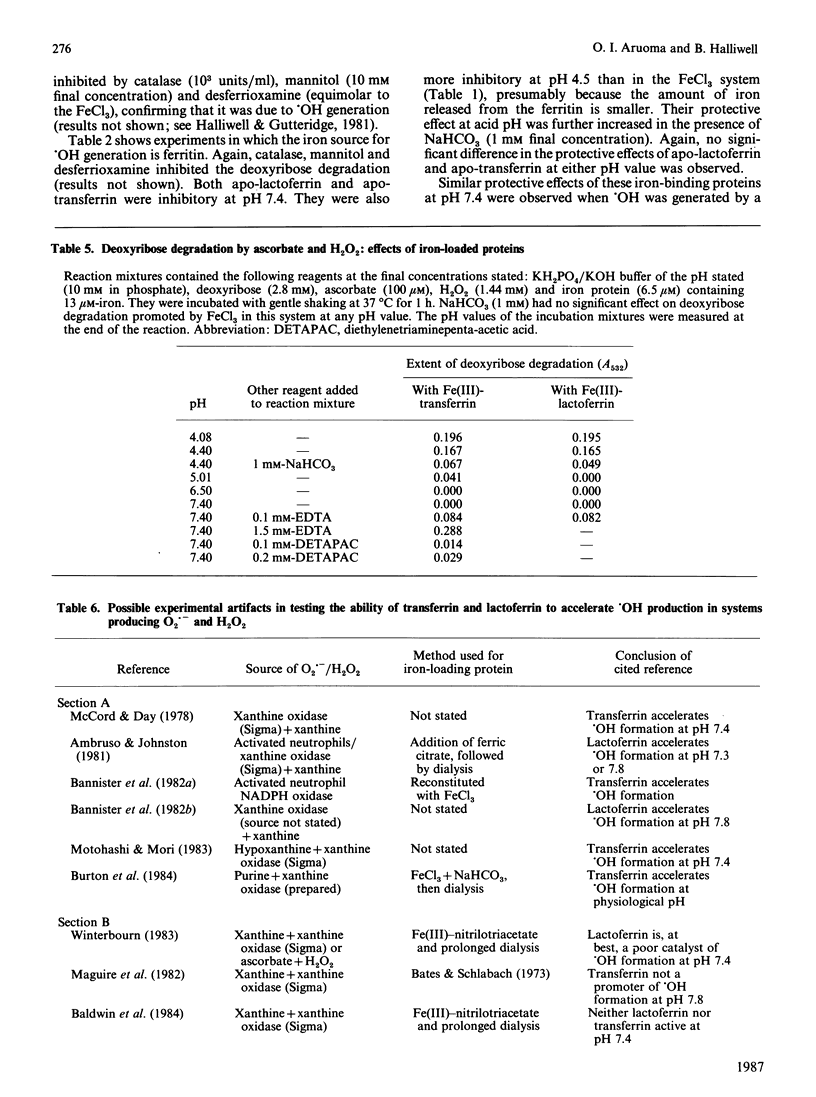
Apo-lactoferrin and apo-transferrin protect against iron-ion-dependent hydroxyl-radical (.OH) generation from H2O2 in the presence of superoxide radicals or ascorbic acid at pH 7.4, whether the necessary iron is added as ionic iron or as ferritin. Iron-loaded transferrin and lactoferrin [2 mol of Fe(III)/mol] show no protective ability, but do not themselves accelerate .OH production unless chelating agents are present in the reaction mixture, especially if the proteins are incorrectly loaded with iron. At acidic pH values, the protective ability of the apoproteins is diminished, and the fully iron-loaded proteins can release some iron in a form able to accelerate .OH generation. The physiological significance of these observations is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankel E., Petering D. H. Iron-chelating agents and the reductive removal of iron from transferrin. Biochem Pharmacol. 1980 Jun 15;29(12):1833–1837. doi: 10.1016/0006-2952(80)90146-x. [DOI] [PubMed] [Google Scholar]
- Baker M. S., Gebicki J. M. The effect of pH on yields of hydroxyl radicals produced from superoxide by potential biological iron chelators. Arch Biochem Biophys. 1986 May 1;246(2):581–588. doi: 10.1016/0003-9861(86)90313-9. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Hill H. A., Thornalley P. J. Enhanced production of hydroxyl radicals by the xanthine-xanthine oxidase reaction in the presence of lactoferrin. Biochim Biophys Acta. 1982 Mar 15;715(1):116–120. doi: 10.1016/0304-4165(82)90056-3. [DOI] [PubMed] [Google Scholar]
- Bannister J. V., Bellavite P., Davoli A., Thornalley P. J., Rossi F. The generation of hydroxyl radicals following superoxide production by neutrophil NADPH oxidase. FEBS Lett. 1982 Dec 27;150(2):300–302. doi: 10.1016/0014-5793(82)80755-2. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Schlabach M. R. The reaction of ferric salts with transferrin. J Biol Chem. 1973 May 10;248(9):3228–3232. [PubMed] [Google Scholar]
- Berglin E. H., Carlsson J. Potentiation by sulfide of hydrogen peroxide-induced killing of Escherichia coli. Infect Immun. 1985 Sep;49(3):538–543. doi: 10.1128/iai.49.3.538-543.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton K. P., McCord J. M., Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984 Jun;246(6 Pt 2):H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- Butler J., Halliwell B. Reaction of iron-EDTA chelates with the superoxide radical. Arch Biochem Biophys. 1982 Oct 1;218(1):174–178. doi: 10.1016/0003-9861(82)90333-2. [DOI] [PubMed] [Google Scholar]
- Carver F. J., Frieden E. Factors affecting the adenosine triphosphate induced release of iron from transferrin. Biochemistry. 1978 Jan 10;17(1):167–172. doi: 10.1021/bi00594a024. [DOI] [PubMed] [Google Scholar]
- Czapski G., Goldstein S. When do metal complexes protect the biological system from superoxide toxicity and when do they enhance it? Free Radic Res Commun. 1986;1(3):157–161. doi: 10.3109/10715768609083147. [DOI] [PubMed] [Google Scholar]
- Etherington D. J., Pugh D., Silver I. A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 1981;40(10-11):1625–1636. [PubMed] [Google Scholar]
- Floyd R. A. DNA-ferrous iron catalyzed hydroxyl free radical formation from hydrogen peroxide. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1209–1215. doi: 10.1016/0006-291x(81)90748-8. [DOI] [PubMed] [Google Scholar]
- Floyd R. A. Direct demonstration that ferrous ion complexes of di- and triphosphate nucleotides catalyze hydroxyl free radical formation from hydrogen peroxide. Arch Biochem Biophys. 1983 Aug;225(1):263–270. doi: 10.1016/0003-9861(83)90029-2. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of the proteins caeruloplasmin, albumin and transferrin. A study of their activity in serum and synovial fluid from patients with rheumatoid arthritis. Biochim Biophys Acta. 1986 Jan 30;869(2):119–127. doi: 10.1016/0167-4838(86)90286-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B., Treffry A., Harrison P. M., Blake D. Effect of ferritin-containing fractions with different iron loading on lipid peroxidation. Biochem J. 1983 Feb 1;209(2):557–560. doi: 10.1042/bj2090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Paterson S. K., Segal A. W., Halliwell B. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981 Oct 1;199(1):259–261. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Richmond R., Halliwell B. Inhibition of the iron-catalysed formation of hydroxyl radicals from superoxide and of lipid peroxidation by desferrioxamine. Biochem J. 1979 Nov 15;184(2):469–472. doi: 10.1042/bj1840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Thiobarbituric acid-reactivity following iron-dependent free-radical damage to amino acids and carbohydrates. FEBS Lett. 1981 Jun 15;128(2):343–346. doi: 10.1016/0014-5793(81)80113-5. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M., Blake D. Metal ions and oxygen radical reactions in human inflammatory joint disease. Philos Trans R Soc Lond B Biol Sci. 1985 Dec 17;311(1152):659–671. doi: 10.1098/rstb.1985.0171. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron chelates: is it a mechanism for hydroxyl radical production in biochemical systems? FEBS Lett. 1978 Aug 15;92(2):321–326. doi: 10.1016/0014-5793(78)80779-0. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Use of desferrioxamine as a 'probe' for iron-dependent formation of hydroxyl radicals. Evidence for a direct reaction between desferal and the superoxide radical. Biochem Pharmacol. 1985 Jan 15;34(2):229–233. doi: 10.1016/0006-2952(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Rinehart A. L., Hereld D., Schwartz R. W., Burke F. P., Salvador A. P. Reduction potential of iron in transferrin. Biochim Biophys Acta. 1985 Mar 8;838(3):295–301. doi: 10.1016/0304-4165(85)90226-0. [DOI] [PubMed] [Google Scholar]
- Lönnerdal B., Keen C. L., Hurley L. S. Iron, copper, zinc, and manganese in milk. Annu Rev Nutr. 1981;1:149–174. doi: 10.1146/annurev.nu.01.070181.001053. [DOI] [PubMed] [Google Scholar]
- Maguire J. J., Kellogg E. W., 3rd, Packer L. Protection against free radical formation by protein bound iron. Toxicol Lett. 1982 Nov;14(1-2):27–34. doi: 10.1016/0378-4274(82)90006-6. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Morgan E. H. Studies on the mechanism of iron release from transferrin. Biochim Biophys Acta. 1979 Oct 24;580(2):312–326. doi: 10.1016/0005-2795(79)90144-2. [DOI] [PubMed] [Google Scholar]
- Motohashi N., Mori I. Superoxide-dependent formation of hydroxyl radical catalyzed by transferrin. FEBS Lett. 1983 Jun 27;157(1):197–199. doi: 10.1016/0014-5793(83)81144-2. [DOI] [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., van Renswoude J., Kempf C., Klausner R. D. Separation of Fe+3 from transferrin in endocytosis. Role of the acidic endosome. FEBS Lett. 1983 Aug 22;160(1-2):213–216. doi: 10.1016/0014-5793(83)80969-7. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Vercellotti G. M., van Asbeck B. S., Jacob H. S. Oxygen radical-induced erythrocyte hemolysis by neutrophils. Critical role of iron and lactoferrin. J Clin Invest. 1985 Sep;76(3):956–962. doi: 10.1172/JCI112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson R. L. Iron, zinc, free radicals and oxygen in tissue disorders and cancer control. Ciba Found Symp. 1976 Dec 7;(51):331–354. doi: 10.1002/9780470720325.ch16. [DOI] [PubMed] [Google Scholar]
- Winston G. W., Eibschutz O. M., Strekas T., Cederbaum A. I. Complex-formation and reduction of ferric iron by 2-oxo-4-thiomethylbutyric acid, and the production of hydroxyl radicals. Biochem J. 1986 Apr 15;235(2):521–529. doi: 10.1042/bj2350521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]


