Abstract
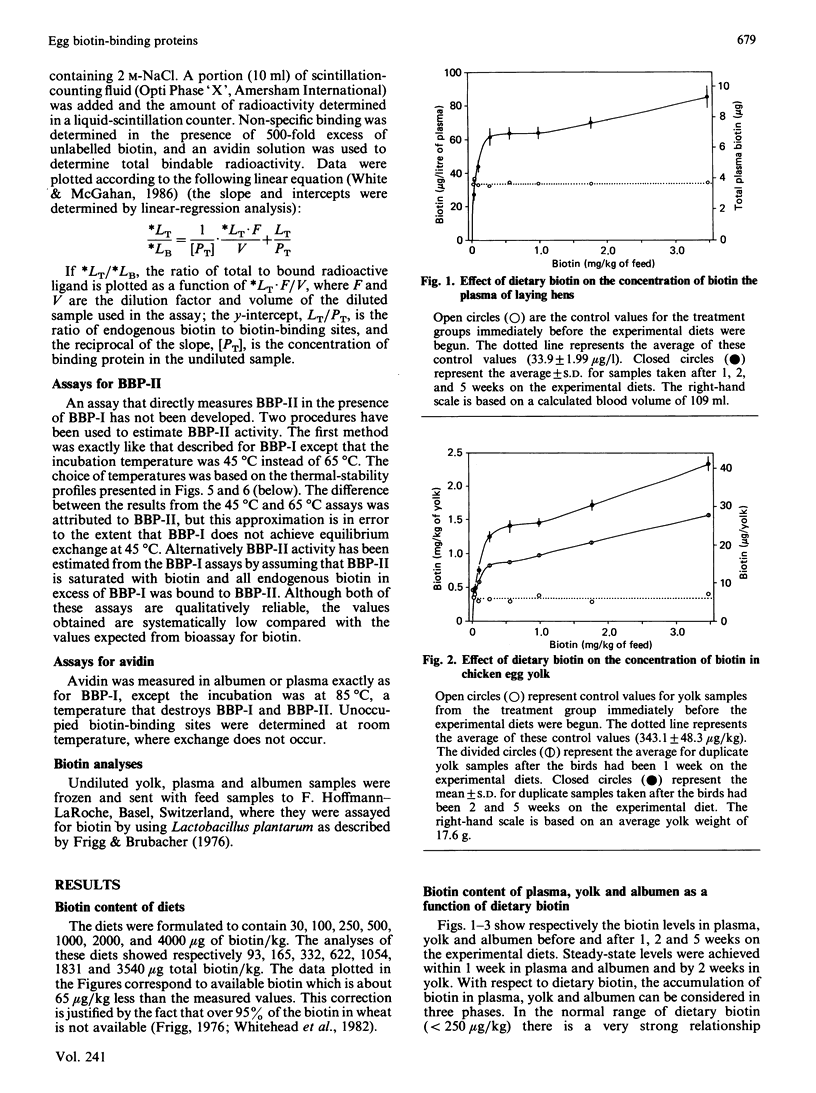
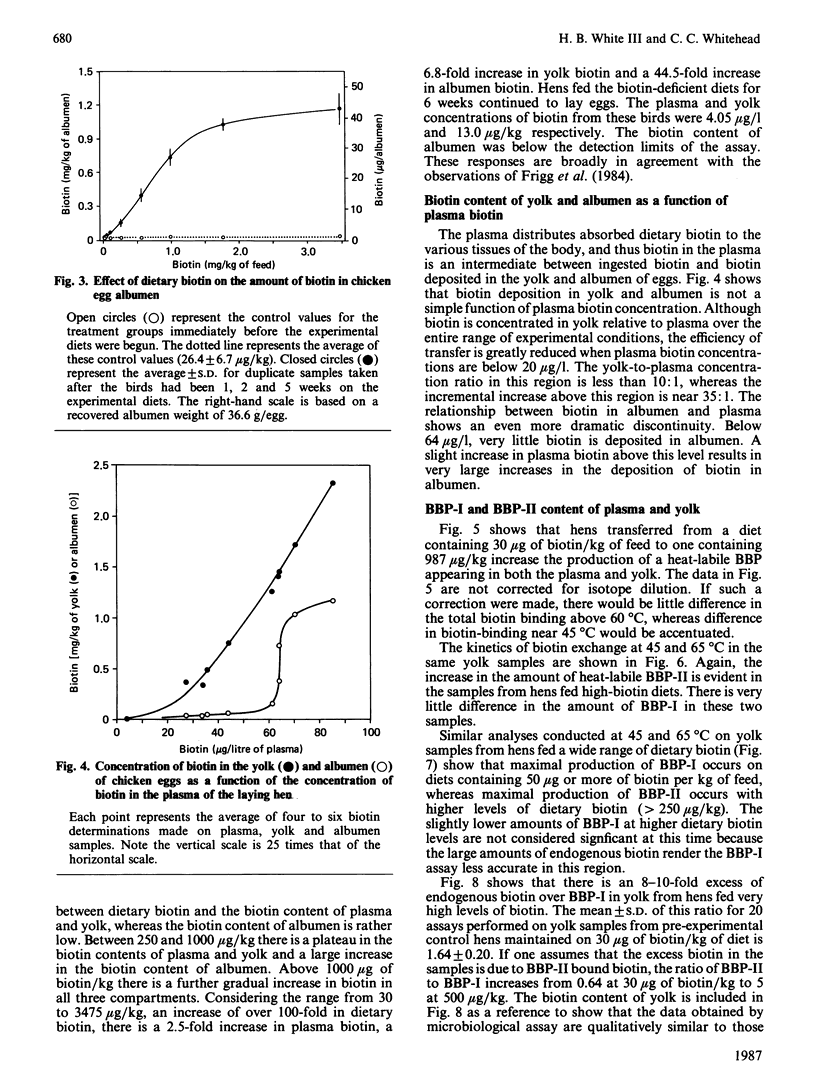
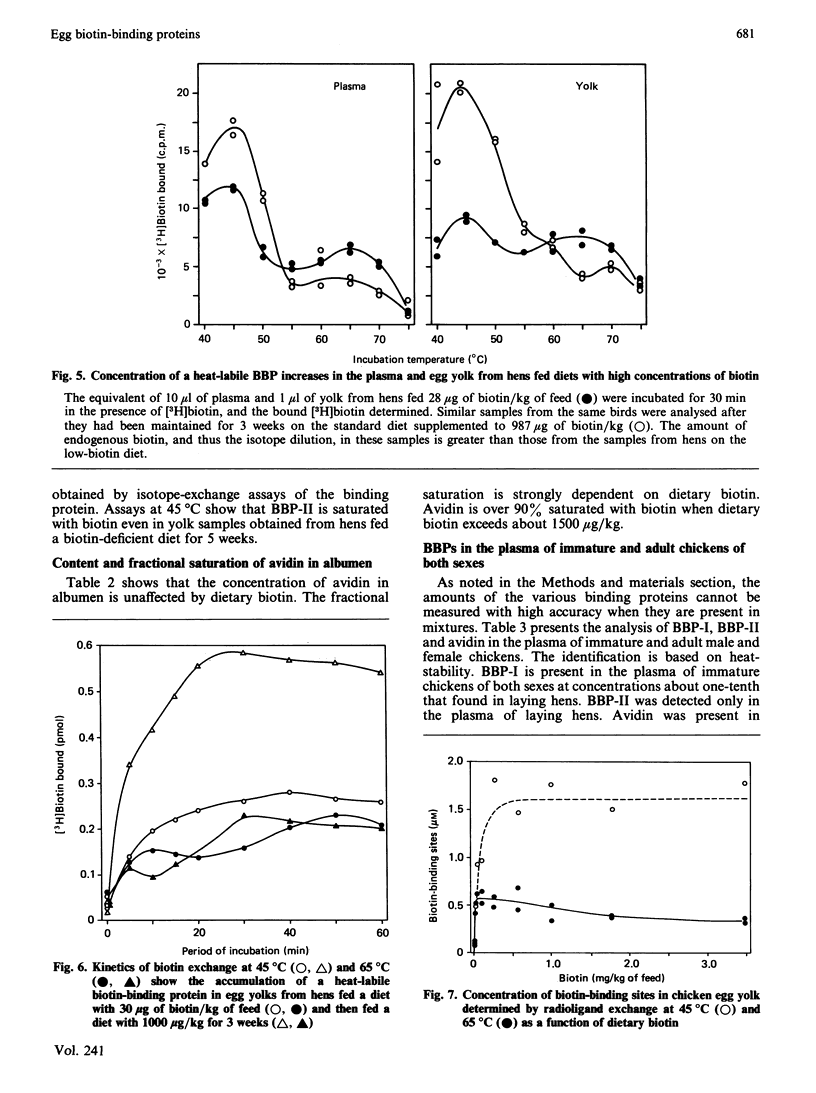
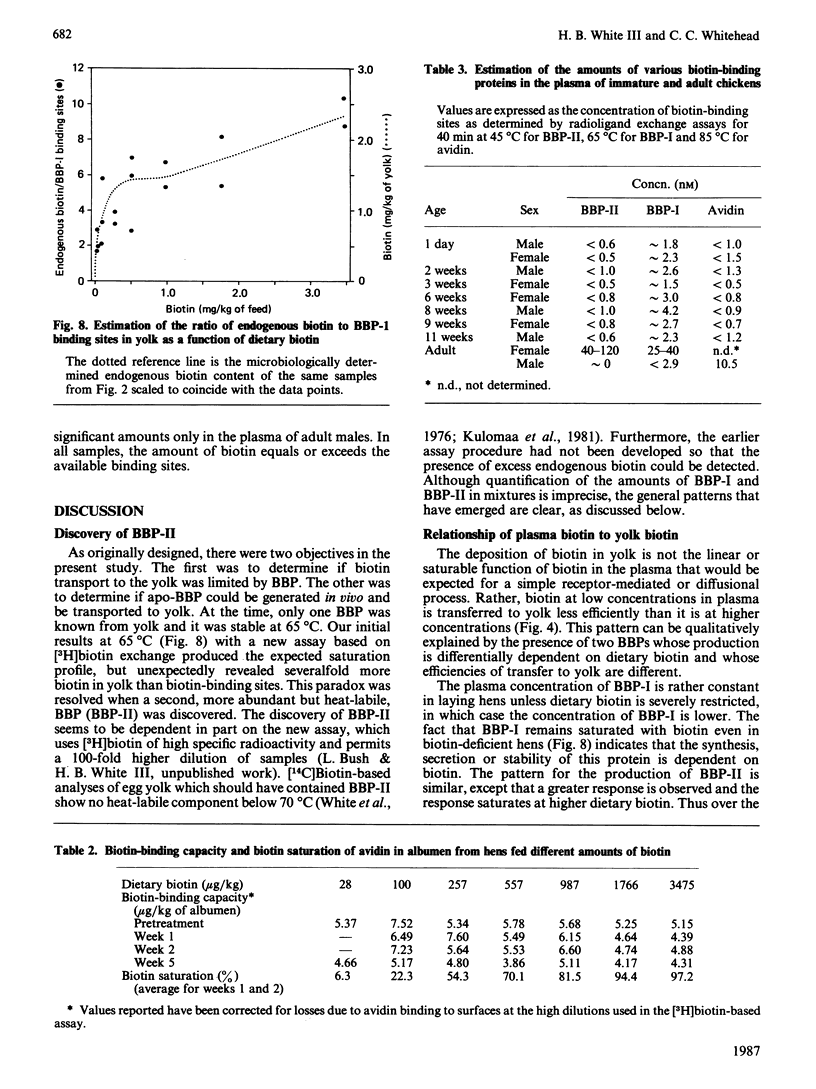
In addition to the previously characterized egg-yolk biotin-binding protein (BBP-I), we have discovered another BBP (BBP-II) in the plasma and yolk from laying hens. BBP-I is stable to 65 degrees C, whereas BBP-II is stable to 45 degrees C. Both proteins are normally saturated with biotin and together they account for most, if not all, of the biotin in hen plasma and yolk, except in hens fed excessive amounts of biotin (greater than 1 mg of biotin/kg of feed). The maximal production of BBP-I is attained at lower levels of dietary biotin (approximately 50 micrograms/kg) than for BBP-II (approximately 250 micrograms/kg); however, the maximal production of BBP-II is severalfold greater than for BBP-I. Consequently, as dietary biotin increases, the ratio of BBP-II to BBP-I increases and becomes constant at dietary intakes of biotin above 250 micrograms/kg. The observation that the amounts of these proteins are limited by biotin in the normal dietary range (less than 250 micrograms/kg) suggests that biotin is required for the synthesis, secretion or stability of these proteins. Although both plasma vitamin-protein complexes are transported to the oocyte and concentrated in the yolk, BBP-II is transferred more efficiently. Thus biotin deposition in the yolk is a function of the amounts and relative concentrations of the two proteins. Dietary biotin above 250 micrograms/kg exceeds the transport capacity of BBP-I and BBP-II in the plasma; however, unbound biotin does not accumulate. Rather it is efficiently scavenged by avidin in the oviduct and transferred to the egg albumen. Only when avidin becomes saturated at high dietary intake does free or weakly bound biotin accumulate in plasma and yolk. The synthesis of avidin is independent of dietary biotin. Small amounts of BBPs with the heat-stability of avidin or BBP-I respectively are present in the plasma of adult males or immature chickens. BBP-II, the major BBP in the plasma and yolk of laying hens, was not detected in the plasma of non-laying chickens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boas M. A. The Effect of Desiccation upon the Nutritive Properties of Egg-white. Biochem J. 1927;21(3):712–724.1. doi: 10.1042/bj0210712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo H. A., Korpela J. The occurrence and production of avidin: a new conception of the high-affinity biotin-binding protein. Comp Biochem Physiol B. 1984;78(1):15–20. doi: 10.1016/0305-0491(84)90137-8. [DOI] [PubMed] [Google Scholar]
- Elo H. A., Kulomaa M. S., Tuohimaa P. J. Avidin induction by tissue injury and inflammation in male and female chickens. Comp Biochem Physiol B. 1979;62(3):237–240. doi: 10.1016/0305-0491(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Emtage J. S. Vitamin D in the avian egg. Its molecular identity and mechanism of incorporation into yolk. Biochem J. 1976 Dec 15;160(3):671–682. doi: 10.1042/bj1600671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger J. A. Maternally derived transferrin in pigeon squabs. Science. 1971 Mar 26;171(3977):1260–1261. doi: 10.1126/science.171.3977.1260. [DOI] [PubMed] [Google Scholar]
- Frigg M., Brubacher G. Biotin deficiency in chicks fed a wheat-based diet. Int J Vitam Nutr Res. 1976;46(3):314–321. [PubMed] [Google Scholar]
- Green N. M. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- Krumins S. A., Roth T. F. High-affinity binding of lower-density lipoproteins to chicken oocyte membranes. Biochem J. 1981 May 15;196(2):481–488. doi: 10.1042/bj1960481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulomaa M. S., Elo H. A., Niemelä A. O., Tuohimaa P. J. Similarity of biotin-binding activity and immunoreactivity in chicken oviduct and non-oviduct avidin. Biochim Biophys Acta. 1981 Sep 29;670(2):207–213. doi: 10.1016/0005-2795(81)90011-8. [DOI] [PubMed] [Google Scholar]
- Loeken M. R., Roth T. F. Analysis of maternal IgG subpopulations which are transported into the chicken oocyte. Immunology. 1983 May;49(1):21–28. [PMC free article] [PubMed] [Google Scholar]
- Lotter S. E., Miller M. S., Bruch R. C., White H. B., 3rd Competitive binding assays for riboflavin and riboflavin-binding protein. Anal Biochem. 1982 Sep 1;125(1):110–117. doi: 10.1016/0003-2697(82)90390-6. [DOI] [PubMed] [Google Scholar]
- Mandella R. D., Meslar H. W., White H. B., 3rd Relationship between biotin-binding proteins from chicken plasma and egg yolk. Biochem J. 1978 Nov 1;175(2):629–633. doi: 10.1042/bj1750629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meslar H. W., Camper S. A., White H. B., 3rd Biotin-binding protein from egg yolk. A protein distinct from egg white avidin. J Biol Chem. 1978 Oct 10;253(19):6979–6982. [PubMed] [Google Scholar]
- Meslar H. W., White H. B., 3rd Egg yolk biotin-binding protein: assay and purification. Methods Enzymol. 1979;62:316–319. doi: 10.1016/0076-6879(79)62236-x. [DOI] [PubMed] [Google Scholar]
- Murthy C. V., Adiga P. R. Purification of biotin-binding protein from chicken egg yolk and comparison with avidin. Biochim Biophys Acta. 1984 May 17;786(3):222–230. doi: 10.1016/0167-4838(84)90092-x. [DOI] [PubMed] [Google Scholar]
- Murty C. V., Adiga P. R. Estrogen induction of biotin-binding protein in immature chicks: kinetics, hormonal specificity and modulation. Mol Cell Endocrinol. 1985 Apr;40(1):79–86. doi: 10.1016/0303-7207(85)90160-1. [DOI] [PubMed] [Google Scholar]
- Perry M. M., Gilbert A. B., Evans A. J. Electron microscope observations on the ovarian follicle of the domestic fowl during the rapid growth phase. J Anat. 1978 Mar;125(Pt 3):481–497. [PMC free article] [PubMed] [Google Scholar]
- Perry M. M., Griffin H. D., Gilbert A. B. The binding of very low density and low density lipoproteins to the plasma membrane of the hen's oocyte. A morphological study. Exp Cell Res. 1984 Apr;151(2):433–446. doi: 10.1016/0014-4827(84)90393-8. [DOI] [PubMed] [Google Scholar]
- Schjeide O. A., Briles W. E., Holshouser S., Jones D. G. Effect of "restricted ovulator" gene on uptake of yolk-precursor protein. Cell Tissue Res. 1976 Feb 6;166(1):109–116. doi: 10.1007/BF00215130. [DOI] [PubMed] [Google Scholar]
- White H. B., 3rd, Armstrong J., Whitehead C. C. Riboflavin-binding protein. Concentration and fractional saturation in chicken eggs as a function of dietary riboflavin. Biochem J. 1986 Sep 15;238(3):671–675. doi: 10.1042/bj2380671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. B., 3rd Biotin-binding proteins and biotin transport to oocytes. Ann N Y Acad Sci. 1985;447:202–211. doi: 10.1111/j.1749-6632.1985.tb18438.x. [DOI] [PubMed] [Google Scholar]
- White H. B., 3rd, Dennison B. A., Della Fera M. A., Whitney C. J., McGuire J. C., Meslar H. W., Sammelwitz P. H. Biotin-binding protein from chicken egg yolk. Assay and relationship to egg-white avidin. Biochem J. 1976 Aug 1;157(2):395–400. doi: 10.1042/bj1570395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C. C., Armstrong J. A., Waddington D. The determination of the availability to chicks of biotin in feed ingredients by a bioassay based on the response of blood pyruvate carboxylase (EC 6.4.1.1) activity. Br J Nutr. 1982 Jul;48(1):81–88. doi: 10.1079/bjn19820090. [DOI] [PubMed] [Google Scholar]
- Whitehead C. C. Performance of laying hens fed on practical diets containing different levels of supplemental biotin during the rearing and laying stages. Br J Nutr. 1980 Sep;44(2):151–159. doi: 10.1079/bjn19800022. [DOI] [PubMed] [Google Scholar]
- Woods J. W., Roth T. F. A specific subunit of vitellogenin that mediates receptor binding. Biochemistry. 1984 Nov 20;23(24):5774–5780. doi: 10.1021/bi00319a016. [DOI] [PubMed] [Google Scholar]
- Yusko S., Roth T. F., Smith T. Receptor-mediated vitellogenin binding to chicken oocytes. Biochem J. 1981 Oct 15;200(1):43–50. doi: 10.1042/bj2000043. [DOI] [PMC free article] [PubMed] [Google Scholar]


