Abstract
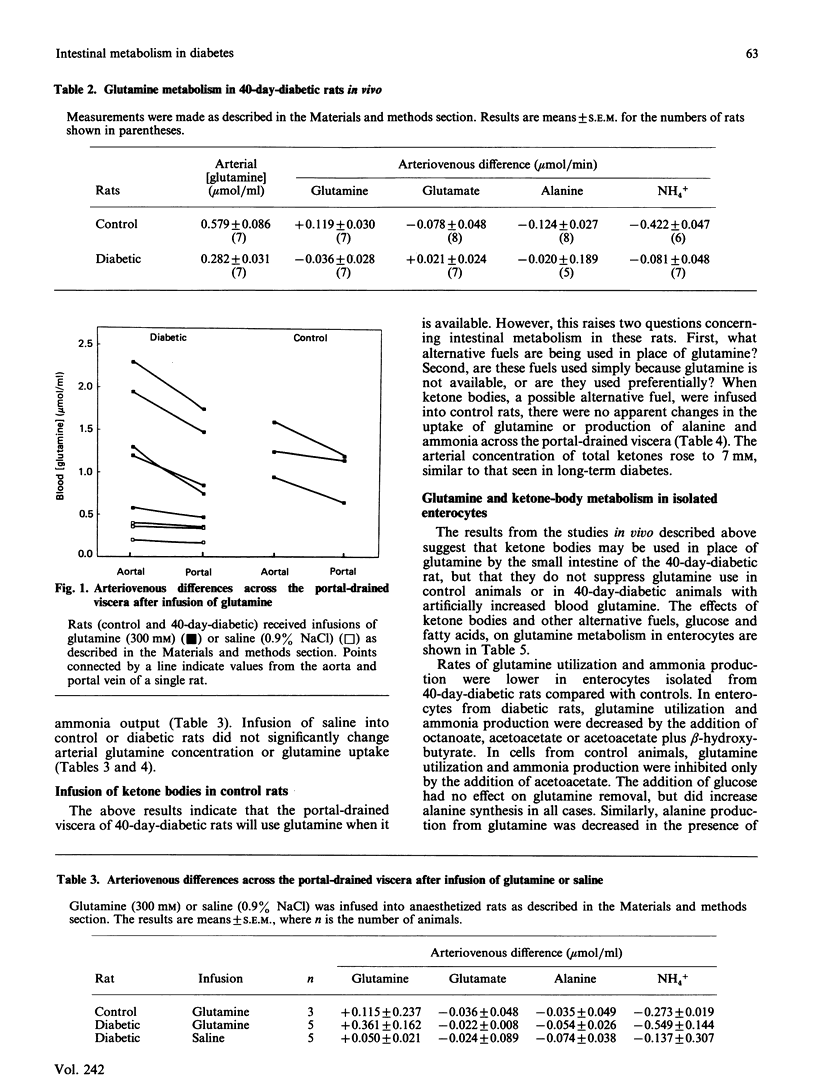
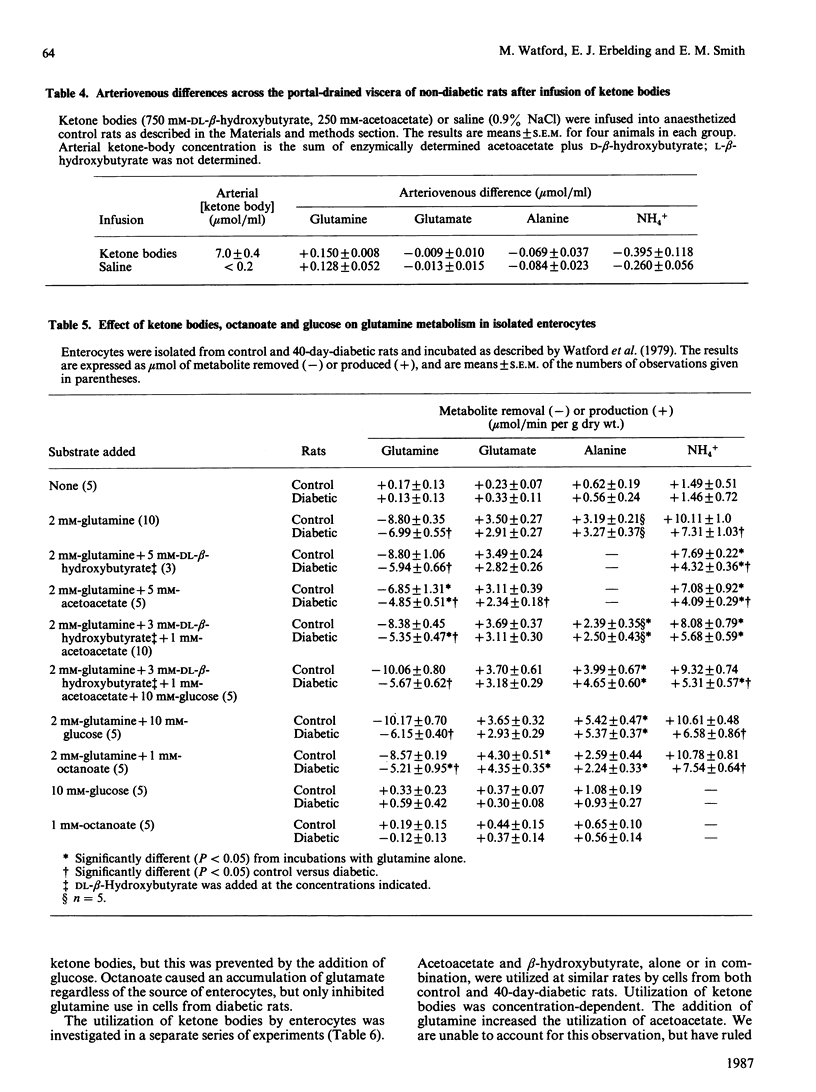
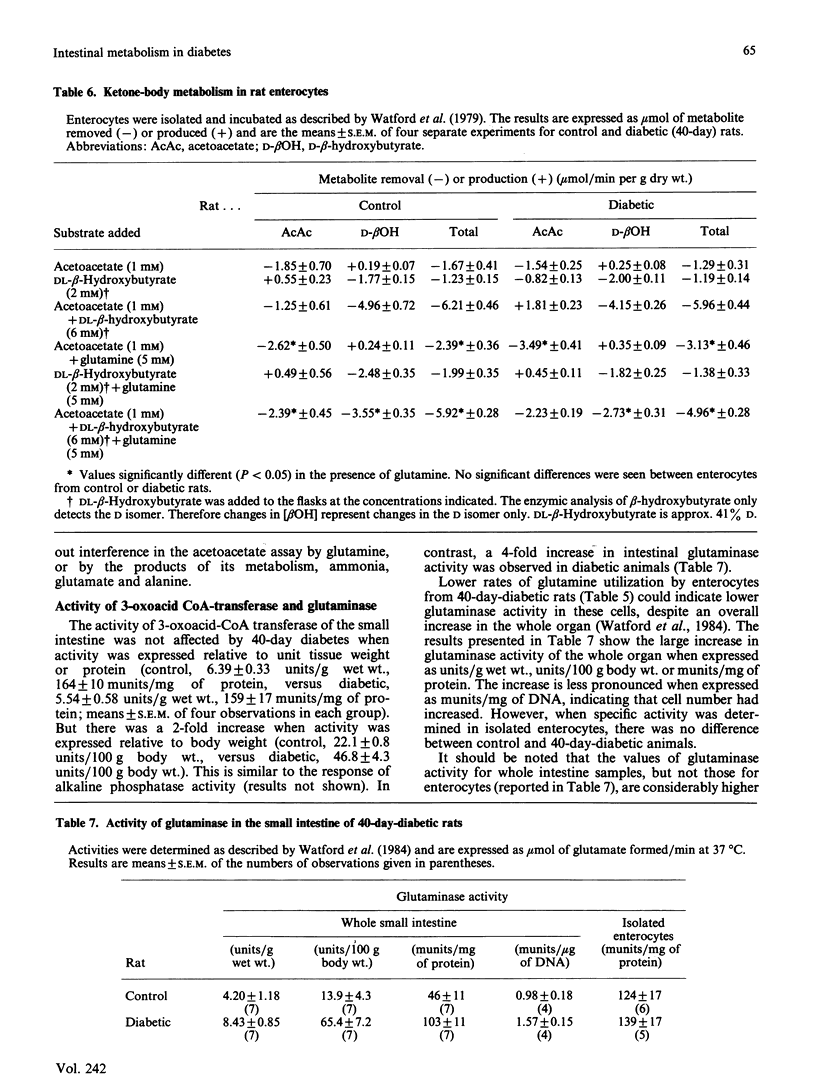
The small intestine is the major site of glutamine utilization in the mammalian body. During prolonged (40-day) streptozotocin-diabetes in the rat there is a marked increase in both the size and the phosphate-activated glutaminase activity of the small intestine. Despite this increased capacity, intestinal glutamine utilization ceases in diabetic rats. Mean arterial glutamine concentration fell by more than 50% in diabetic rats, suggesting that substrate availability is responsible for the decrease in intestinal glutamine use. When arterial glutamine concentrations in diabetic rats were elevated by infusion of glutamine solutions, glutamine uptake across the portal-drained viscera was observed. The effect of other respiratory fuels on intestinal glutamine metabolism was examined. Infusions of ketone bodies did not affect glutamine use by the portal-drained viscera of non-diabetic rats. Prolonged diabetes had no effect on the activity of 3-oxoacid CoA-transferase in the small intestine or on the rate of ketone-body utilization in isolated enterocytes. Glutamine (2 mM) utilization was decreased in enterocytes isolated from diabetic rats as compared with those from control animals. However, glutaminase activity in homogenates of enterocytes was unchanged by diabetes. In enterocytes isolated from diabetic rats the addition of ketone bodies or octanoate decreased glutamine use. It is proposed that during prolonged diabetes ketone bodies, and possibly fatty acids, replace glutamine as the major respiratory fuel of the small intestine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Man K. C., Hall D. E., Colbourne S. A., Brosnan M. E. Interorgan metabolism of amino acids in streptozotocin-diabetic ketoacidotic rat. Am J Physiol. 1983 Feb;244(2):E151–E158. doi: 10.1152/ajpendo.1983.244.2.E151. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr Starvation in man. N Engl J Med. 1970 Mar 19;282(12):668–675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- Caldecourt M. A., Cox D. J., Sugden M. C., Palmer T. N. Glycolytic origin of alanine formed in rat diaphragm muscle in vitro. Biochem J. 1985 Nov 1;231(3):801–804. doi: 10.1042/bj2310801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E., Williams P. E., Radosevich P. M., Hoxworth B. T., Lacy W. W., Abumrad N. N. Role of glutamine in adaptations in nitrogen metabolism during fasting. Am J Physiol. 1986 Jun;250(6 Pt 1):E622–E628. doi: 10.1152/ajpendo.1986.250.6.E622. [DOI] [PubMed] [Google Scholar]
- Cersosimo E., Williams P., Hoxworth B., Lacy W., Abumrad N. Glutamine blocks lipolysis and ketogenesis of fasting. Am J Physiol. 1986 Mar;250(3 Pt 1):E248–E252. doi: 10.1152/ajpendo.1986.250.3.E248. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- Hanson P. J., Carrington J. M. Activity of 3-oxo acid CoA-transferase, D-3-hydroxybutyrate dehydrogenase, hexokinase and carnitine palmitoyltransferase in the stomach and small and large intestine of the rat. Biochem J. 1981 Nov 15;200(2):349–355. doi: 10.1042/bj2000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. Factors affecting the utilization of ketone bodies and other substrates by rat jejunum: effects of fasting and of diabetes. J Physiol. 1978 May;278:55–67. doi: 10.1113/jphysiol.1978.sp012292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976 Mar;255(3):775–795. doi: 10.1113/jphysiol.1976.sp011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson P. J., Parsons S. Metabolism and transport of glutamine and glucose in vascularly perfused small intestine rat. Biochem J. 1977 Sep 15;166(3):509–519. doi: 10.1042/bj1660509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsmann W. C., Breeman W. A., Stam H., Kort W. J. Comparative study of chylomicron and fatty acid utilization in small intestine and heart. Biochim Biophys Acta. 1981 Feb 23;663(2):373–379. doi: 10.1016/0005-2760(81)90166-1. [DOI] [PubMed] [Google Scholar]
- Hülsmann W. C., Iemhoff W. G., van den Berg J. W., de Pijper A. M. Unequal rates of development of mitochondrial enzymes in rat small intestinal epithelium. Biochim Biophys Acta. 1970 Sep 22;215(3):553–555. doi: 10.1016/0304-4165(70)90108-x. [DOI] [PubMed] [Google Scholar]
- Hülsmann W. C. Preferential oxidation of fatty acids by rat small intestine. FEBS Lett. 1971 Sep 15;17(1):35–38. doi: 10.1016/0014-5793(71)80557-4. [DOI] [PubMed] [Google Scholar]
- Iemhoff W. G., Hülsmann W. C. Development of mitochondrial enzyme activities in rat-small-intestinal epithelium. Eur J Biochem. 1971 Dec 10;23(3):429–434. doi: 10.1111/j.1432-1033.1971.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z., McGivan J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983 Apr;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- Mallet R. T., Kelleher J. K., Jackson M. J. Substrate metabolism of isolated jejunal epithelium: conservation of three-carbon units. Am J Physiol. 1986 Feb;250(2 Pt 1):C191–C198. doi: 10.1152/ajpcell.1986.250.2.C191. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L. Glycolysis, glutaminolysis and cell proliferation. Cell Biol Int Rep. 1982 Jul;6(7):635–650. doi: 10.1016/0309-1651(82)90125-4. [DOI] [PubMed] [Google Scholar]
- NEPTUNE E. M., Jr RESPIRATION AND OXIDATION OF VARIOUS SUBSTRATES BY ILEUM IN VITRO. Am J Physiol. 1965 Aug;209:329–332. doi: 10.1152/ajplegacy.1965.209.2.329. [DOI] [PubMed] [Google Scholar]
- Nagy L. E., Kretchmer N. Effect of diabetic ketosis on jejunal glutaminase. Arch Biochem Biophys. 1986 Jul;248(1):80–88. doi: 10.1016/0003-9861(86)90403-0. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985 Oct;70(4):473–489. doi: 10.1113/expphysiol.1985.sp002935. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985 May;5(5):393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- Pinkus L. M., Windmueller H. G. Phosphate-dependent glutaminase of small intestine: localization and role in intestinal glutamine metabolism. Arch Biochem Biophys. 1977 Aug;182(2):506–517. doi: 10.1016/0003-9861(77)90531-8. [DOI] [PubMed] [Google Scholar]
- Porteous J. W. Glutamate, glutamine, aspartate, asparagine, glucose and ketone-body metabolism in chick intestinal brush-border cells. Biochem J. 1980 Jun 15;188(3):619–632. doi: 10.1042/bj1880619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Williamson D. H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol Rev. 1980 Jan;60(1):143–187. doi: 10.1152/physrev.1980.60.1.143. [DOI] [PubMed] [Google Scholar]
- Rofe A. M., Bais R., Conyers R. A. Ketone-body metabolism in tumour-bearing rats. Biochem J. 1986 Jan 15;233(2):485–491. doi: 10.1042/bj2330485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler C. M., Pugh-Humphreys G. P., Porteous J. W. Characterization of columnar absorptive epithelial cells isolated from rat jejunum. J Cell Sci. 1978 Feb;29:53–75. doi: 10.1242/jcs.29.1.53. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Wagenmakers A. J., Salden H. J., Veerkamp J. H. The metabolic fate of branched-chain amino acids and 2-oxo acids in rat muscle homogenates and diaphragms. Int J Biochem. 1985;17(9):957–965. doi: 10.1016/0020-711x(85)90240-x. [DOI] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M., Smith E. M., Erbelding E. J. The regulation of phosphate-activated glutaminase activity and glutamine metabolism in the streptozotocin-diabetic rat. Biochem J. 1984 Nov 15;224(1):207–214. doi: 10.1042/bj2240207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Bates M. W., Page M. A., Krebs H. A. Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J. 1971 Jan;121(1):41–47. doi: 10.1042/bj1210041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Kunenzel P. The nature of the cytoplasmic-3-hydroxybutyrate dehydrogenase from sheep kidney. Biochem J. 1971 Feb;121(3):569–570. [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem. 1978 Jan 10;253(1):69–76. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from blood. Arch Biochem Biophys. 1975 Dec;171(2):662–672. doi: 10.1016/0003-9861(75)90078-8. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980 Jan 10;255(1):107–112. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]
- Zielke H. R., Zielke C. L., Ozand P. T. Glutamine: a major energy source for cultured mammalian cells. Fed Proc. 1984 Jan;43(1):121–125. [PubMed] [Google Scholar]


