Abstract
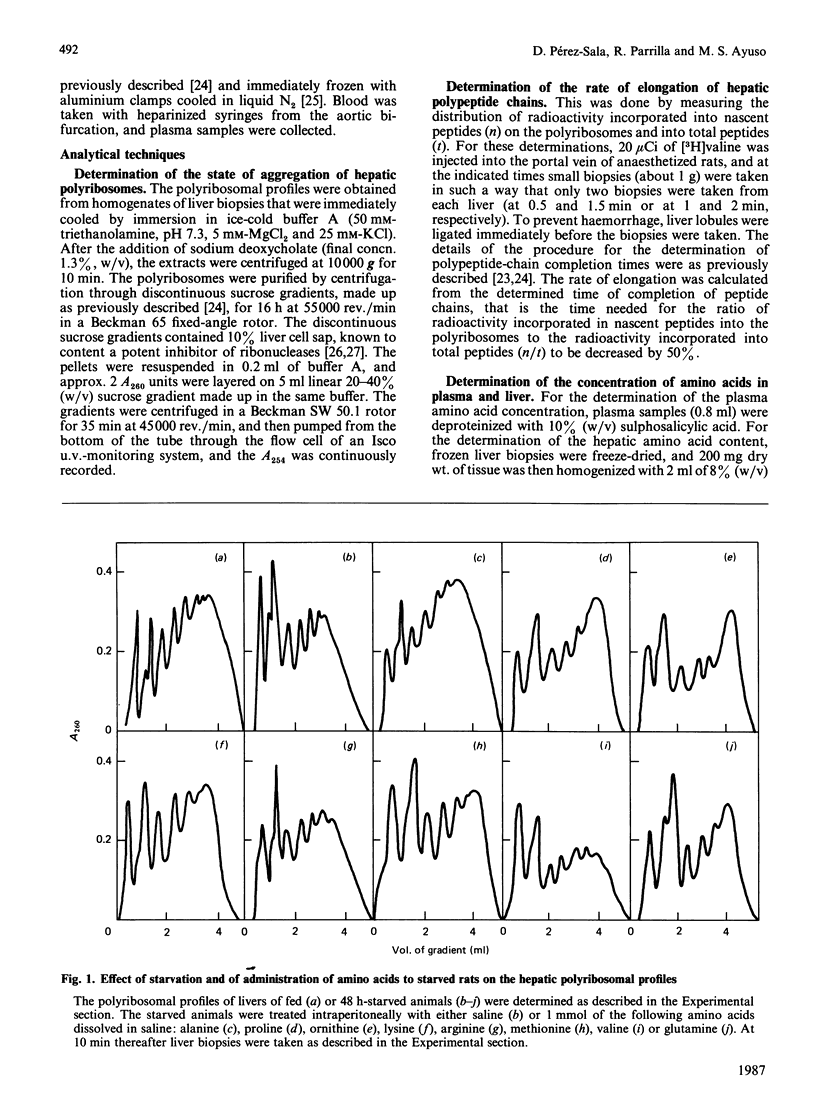
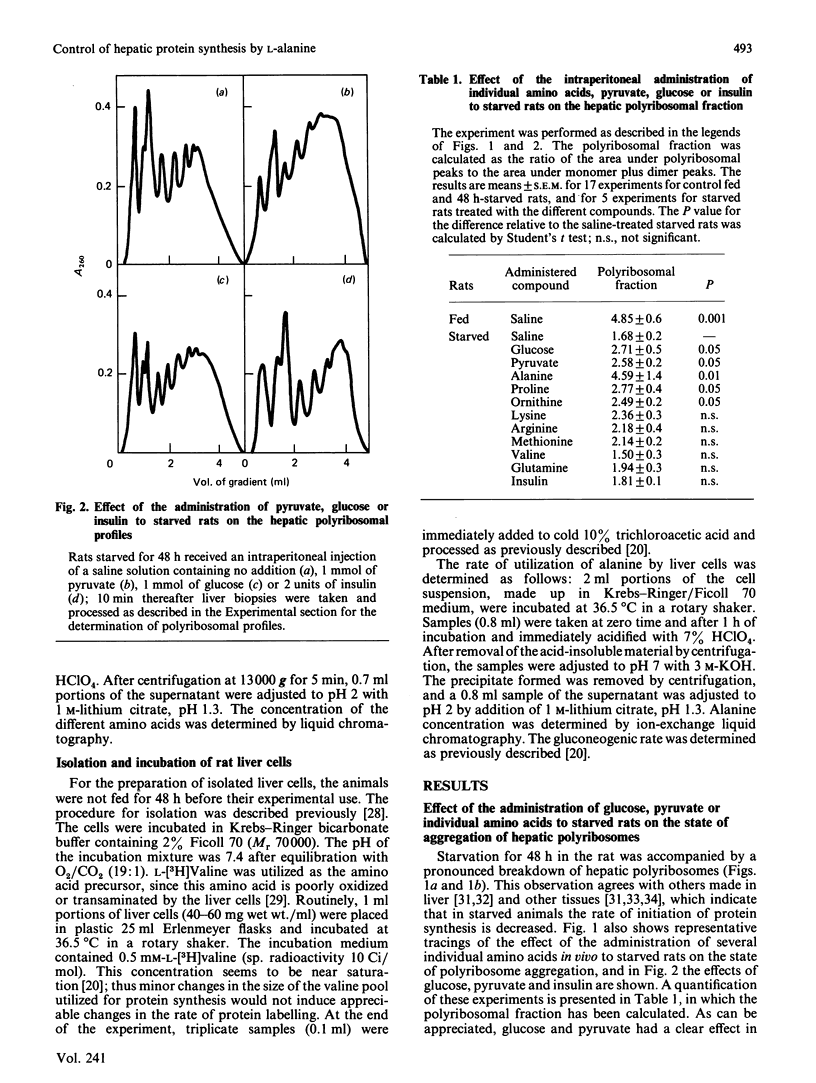
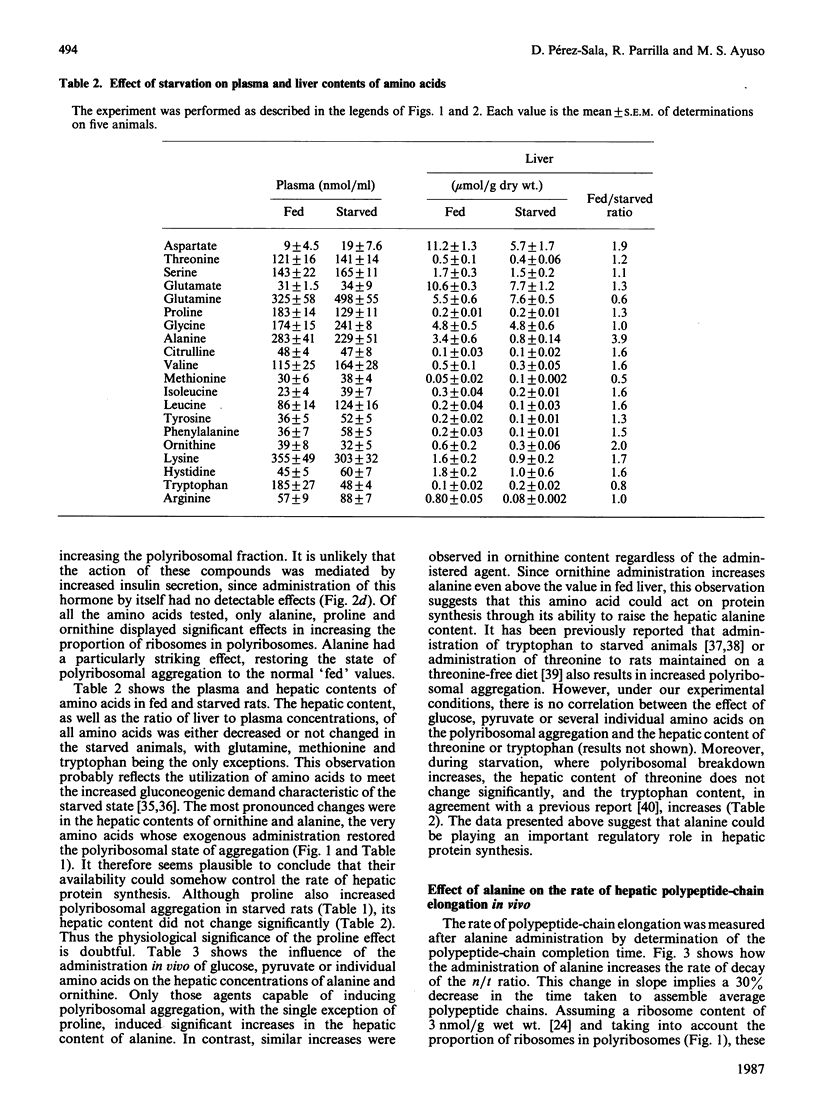
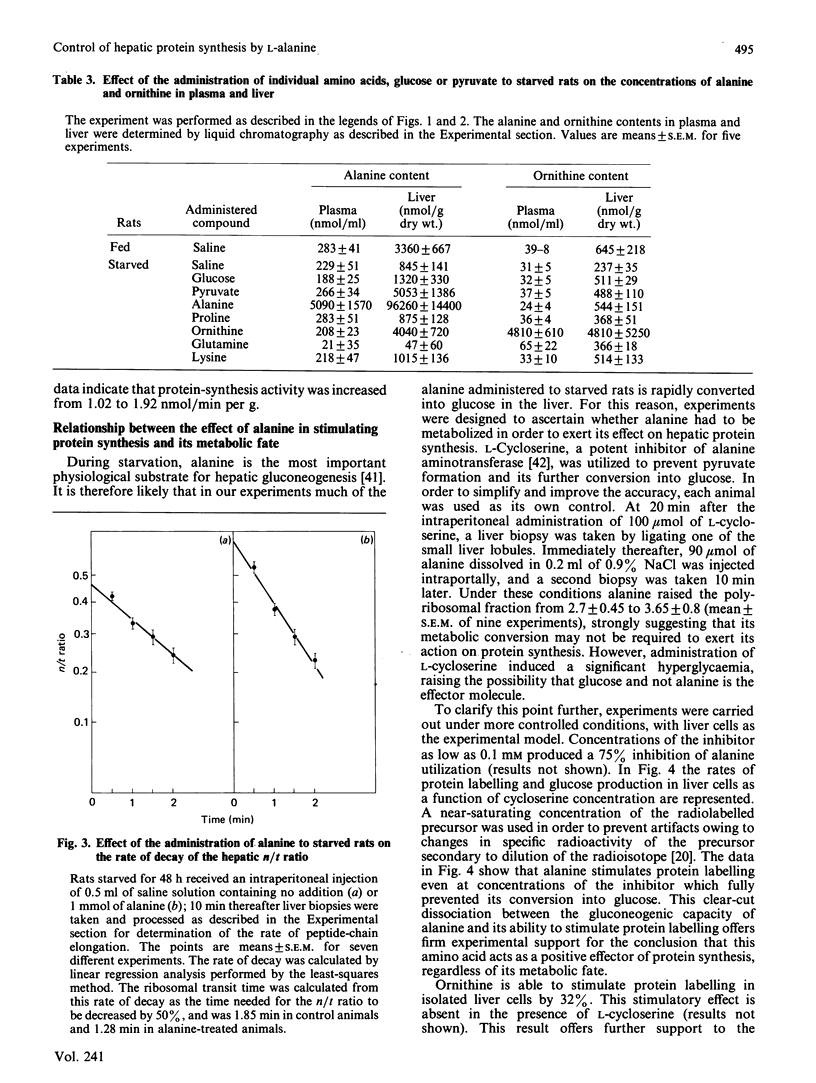
We investigated the effects of administration of single amino acids to starved rats on the regulation of protein synthesis in the liver. Of all the amino acids tested, only alanine, ornithine and proline promoted statistically significant increases in the extent of hepatic polyribosome aggregation. The most effective of these was alanine, whose effect of promoting polyribosomal aggregation was accompanied by a decrease in the polypeptide-chain elongation time. The following observations indicate that alanine plays an important physiological role in the regulation of hepatic protein synthesis. Alanine was the amino acid showing the largest decrease in hepatic content in the transition from high (fed) to low (starved) rates of protein synthesis. The administration of glucose or pyruvate is also effective in increasing liver protein synthesis in starved rats, and their effects were accompanied by an increased hepatic alanine content. An increase in hepatic ornithine content does not lead to an increased protein synthesis, unless it is accompanied by an increase of alanine. The effect of alanine is observed either in vivo, in rats pretreated with cycloserine to prevent its transamination, or in isolated liver cells under conditions in which its metabolic transformation is fully impeded.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin S. A., Clemens M. J. The regulation of protein synthesis in mammalian cells by amino acid supply. Biosci Rep. 1981 Jan;1(1):35–44. doi: 10.1007/BF01115147. [DOI] [PubMed] [Google Scholar]
- Austin S. A., Pain V. M., Lewis J. A., Clemens M. J. Investigation of the role of uncharged tRNA in the regulation of polypeptide chain initiation by amino acid starvation in cultured mammalian cells; a reappraisal. Eur J Biochem. 1982 Mar 1;122(3):519–526. doi: 10.1111/j.1432-1033.1982.tb06468.x. [DOI] [PubMed] [Google Scholar]
- Ayuso-Parrilla M. S., Martín-Requero A., Pérez-Días J., Parrilla R. Role of glucagon on the control of hepatic protein synthesis and degradation in the rat in vivo. J Biol Chem. 1976 Dec 25;251(24):7785–7790. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer W. W., Chance R. E. Zinc glucagon depression of blood amino acids in rabbits. Diabetes. 1969 Nov;18(11):748–754. doi: 10.2337/diab.18.11.748. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Removal of ammonia by formation of amino acids in rat liver. Biochem J. 1967 Sep;104(3):43P–44P. [PMC free article] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Goldberg A. L. The origin of alanine produced in skeletal muscle. J Biol Chem. 1978 May 25;253(10):3677–3684. [PubMed] [Google Scholar]
- Cornell N. W., Lund P., Krebs H. A. The effect of lysine on gluconeogenesis from lactate in rat hepatocytes. Biochem J. 1974 Aug;142(2):327–337. doi: 10.1042/bj1420327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Foster A., Lanthorn T. An overview of glutamate as a neurotransmitter. Adv Biochem Psychopharmacol. 1981;27:1–27. [PubMed] [Google Scholar]
- Damuni Z., Caudwell F. B., Cohen P. Regulation of the aminoacyl-tRNA synthetase complex of rat liver by phosphorylation/dephosphorylation in vitro and in vivo. Eur J Biochem. 1982 Dec;129(1):57–65. doi: 10.1111/j.1432-1033.1982.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Felig P. Amino acid metabolism in man. Annu Rev Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Flaim K. E., Peavy D. E., Everson W. V., Jefferson L. S. The role of amino acids in the regulation of protein synthesis in perfused rat liver. I. Reduction in rates of synthesis resulting from amino acid deprivation and recovery during flow-through perfusion. J Biol Chem. 1982 Mar 25;257(6):2932–2938. [PubMed] [Google Scholar]
- Friedrichs D. On the stimulation of gluconeogenesis by L-lysine in isolated rat kidney cortex tubules. Biochim Biophys Acta. 1975 Jun 12;392(2):255–270. doi: 10.1016/0304-4165(75)90007-0. [DOI] [PubMed] [Google Scholar]
- Girbes T., Susin A., Ayuso M. S., Parrilla R. Acute effects of ethanol in the control of protein synthesis in isolated rat liver cells. Arch Biochem Biophys. 1983 Oct 1;226(1):37–49. doi: 10.1016/0003-9861(83)90269-2. [DOI] [PubMed] [Google Scholar]
- Harmon C. S., Proud C. G., Pain V. M. Effects of starvation, diabetes and acute insulin treatment on the regulation of polypeptide-chain initiation in rat skeletal muscle. Biochem J. 1984 Nov 1;223(3):687–696. doi: 10.1042/bj2230687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw E. C., Hirsch C. A., Morton B. E., Hiatt H. H. Control of protein synthesis in mammalian tissues through changes in ribosome activity. J Biol Chem. 1971 Jan 25;246(2):436–446. [PubMed] [Google Scholar]
- Ip C. C., Harper A. E. Effect of threonine supplementation on hepatic polysome patterns and protein synthesis of rats fed a threonine-deficient diet. Biochim Biophys Acta. 1973 Dec 7;331(2):251–263. doi: 10.1016/0005-2787(73)90438-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Aikawa T., Matsutaka H. The roles of alanine as a major precursor among amino acids for hepatic gluconeogenesis and as a major end product of the degradation of amino acids in rat tissues. J Biochem. 1972 Jun;71(6):1097–1099. doi: 10.1093/oxfordjournals.jbchem.a129862. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Korner A. Influence of amino acid supply on ribosomes and protein synthesis of perfused rat liver. Biochem J. 1969 Mar;111(5):703–712. doi: 10.1042/bj1110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSTEN E., KIRSTEN R., HOHORST H. J., BUECHER T. Free amino acids in alloxan diabetic rat livers. Biochem Biophys Res Commun. 1961 Mar 10;4:169–174. doi: 10.1016/0006-291x(61)90264-9. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Stimulation of hepatic glycogen synthesis by amino acids. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3433–3437. doi: 10.1073/pnas.73.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Lund P. Some regulatory mechanisms in the synthesis of urea in the mammalian liver. Adv Enzyme Regul. 1973;11:361–377. doi: 10.1016/0065-2571(73)90024-1. [DOI] [PubMed] [Google Scholar]
- Li J. B., Higgins J. E., Jefferson L. S. Changes in protein turnover in skeletal muscle in response to fasting. Am J Physiol. 1979 Mar;236(3):E222–E228. doi: 10.1152/ajpendo.1979.236.3.E222. [DOI] [PubMed] [Google Scholar]
- Mallet L. E., Exton J. H., Park C. R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5713–5723. [PubMed] [Google Scholar]
- McGivan J. D., Bradford N. M., Beavis A. D. Factors influencing the activity of ornithine aminotransferase in isolated rat liver mitochondria. Biochem J. 1977 Jan 15;162(1):147–156. doi: 10.1042/bj1620147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Katz J. Effect of mercaptopicolinic acid and of transaminase inhibitors on glycogen synthesis by rat hepatocytes. Biochem Biophys Res Commun. 1979 Mar 15;87(1):155–162. doi: 10.1016/0006-291x(79)91660-7. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Lewis J. A., Huvos P., Henshaw E. C., Clemens M. J. The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem. 1980 Feb 25;255(4):1486–1491. [PubMed] [Google Scholar]
- Parrilla R. Flux of metabolic fuels during starvation in the rat. Pflugers Arch. 1978 Apr 25;374(1):3–7. doi: 10.1007/BF00585690. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N. Nitrogen metabolism in the isolated perfused rat liver. Nitrogen balance, redox state and rates of proteolysis. Biochem J. 1974 Mar;138(3):341–348. doi: 10.1042/bj1380341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N., Toews C. J. The effect of anoxia on nitrogen metabolism in the isolated perfused rat liver. Pflugers Arch. 1977 Jun 8;369(2):167–175. doi: 10.1007/BF00591573. [DOI] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH J. S. Ribonuclease. VII. Partial purification and characterization of a ribonuclease inhibitor in rat liver supernatant fraction. J Biol Chem. 1958 Apr;231(2):1085–1095. [PubMed] [Google Scholar]
- SHORTMAN K. Studies on cellular inhibitors of ribonuclease. II. Some properties of the inhibitor from rat liver. Biochim Biophys Acta. 1962 Jan 22;55:88–96. doi: 10.1016/0006-3002(62)90934-4. [DOI] [PubMed] [Google Scholar]
- Scornik O. A. In vivo rate of translation by ribosomes of normal and regenerating liver. J Biol Chem. 1974 Jun 25;249(12):3876–3883. [PubMed] [Google Scholar]
- Seglen P. O., Solheim A. E. Effects of aminooxyacetate, alanine and other amino acids on protein synthesis in isolated rat hepatocytes. Biochim Biophys Acta. 1978 Oct 24;520(3):630–641. doi: 10.1016/0005-2787(78)90148-x. [DOI] [PubMed] [Google Scholar]
- Shenoy S. T., Rogers Q. R. Effect of dietary amino acids on transfer ribonucleic acid charging levels in rat liver. J Nutr. 1978 Sep;108(9):1412–1421. doi: 10.1093/jn/108.9.1412. [DOI] [PubMed] [Google Scholar]
- Sidransky H., Sarma D. S., Bongiorno M., Verney E. Effect of dietary tryptophan on hepatic polyribosomes and protein synthesis in fasted mice. J Biol Chem. 1968 Mar 25;243(6):1123–1132. [PubMed] [Google Scholar]
- Stubbs M., Krebs H. A. The accumulation of aspartate in the presence of ethanol in rat liver. Biochem J. 1975 Jul;150(1):41–45. doi: 10.1042/bj1500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Desautels M., Goldberg A. L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982 Feb 25;257(4):1613–1621. [PubMed] [Google Scholar]
- Tolman E. L., Schworer C. M., Jefferson L. S. Effects of hypophysectomy on amino acid metabolism and gluconeogenesis in the perfused rat liver. J Biol Chem. 1973 Jul 10;248(13):4552–4560. [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]
- Wunner W. H., Bell J., Munro H. N. The effect of feeding with a tryptophan-free amino acid mixture on rat-liver polysomes and ribosomal ribonucleic acid. Biochem J. 1966 Nov;101(2):417–428. doi: 10.1042/bj1010417. [DOI] [PMC free article] [PubMed] [Google Scholar]


