Abstract
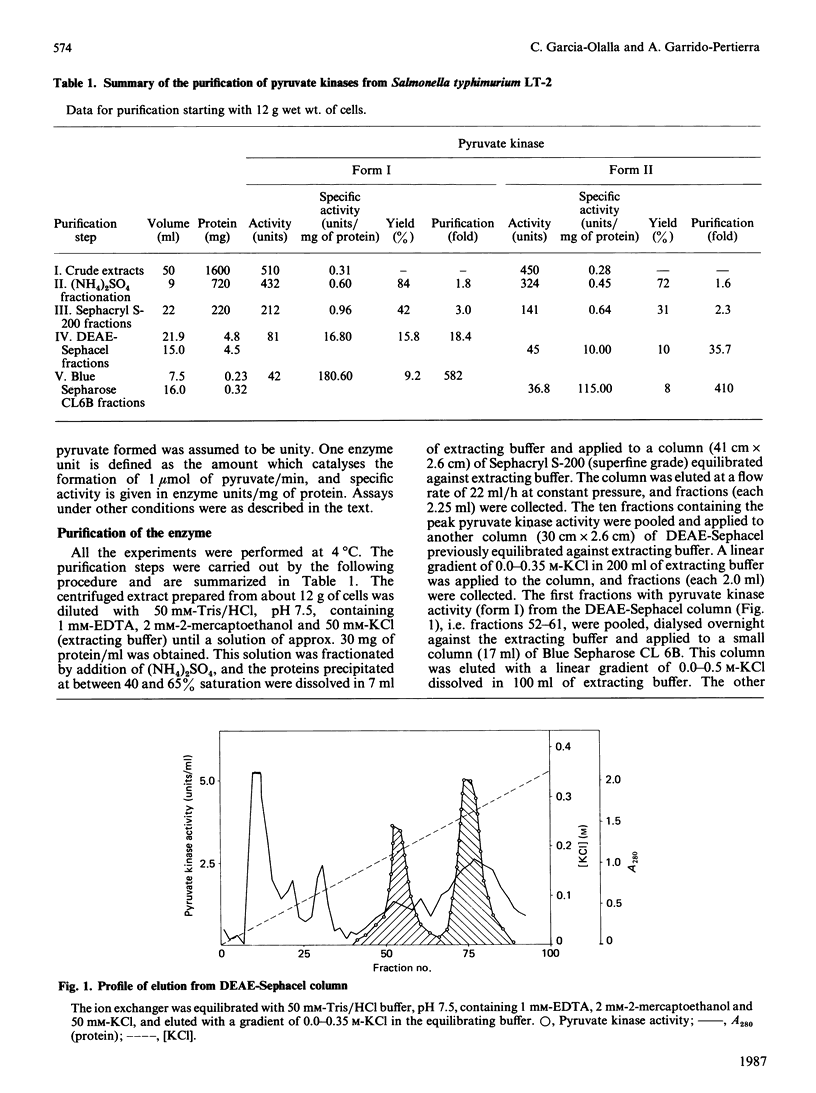
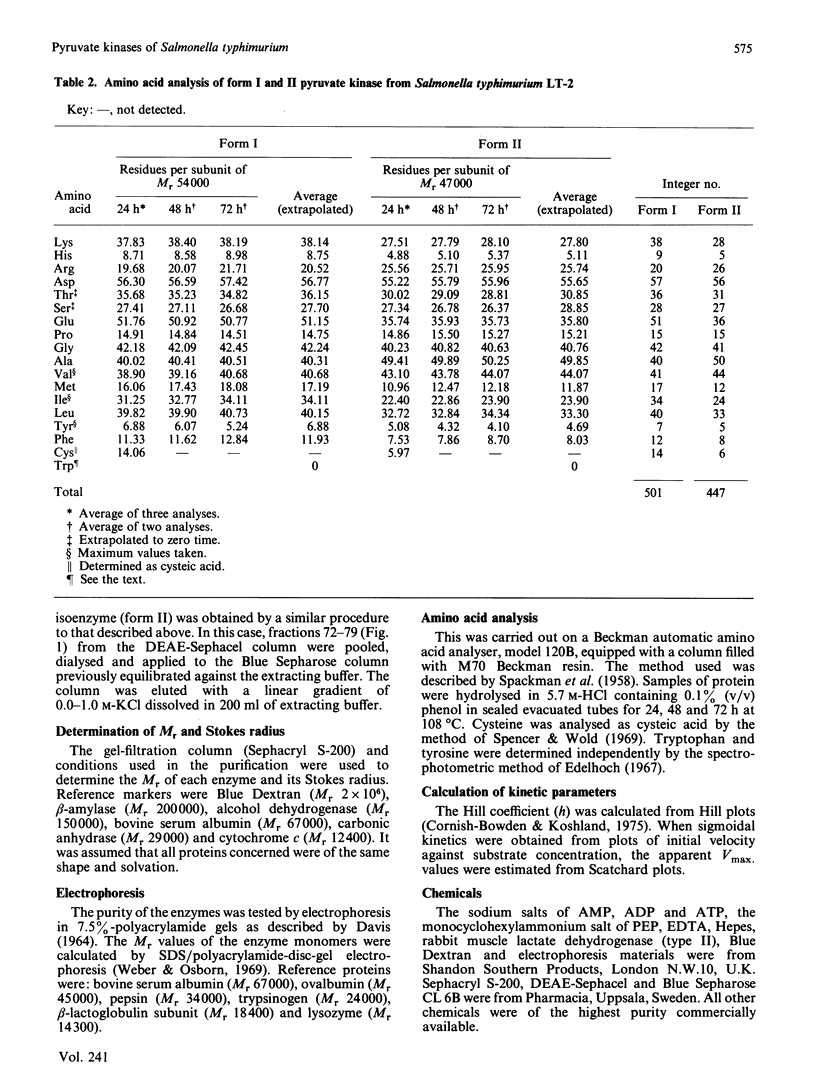
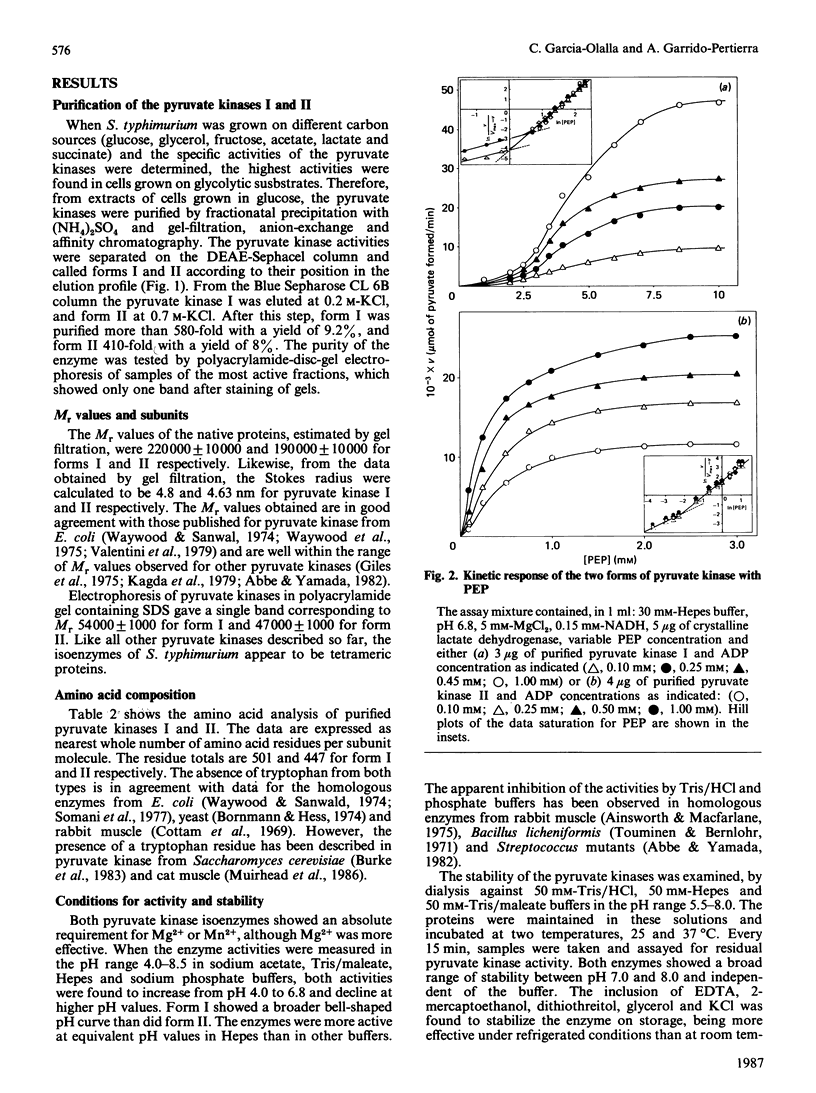
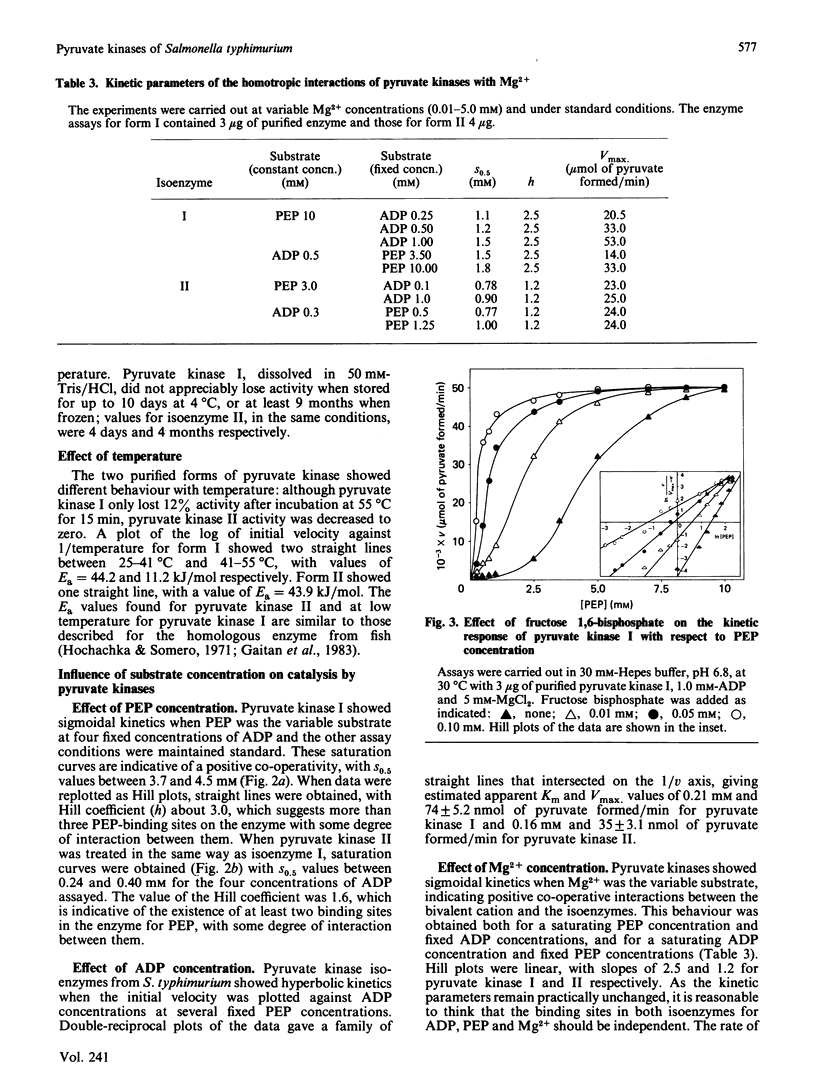
Two forms of pyruvate kinase (ATP: pyruvate 2-O-phosphotransferase, EC 2.7.1.40) present in Salmonella typhimurium were purified to homogeneity from the same cultures by (NH4)2SO4 fractionation and gel filtration, anion-exchange and affinity chromatography. Mr values, subunit structure, amino acid composition and activity and stability conditions were determined for the two forms. Kinetic and regulatory properties of the two purified isoenzymes were studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbe K., Yamada T. Purification and properties of pyruvate kinase from Streptococcus mutans. J Bacteriol. 1982 Jan;149(1):299–305. doi: 10.1128/jb.149.1.299-305.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth S., Macfarlane N. Activation and inhibition of rabbit muscle pyruvate kinase by transition-metal ions. Biochem J. 1975 Jan;145(1):63–71. doi: 10.1042/bj1450063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm C. A., Bryant C. Regulatory properties of a partially purified preparation of pyruvate kinase from Fasciola hepatica. Int J Parasitol. 1980 Apr;10(2):107–114. doi: 10.1016/0020-7519(80)90020-x. [DOI] [PubMed] [Google Scholar]
- Benziman M. Factors afecting the activity of pyruvate kinase of Acetobacter xylinum. Biochem J. 1969 May;112(5):631–636. doi: 10.1042/bj1120631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornmann L., Hess B. The subunit structure of yeast pyruvate kinase. Eur J Biochem. 1974 Aug 15;47(1):1–4. doi: 10.1111/j.1432-1033.1974.tb03660.x. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Tekamp-Olson P., Najarian R. The isolation, characterization, and sequence of the pyruvate kinase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Feb 25;258(4):2193–2201. [PubMed] [Google Scholar]
- Cornish-Bowden A., Koshland D. E., Jr Diagnostic uses of the Hill (Logit and Nernst) plots. J Mol Biol. 1975 Jun 25;95(2):201–212. doi: 10.1016/0022-2836(75)90390-3. [DOI] [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Flynn I. W., Bowman I. B. Purification and characterization of pyruvate kinase from Trypanosoma brucei. Arch Biochem Biophys. 1980 Apr 1;200(2):401–409. doi: 10.1016/0003-9861(80)90370-7. [DOI] [PubMed] [Google Scholar]
- Gaitán S., Tejero C., Ruiz-Amil M. Some comparative properties of pyruvate kinase in haematopoietic cells and erythrocytes from rainbow trout (Salmo gairdneri R). Comp Biochem Physiol B. 1983;74(4):801–805. doi: 10.1016/0305-0491(83)90149-9. [DOI] [PubMed] [Google Scholar]
- Garrido-Pertierra A., Cooper R. A. Evidence for two distinct pyruvate kinase genes in Escherichia coli K-12. FEBS Lett. 1983 Oct 17;162(2):420–422. doi: 10.1016/0014-5793(83)80799-6. [DOI] [PubMed] [Google Scholar]
- Giles I. G., Poat P. C., Munday K. A. A kinetic analysis of the fructose 1,6-diphosphate-activated pyruvate kinase from the hepatopancreas of Carcinus maenas. Biochem Soc Trans. 1975;3(5):714–716. doi: 10.1042/bst0030714. [DOI] [PubMed] [Google Scholar]
- Hall E. R., Cottam G. L. Isozymes of pyruvate kinase in vertebrates: their physical, chemical, kinetic and immunological properties. Int J Biochem. 1978;9(11):785–793. doi: 10.1016/0020-711x(78)90027-7. [DOI] [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- Ibsen K. H. Interrelationships and functions of the pyruvate kinase isozymes and their variant forms: a review. Cancer Res. 1977 Feb;37(2):341–353. [PubMed] [Google Scholar]
- Kajda P. K., Birch N. J., O'Brien M. J., Hullin R. P. Rat-brain pyruvate kinase: purification and effects of lithium. J Inorg Biochem. 1979 Dec;11(4):361–366. doi: 10.1016/s0162-0134(00)80191-9. [DOI] [PubMed] [Google Scholar]
- Kier L. D., Weppelman R., Ames B. N. Resolution and purification of three periplasmic phosphatases of Salmonella typhimurium. J Bacteriol. 1977 Apr;130(1):399–410. doi: 10.1128/jb.130.1.399-410.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarz D., Garreau H., Buc H. Regulation of the amount and of the activity of phosphofructokinases and pyruvate kinases in Escherichia coli. Biochim Biophys Acta. 1975 Feb 13;381(2):257–268. doi: 10.1016/0304-4165(75)90232-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. The regulation of pyruvate kinase of Escherichia coli by fructose diphosphate and adenylic acid. J Biol Chem. 1968 Jan 25;243(2):448–450. [PubMed] [Google Scholar]
- Malcovati M., Valentini G., Kornberg H. L. Two forms of pyruvate kinase in E. coli: their properties and regulation. Acta Vitaminol Enzymol. 1973;27(1):96–111. [PubMed] [Google Scholar]
- Morris C. N., Ainsworth S., Kinderlerer J. The regulatory properties of yeast pyruvate kinase. Biochem J. 1984 Feb 1;217(3):641–647. doi: 10.1042/bj2170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead H., Clayden D. A., Barford D., Lorimer C. G., Fothergill-Gilmore L. A., Schiltz E., Schmitt W. The structure of cat muscle pyruvate kinase. EMBO J. 1986 Mar;5(3):475–481. doi: 10.1002/j.1460-2075.1986.tb04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S. K., Hamilton I. R. Purification and regulatory properties of pyruvate kinase from Veillonella parvula. J Bacteriol. 1975 Jun;122(3):1274–1282. doi: 10.1128/jb.122.3.1274-1282.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J Biochem. 1969 Sep;66(3):297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- Pertierra A. G., Cooper R. A. Pyruvate formation during the catabolism of simple hexose sugars by Escherichia coli: studies with pyruvate kinase-negative mutants. J Bacteriol. 1977 Mar;129(3):1208–1214. doi: 10.1128/jb.129.3.1208-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Wentzel D. L., Feucht B. U., Judice J. J. A transport system for phosphoenolpyruvate, 2-phosphoglycerate, and 3-phosphoglycerate in Salmonella typhimurium. J Biol Chem. 1975 Jul 10;250(13):5089–5096. [PubMed] [Google Scholar]
- Somani B. L., Valentini G., Malcovati M. Purification and molecular properties of the AMP-activated pyruvate kinase from Escherichia coli. Biochim Biophys Acta. 1977 May 12;482(1):52–63. doi: 10.1016/0005-2744(77)90353-9. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J Biol Chem. 1971 Mar 25;246(6):1746–1755. [PubMed] [Google Scholar]
- Valentini G., Iadarola P., Somani B. L., Malcovati M. Two forms of pyruvate kinase in Escherichia coli. A comparison of chemical and molecular properties. Biochim Biophys Acta. 1979 Oct 11;570(2):248–258. doi: 10.1016/0005-2744(79)90145-1. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Rayman M. K., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. II. Effectors and regulatory properties of the enzyme activated by ribose 5-phosphate. Can J Biochem. 1975 Apr;53(4):444–454. doi: 10.1139/o75-061. [DOI] [PubMed] [Google Scholar]
- Waygood E. B., Sanwal B. D. The control of pyruvate kinases of Escherichia coli. I. Physicochemical and regulatory properties of the enzyme activated by fructose 1,6-diphosphate. J Biol Chem. 1974 Jan 10;249(1):265–274. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


