Abstract
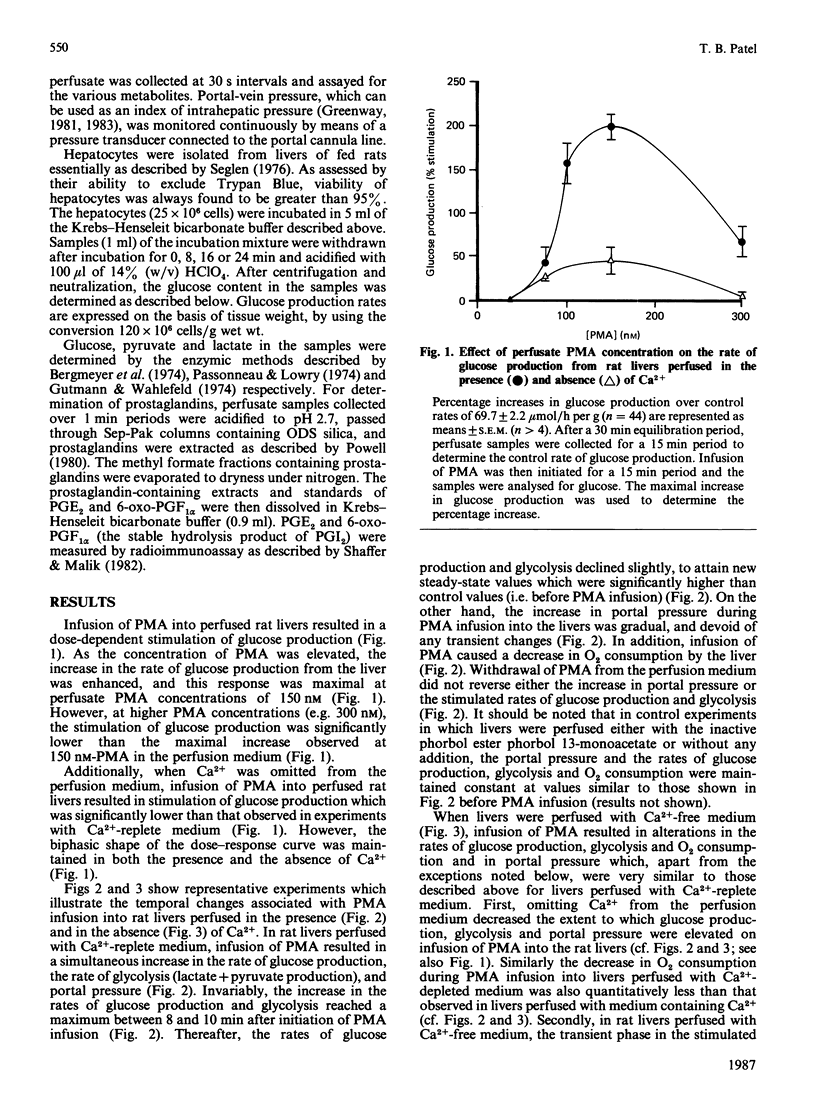
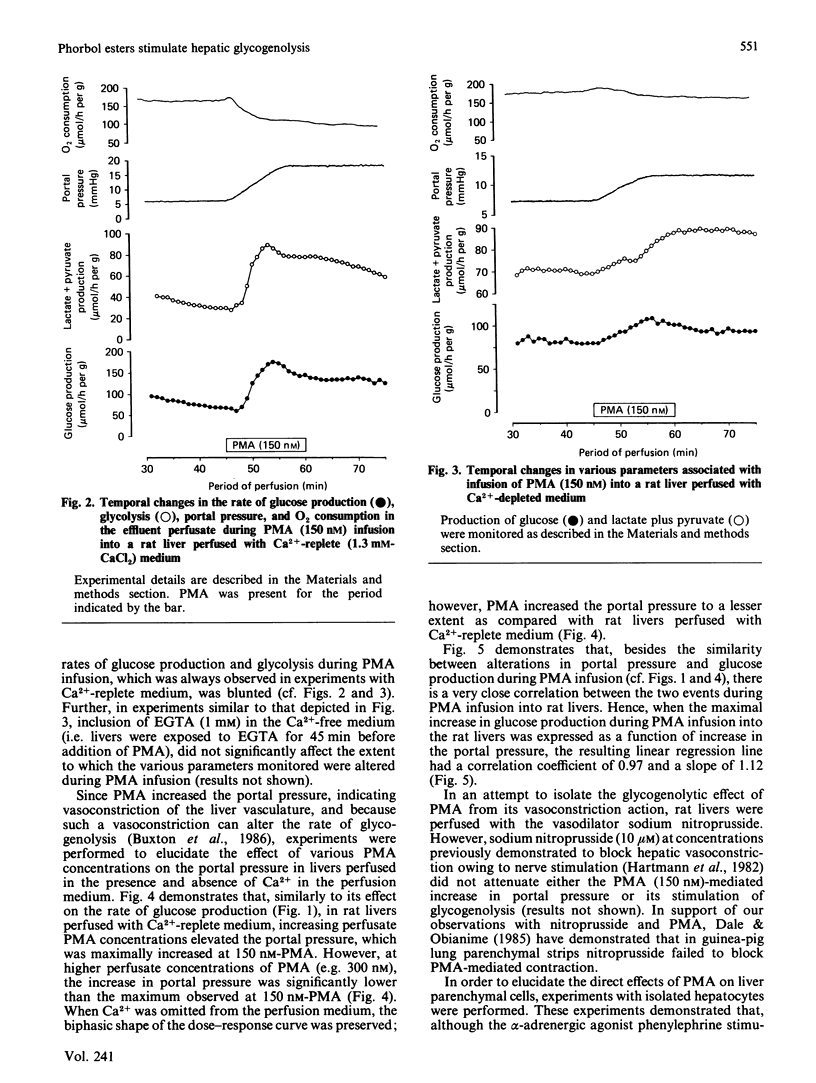
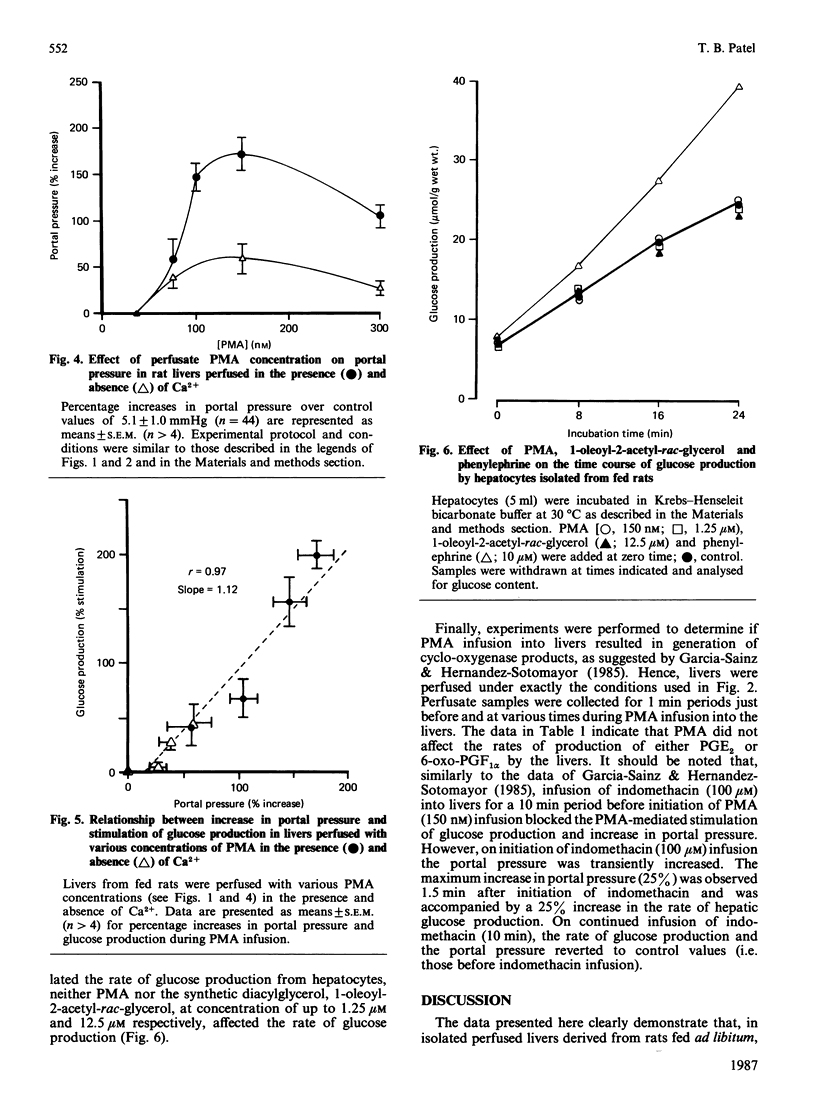
In isolated perfused rat livers, infusion of phorbol 12-myristate 13-acetate (PMA) (150 nM) resulted in a 3-fold stimulation of the rate of glucose production. This response was maximal at a perfusate PMA concentration of 150 nM, and was significantly diminished at higher concentrations of PMA (e.g. 300 nM). Stimulation of glycogenolysis by PMA was greatly decreased in livers perfused with Ca2+-free medium. PMA infusion into livers perfused in the absence of Ca2+ did not result in Ca2+ efflux from the livers. Additionally, in hepatocytes isolated from livers of fed rats, neither PMA nor 1-oleoyl-2-acetyl-rac-glycerol stimulated the rate of glucose production. Although indomethacin has been demonstrated to block PMA-stimulated hepatic glycogenolysis [Garcia-Sainz & Hernandez-Sotomayor (1985) Biochem. Biophys. Res. Commun. 132, 204-209], infusion of PMA into perfused rat livers did not alter the rates of production of either prostaglandin E2 or 6-oxo-prostaglandin F1 alpha in the livers. These data, along with the observed increases in the perfusion pressure and decrease in O2 consumption in isolated perfused livers suggest that phorbol-ester-stimulated glycogenolysis is not a consequence of a direct effect of phorbol ester on liver parenchymal cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackmore P. F., Dehaye J. P., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. The role of mitochondrial calcium release in alpha-adrenergic activation of phosphorylase in perfused rat liver. J Biol Chem. 1979 Aug 10;254(15):6945–6950. [PubMed] [Google Scholar]
- Brass E. P., Garrity M. J. Effect of nonsteroidal anti-inflammatory drugs on glycogenolysis in isolated hepatocytes. Br J Pharmacol. 1985 Oct;86(2):491–496. doi: 10.1111/j.1476-5381.1985.tb08919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton D. B., Fisher R. A., Hanahan D. J., Olson M. S. Platelet-activating factor-mediated vasoconstriction and glycogenolysis in the perfused rat liver. J Biol Chem. 1986 Jan 15;261(2):644–649. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cooper R. H., Coll K. E., Williamson J. R. Differential effects of phorbol ester on phenylephrine and vasopressin-induced Ca2+ mobilization in isolated hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3281–3288. [PubMed] [Google Scholar]
- Dale M. M., Obianime W. Phorbol myristate acetate causes in guinea-pig lung parenchymal strip a maintained spasm which is relatively resistant to isoprenaline. FEBS Lett. 1985 Oct 7;190(1):6–10. doi: 10.1016/0014-5793(85)80415-4. [DOI] [PubMed] [Google Scholar]
- Endo T., Naka M., Hidaka H. Ca2+-phospholipid dependent phosphorylation of smooth muscle myosin. Biochem Biophys Res Commun. 1982 Apr 14;105(3):942–948. doi: 10.1016/0006-291x(82)91061-0. [DOI] [PubMed] [Google Scholar]
- Forder J., Scriabine A., Rasmussen H. Plasma membrane calcium flux, protein kinase C activation and smooth muscle contraction. J Pharmacol Exp Ther. 1985 Nov;235(2):267–273. [PubMed] [Google Scholar]
- García-Sáinz J. A., Hernández-Sotomayor S. M. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoyl-phorbol-13-acetate (TPA) via cyclooxygenase products. Biochem Biophys Res Commun. 1985 Oct 15;132(1):204–209. doi: 10.1016/0006-291x(85)91008-3. [DOI] [PubMed] [Google Scholar]
- Garrison J. C., Johnsen D. E., Campanile C. P. Evidence for the role of phosphorylase kinase, protein kinase C, and other Ca2+-sensitive protein kinases in the response of hepatocytes to angiotensin II and vasopressin. J Biol Chem. 1984 Mar 10;259(5):3283–3292. [PubMed] [Google Scholar]
- Goueli S. A., Ahmed K. Indomethacin and inhibition of protein kinase reactions. Nature. 1980 Sep 11;287(5778):171–172. doi: 10.1038/287171a0. [DOI] [PubMed] [Google Scholar]
- Greenway C. V. Mechanisms and quantitative assessment of drug effects on cardiac output with a new model of the circulation. Pharmacol Rev. 1981 Dec;33(4):213–251. [PubMed] [Google Scholar]
- Greenway C. V. Role of splanchnic venous system in overall cardiovascular homeostasis. Fed Proc. 1983 Apr;42(6):1678–1684. [PubMed] [Google Scholar]
- Hartmann H., Beckh K., Jungermann K. Direct control of glycogen metabolism in the perfused rat liver by the sympathetic innervation. Eur J Biochem. 1982 Apr;123(3):521–526. doi: 10.1111/j.1432-1033.1982.tb06562.x. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Control of hepatic glycogenolysis. Physiol Rev. 1980 Jan;60(1):1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Inagaki M., Kanamaru K., Hidaka H. Phosphorylation of smooth muscle myosin light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J Biol Chem. 1985 Apr 25;260(8):4547–4550. [PubMed] [Google Scholar]
- Kantor H. S., Hampton M. Indomethacin in submicromolar concentrations inhibits cyclic AMP-dependent protein kinase. Nature. 1978 Dec 21;276(5690):841–842. doi: 10.1038/276841a0. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kimura S., Nagasaki K., Adachi I., Yamaguchi K., Fujiki H., Abe K. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoylphorbol-13-acetate (TPA) via a calcium requiring process. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1057–1064. doi: 10.1016/0006-291x(84)91198-7. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Lynch C. J., Charest R., Bocckino S. B., Exton J. H., Blackmore P. F. Inhibition of hepatic alpha 1-adrenergic effects and binding by phorbol myristate acetate. J Biol Chem. 1985 Mar 10;260(5):2844–2851. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Northover B. J. Indomethacin--a calcium antagonist. Gen Pharmacol. 1977;8(5-6):293–296. doi: 10.1016/0306-3623(77)90001-5. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Stabel S., Waterfield M. D. Purification to homogeneity of protein kinase C from bovine brain--identity with the phorbol ester receptor. EMBO J. 1984 May;3(5):953–959. doi: 10.1002/j.1460-2075.1984.tb01913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of oxygenated metabolites of arachidonic acid from biological samples using octadecylsilyl silica. Prostaglandins. 1980 Nov;20(5):947–957. doi: 10.1016/0090-6980(80)90144-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Forder J., Kojima I., Scriabine A. TPA-induced contraction of isolated rabbit vascular smooth muscle. Biochem Biophys Res Commun. 1984 Jul 31;122(2):776–784. doi: 10.1016/s0006-291x(84)80101-1. [DOI] [PubMed] [Google Scholar]
- Reilly F. D., McCuskey R. S., Cilento E. V. Hepatic microvascular regulatory mechanisms. I. Adrenergic mechanisms. Microvasc Res. 1981 Jan;21(1):103–116. doi: 10.1016/0026-2862(81)90008-x. [DOI] [PubMed] [Google Scholar]
- Scholz R., Hansen W., Thurman R. G. Interaction of mixed-function oxidation with biosynthetic processes. 1. Inhibition of gluconeogenesis by aminopyrine in perfused rat liver. Eur J Biochem. 1973 Sep 21;38(1):64–72. doi: 10.1111/j.1432-1033.1973.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shaffer J. E., Malik K. U. Enhancement of prostaglandin output during activation of beta-1 adrenoceptors in the isolated rabbit heart. J Pharmacol Exp Ther. 1982 Dec;223(3):729–735. [PubMed] [Google Scholar]
- Theen J., Gilboe D. P., Nuttall F. Q. Liver glycogen synthase and phosphorylase changes in vivo with hypoxia and anesthetics. Am J Physiol. 1982 Sep;243(3):E182–E187. doi: 10.1152/ajpendo.1982.243.3.E182. [DOI] [PubMed] [Google Scholar]


